Abstract
Background
This study examined whether mobile phone-based, one-way video messages about diabetes self-care improve hemoglobin A1c (A1C) and self-monitoring of blood glucose (SMBG).
Methods
This was a 1-year prospective randomized trial with two groups. The active intervention lasted 6 months. The study enrolled 65 people with A1C >8.0% who were established (>6 months) patients in the endocrinology clinics of the Walter Reed Health Care System. Participants were randomized to receive “usual care” or self-care video messages from their diabetes nurse practitioner. Video messages were sent daily to cell phones of study participants. Hemoglobin A1c and SMBG data were collected at 0, 3, 6, 9, and 12 months.
Results
Participants who received the messages had a larger rate of decline in A1C than people who received usual care (0.2% difference over 12 months, adjusting for covariates; p = .002 and p = .004 for the interaction between time and group and for the quadratic effect of time by group, respectively). Hemoglobin A1c decline was greatest among participants who received video messages and viewed >10 a month (0.6% difference over 12 months, adjusting for covariates; p < .001 for the interaction between time and group and the quadratic effect). Self-monitoring of blood glucose metrics were not related to the intervention.
Conclusions
A one-way intervention using mobile phone-based video messages about diabetes self-care can improve A1C. Engagement with the technology is an important predictor of its success. This intervention is simple to implement and sustain.
Keywords: diabetes education, lifestyle, mobile health, telemedicine
Introduction
Despite the well-documented benefits of glycemic control1,2 and a secular trend to overall improvement in people with diabetes,3 glycemic control is still suboptimal in many patients. According to the National Health and Nutrition Examination Survey, 43.2% of people with diabetes had hemoglobin A1c (A1C) levels greater than or equal to the generally recommended target of 7.0%.3 Achieving target glycemic control typically requires a multifactorial approach with considerable commitment from the person with diabetes to examine and interpret random blood glucose readings correctly, take medications as prescribed, follow a balanced, whole foods-based diet, and engage in regular physical activity. For a variety of reasons, many people with diabetes do not adhere to these requirements;4–8 failure to do so may be due to inadequate education about the purpose and outcomes of such behaviors and the absence of support and/or reminders.
Researchers have sought to determine whether mobile health (mhealth) on a cell phone can support diabetes management and self-care.9 Such a solution is attractive because cell phones are ubiquitous, mobile (support can be available anytime and anywhere), and increasingly “smart.” The “smart” features of cell phones allow patients to upload or manually type in-home monitoring data, receive provider feedback via a phone call or short message service (SMS), receive reminders and tips, and access information at a Web site through the cell phone’s browser. Thus far, some but not all mhealth research suggest that mobile phone-based interventions to support diabetes care result in favorable clinical outcomes, particularly if the intervention involves two-way communication with data inputs from the patients and individualized feedback from a health care provider.9–15
In the present study, people with diabetes received daily, asynchronous one-way videos of diabetes-related tips and reminders delivered via cell phones. The intervention was an adjunct to usual and specialty diabetes care, aimed at providing generalized lifestyle support to people who were not meeting glycemic targets despite receiving specialty diabetes care. The primary study hypothesis was that those subjects who received daily video messages on their mobile phones about diabetes self-care over 6 months would improve their glycemic control at 6 months and that it would continue over the ensuing 6 months. In addition, we hypothesized that the intervention would be associated with greater adherence to SMBG and better glycemic metrics derived from self-monitoring data.
Methods
Design Overview
The study was a 1-year prospective randomized trial, with active intervention during the first 6 months.
Participants and Recruitment
Patients with poorly controlled type 1 or type 2 diabetes (i.e., A1C >8.0%) were recruited from the outpatient clinics of the Diabetes Institute in the Walter Reed Health Care System, Washington, DC. The Walter Reed Health Care System treats active duty military, retirees from the military, and their dependents. All diabetes supplies, including meters and strips, are provided to patients without charge.
To be eligible for the study, patients had to be at least 18 years of age, had to have received care from a nurse practitioner (NP) of the Diabetes Institute for at least 6 months and still be poorly controlled (A1C >8%), and had to be taking oral hypoglycemic medications and/or insulin. All patients were able to demonstrate their ability to use a mobile phone and were provided with a mobile phone and subscription for 6 months. Patients who were pregnant, lactating, planning to become pregnant, without reliable contraception, or using glucocorticoids, amphetamines, anabolic, or weight-reducing agents were excluded.
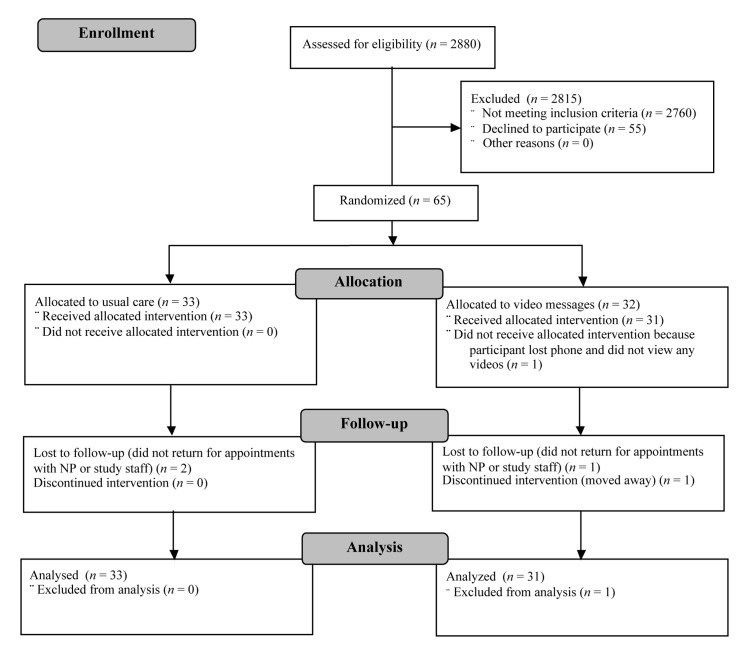
Recruitment took place from November 2007 to February 2009 (Figure 1). Study staff examined the appointment schedules of the Diabetes Institute’s NPs for upcoming appointments and determined the eligibility of these scheduled patients by looking in the electronic medical record. Study staff then contacted all eligible patients by phone or in person to describe the study. The study was approved by the Human Use Committee/Institutional Review Board at the Walter Reed Army Medical Center. All eligible and interested patients provided written, informed consent.
Figure 1.
CONSORT diagram.
The study enrolled 65 participants. The achieved power for this study is 0.93 given a medium effect of 0.25, an alpha of 0.05, a correlation of 0.40 between repeated factors, and a correction for nonsphericity in which epsilon is 0.40. One participant had a baseline A1C that was greater than 15%, an outlying value, so the analyses exclude those data (n = 64).
Intervention
Following enrollment, participants were randomized to receive usual care (defined as the care that would be provided if the patient was not in the study) or video messages daily from their own NP. The study used block randomization, which assumed the ratio of active intervention to control was balanced.
Six NPs created 540 30- to 60-second videos covering self-care topics outlined by the American Association of Diabetes Educators,7—e.g., healthy eating, being active, monitoring.16 Samples of the scripts for the videos are in the online Appendix (Table 1), and sample videos are available at: http://www.wramc.army.mil/Patients/healthcare/medicine/diabetes/Pages/default.aspx. Video messages of the NPs were sent to their patients in random order, at the time of day determined by the participants after randomization. Each video could be viewed multiple times throughout the 24-hour period before the next video was sent.
All enrolled participants received a broadband-enabled cell phone and service for 6 months, paid for by the study.
Measures
Participants were seen by the study staff at baseline and quarterly thereafter for the collection of study metrics. The primary research outcome was glycemic control as measured by A1C. The A1C was measured using a COBAS® C 111 analyzer (Roche Diagnostics, Indianapolis, IN) with a Tina-quant® HbA1c Gen. 2 whole blood assay (Roche Diagnostics) in the Walter Reed Clinical Laboratory. The secondary research outcomes were change in weight, change in blood pressure (BP), whether the participants provided SMBG measurement data (as a proxy for whether they collected it), the proportion of SMBG measurements that were above 180 mg/dl and below 70 mg/dl, and the mean of participants’ SMBG values at each quarterly visit.
We counted the number of videos each participant viewed per month and then grouped participants as follows: (1) did not view videos at all or did so briefly at the beginning of their participation and then stopped in the first 2 months (early cessation; n = 11); (2) viewed the videos throughout the active intervention but <10/month, sometimes missing whole weeks (intermittent viewers; n = 10); and (3) viewed 10+ videos/month (persistent viewers; n = 10).
We obtained age, gender, race/ethnicity, duration of diabetes, type of diabetes, and medications used to manage diabetes at baseline from the medical record.
Statistical Analysis
The analyses examined group differences in background characteristics and changes from baseline of the outcome measures using t-tests and chi-square tests. Next, the analyses estimated multilevel (i.e., mixed or individual growth) models for repeated measures to characterize within- and inter-individual change in actual A1C values. These models included potentially confounding background characteristics defined as such by clinical experience (e.g., type of diabetes) or demographics (e.g., gender, age) and quadratic effects for time, which permitted analyses of the anticipated leveling of change in A1C after cessation of the intervention. The analyses then used chi-square tests or Fisher’s exact test to examine group differences in the provision of SMBG data and analysis of variance to test for within- and between-group differences in the SMBG metrics. All statistical analyses used SAS® 9.2 (SAS Institute Inc., Cary, NC).
Results
Characteristics of the study population are shown in Table 1. Mean age of the participants was 55 (video messages group) and 60 years (usual care group). Overall, most participants attended at least some college, were African American, had type 2 diabetes, and were obese. Mean years since diabetes diagnosis and medication usage were similar between the two groups.
Table 1.
Baseline Characteristics of the Study Participants, Total and by Groupa
| Measure | Total sample (n = 64) | Video messages group (n = 31) | Usual care group (n = 33) | p value |
|---|---|---|---|---|
| Age (mean, SD) | 58 (11) | 55 (10) | 60 (11) | .06 |
| Male (n, %) | 35 (55%) | 15 (48%) | 20 (61%) | .33 |
| Education (n, %): | .23 | |||
| Less than HS grad | 4 (6%) | 1 (3%) | 3 (9%) | |
| Completed HS | 8 (13%) | 4 (13%) | 4 (12%) | |
| Some college | 28 (44%) | 17 (55%) | 11 (33%) | |
| College grad or higher | 23 (36%) | 8 (26%) | 15 (45%) | |
| Ethnicity (n): | .78 | |||
| Black | 37 (58%) | 19 (61%) | 18 (55%) | |
| Asian | 3 (5%) | 2 (6%) | 1 (3%) | |
| Hispanic | 4 (6%) | 2 (6%) | 2 (6%) | |
| White | 20 (31%) | 8 (26%) | 12 (36%) | |
| Type 2 (%) | 59 (92%) | 27 (87%) | 32 (97%) | .14 |
| Years since diagnosis (mean, SD) | 13 (9) | 14 (9) | 13 (9) | .64 |
| Systolic BP (mean, SD) | 136 (19) | 132 (21) | 139 (17) | .16 |
| Diastolic BP | 78 (11) | 77 (10) | 80 (12) | .20 |
| Body mass index (mean, SD) | 34 (7) | 33 (6) | 35 (8) | .29 |
| Medications—taking (n, %): | ||||
| Exenatide (Byetta®) | 4 (6%) | 2 (6%) | 2 (6%) | .95 |
| Sitagliptin (Januvia®) | 1 (2%) | 1 (3%) | 0 (0%) | .30 |
| Metformin | 34 (53%) | 18 (58%) | 16 (48%) | .44 |
| Sulfonylurea | 25 (39%) | 11 (35%) | 14 (42%) | .57 |
| Thiazolidinedione | 8 (13%) | 3 (10%) | 5 (15%) | .51 |
| Basal insulin +/- other medication | 28 (44%) | 15 (48%) | 13 (39%) | .54 |
| Prandial insulin +/- basal insulin | 45 (70%) | 22 (71%) | 23 (70%) | .91 |
| A1C at baseline (mean, SD) | 9.3 (1.3) | 9.6 (1.5) | 9.0 (0.9) | .07 |
One subject was excluded from analyses because s/he had an outlying A1C value at baseline. Not all columns total 64 because of missing data resulting from nonresponse. Subjects were often taking multiple medications, so the sum of the percentages exceeds 100. P values are for the statistical comparisons of the two treatment groups. These comparisons required chi-square tests and t-tests, depending on the level of measurement.
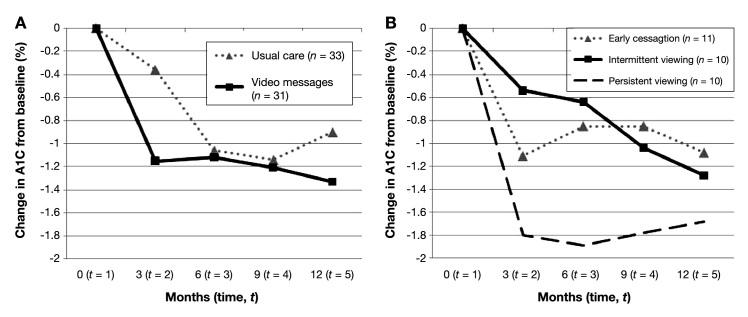
Both groups experienced declines in A1C (Figure 2A). For the video messages group, mean [standard deviation (SD)] decline in A1C from baseline was 1.2% (1.8%), 1.1% (2.3%), 1.2% (2.2%), and 1.3% (1.8%) at 3, 6, 9, and 12 months, respectively. For the usual care group, it was 0.4% (1.2%), 1.1% (1.6%), 1.1% (1.7%), and 0.9% (1.6%) at 3, 6, 9, and 12 months. Post hoc analyses of covariance indicated that the change in A1C from baseline to 3 months, with the baseline A1C included, was significantly different (p = .02) between the two groups.
Figure 2.
Mean change in A1C from baseline, by treatment group and over time. Change = later A1C – baseline A1C. (A) Two main treatment groups, video messages vs usual care. (B) Viewership groups within the video messages group, with the usual care group indicated as reference [note that this line is identical to the line in (A)]. The intervention ended at 6 months.
The rates of change in A1C over 12 months were significantly different from zero for both treatment groups after controlling for A1C level at the time of enrollment, age, gender, and type of diabetes [(a) p < .002 for time × usual care and p = .01 for time × time × usual care; and (b) p = .002 for time × video messages and p = .004 for time × time × video messages]. The 12-month, adjusted rate of change was greater at all time points for the video messages group, but the group differences were modest—approximately 0.1–0.2% per time point, with a cumulative decline in A1C at 12 months of 1.2% for the video message group and 1.0% for the usual care group. Age was also significant; i.e., older age was related to decreasing A1C. Gender and type of diabetes were not significant.
Analysis of A1C by viewership found that the consistent viewers experienced the greatest improvement (Figure 2B). Mean (SD) A1C reduction between baseline and 6 months—the period of time in which decline was greatest—was 0.8% (2.2%) for the subjects in the early cessation group, 0.6% (1.4%) for the intermittent viewers, and 1.9% (3.1%) for the persistent viewers. As of 12 months, mean (SD) A1C decline from baseline for the subjects in the early cessation group was 1.1% (1.9%), 1.3% (1.3%) for the intermittent viewers, and 1.7% (2.4%) for the persistent viewers. The changes suggested by the means were supported by the adjusted models. Specifically, for the early cessation group and the persistent viewers, the 12-month rate of change in A1C and the quadratic effect of time were statistically significant [(a) p < .001 for time × cessation group and p = .004 for time × time × cessation group; and (b) p < .001 for time × persistent group and p < .001 for time × time × persistent group]. The cumulative, adjusted decline in A1C over 12 months was 0.6% greater for the persistent viewership group than for the early cessation group, which is a clinically meaningful difference.
From the multilevel models for Figure 2A, the equations for the two treatments are as follows:
(1) Video Group A1C Over Time = 13.2 – 1.19 (time) + .15 (time) (time) – 0.02 (age) + 0.02 (male) – 0.53 (diabetes type 1); and (2) Usual Care Group A1C Over Time = 12.7 – 0.97 (time) + 0.12 (time) (time) – 0.02 (age) + 0.02 (male) – 0.53 (diabetes type 1).
From the multilevel models for Figure 2B, the equations for the viewership groups are as follows:
(1) Early Cessation Group A1C Over Time = 12.8 – 0.94 (time) + 0.11 (time) (time) - 0.01 (age) + 0.23 (male) – 1.25 (diabetes type 1); (2) Intermittent Group A1C Over Time = 12.0 – 0.20 (time) – 0.02 (time) (time) – 0.01 (age) + 0.23 (male) – 1.25 (diabetes type 1); and (3) Persistent Group A1C Over Time = 15.0 – 2.70 (time) + 0.38 (time) (time) – 0.01 (age) + 0.23 (male) – 1.25 (diabetes type 1).
The study groups did not differ in terms of whether they provided SMBG data or glycemia metrics—the amount of hyperglycemia (>180 mg/dl or >240 mg/dl) identified by those data. The data are available in the online Appendix (Table 2). Hypoglycemia (<70 mg/dl) was slightly more frequent for the video messages group (p = .05 for both time ranges). Further analyses of hypoglycemia indicate that the highest frequency of hypoglycemic readings was observed for the subjects in the group that did not view the videos (early cessation group). There were no significant within-group changes in SMBG metrics over the first 6 months or the subsequent 6 months.
Weight and BP did not change during the study period (data not shown).
Discussion
This study sought to determine whether mobile phone-based, one-way video messages about diabetes self-care improve A1C and SMBG. The study enrolled people with diabetes who, despite having received specialty diabetes care for at least 6 months and being on medications, were not meeting the A1C goals promulgated by all professional associations. The overall purpose of the video messages was to augment primary and specialty diabetes care. We found that participants in the video messages group experienced a greater rate of decline in A1C over time than those who received usual care, especially in the first 3 months. However, the rate of decline was greatest among people who received the videos and viewed them consistently; this difference was statistically significant and clinically meaningful (i.e., 1.1% difference in unadjusted means and 0.6% cumulative, adjusted difference between those who received messages and did not watch them at all or stopped in the first 2 months of the study). Participants’ improvement continued in the 6 months following cessation of the intervention despite no longer having access to the videos, suggesting a legacy effect.
A limitation of this study is that the average A1C for the video messages group was higher at baseline than that of the usual care group (p = .07), and it is well known that people with higher A1Cs are more likely to experience larger improvements in A1C than people with A1Cs closer to generally recognized targets. The analyses accommodated this difference through the use of multilevel models. These models allowed us to examine all data over time to get an overall sense of group differences in rates of change, not just mean changes from baseline at each individual time point. Additionally, they included treatment group as a fixed effect and generated a result for this effect, which represented the mean difference of the outcome between the two groups at baseline; in other words, the model adjusted for possible baseline differences in the outcome between the groups. Lastly, the models specified covariance structures for repeated measurements of the participants over time; the best covariance structure in this case was autoregressive order 1, which recognized that temporally proximate observations/values have higher correlations than distant observations/values.
We designed the study to investigate the effect of a mobile intervention that would augment usual and specialty diabetes care, because mhealth has been shown to be successful in chronic disease management, including asthma, cystic fibrosis, smoking cessation, and others.9 Application of mhealth in diabetes care has varied in focus: one study compared cell phone-based support to internet-based support and found both modes were related to improvement in glycemic control;17 one examined email reminders for blood glucose readings versus SMS and found participants responded more to SMS;18 another qualitative study found that study participants adjusted their medication, food habits, and/or physical activity while using a new cell phone system for diabetes self-care.19 Results from randomized trials comparing a cell phone intervention with usual care are mixed, with some showing no group differences in glycemic control based on intention-to-treat analyses10 and some showing marked improvements in glycemic control,12,13 especially when study participants received individualized support.14,15
Our findings for A1C were consistent with previous examinations of mobile phones for diabetes management documenting a decline in A1C, but the decline we report here is not as great. An important difference between our intervention and others is that A1C improved with one-way support, meaning there was no additional input from the health care providers as part of the intervention after the creation of the videos, and all the participants received the same videos irrespective of their particular interests or needs. As noted above, other mobile phone-based diabetes interventions that also achieved improvement in A1C had included individualized support from health care providers. Although these two-way interventions led to a greater drop in A1C than we found, the continual input needed from health care providers as part of the interventions is more costly and difficult to implement widely than our approach, especially if patients use their own phones and service. A more individualized strategy in using our approach, but one that does not require continual response from health care providers, would be to send only those videos to patients that address their specific needs or interests. This can be accomplished through querying the patients and further software development. Due to our study design, it cannot be determined whether the effectiveness of the video messages is, in part, related to the familiarity that the patients had with the NP in the video. Further studies may be able to determine whether or not a generic provider would be equally effective.
Our SMBG findings differed from other studies where mobile phone-based interventions improved self-care behavior, such as SMBG.18–21 The reason for this might be due to limitations in study design; we did not have SMBG data for the months preceding enrollment, thereby restricting our ability to examine change, and the study design did not require the participants to monitor their blood glucose and record the values on the same days at the same time. Such a prospective and/or systematic design might have resulted in a better understanding of whether the videos increased the frequency of SMBG, how often hypo- and hyperglycemia occurred, as well as the daily glucose pattern.
None of the participants allocated to the video messages group watched the videos daily, i.e., per protocol, yet many experienced improvement in A1C. This suggests that intermittent reinforcement may be a more practical yet equally effective strategy. The rationale for intermittent reinforcement is that frequent—but not daily—contact might be most effective for providing diabetes self-care support, because it is more likely to grab the recipient’s attention and keep them engaged for a longer period of time. Further research may show that patients will benefit as much (or more) from less frequent messages.
One of the strengths of our study is that the results would appear to be applicable to most patients with diabetes, because the demographics, clinical characteristics, and medication usage is typical of those patients treated in most outpatient settings.
Conclusions
A one-way intervention using mobile phone-based video messages about diabetes self-care can modestly improve A1C. Engagement with the technology is an important predictor of its success. This intervention is simple to implement and sustain.
Acknowledgments
We are indebted to the dedicated research staff and subjects who participated in this clinic research project.
Glossary
Abbreviations
- (A1C)
hemoglobin A1c
- (BP)
blood pressure
- (mhealth)
mobile health
- (NP)
nurse practitioner
- (SD)
standard deviation
- (SMBG)
self-monitoring of blood glucose
- (SMS)
short message service
Appendix
Table 1.
Example Text for Videos
| Category | Number | Content |
|---|---|---|
| Healthy eating | 1 | Including more soluble fiber with your meals and snacks will help control your blood glucose and cholesterol levels better. Examples of foods with soluble fiber are grains, such as oat and barley, dried beans and peas, and vegetables and fruits. |
| 2 | According to the American Diabetes Association, a healthy diet has multiple servings of fruits and vegetables, whole grains, low-fat dairy foods, fish, lean meats, poultry, and healthy fats. | |
| 3 | Cholesterol is found only in animal products. There is no cholesterol in plant foods. You can reduce your intake of cholesterol by making up your meals using mainly plant sources and including only low-fat, low-cholesterol meats, meat products, and dairy. | |
| 4 | Here is a tip on portion sizes. One ounce of meat looks like a small matchbox, and 3 ounces of meat looks like a deck of cards. A medium potato is about the size of a computer mouse. One cup of cooked rice is about the size of an adult’s fist. One ounce of cheese or a tablespoon of salad dressing is about the size of an adult’s thumb. | |
| 5 | The average American gains about 2 pounds of weight every year. This average weight gain can be the result of eating an extra 19 calories a day. Nineteen calories per day! | |
| Being active | 1 | Regular exercise will help with control blood sugar levels, reduce risk of heart disease and stroke, control weight, and boost energy levels. Just 30 minutes a day or two 15-minute sessions can make a big difference in your well-being. |
| 2 | Regular exercise will improve your blood sugar levels by helping your body’s own insulin to move the sugar out of your blood and into your cells. The end result is lower blood sugar levels. | |
| 3 | Be sure to talk to your health care provider about what type of exercise is best for you. In general, aerobic exercises are the best because they involve using your large muscles nonstop for at least 15 minutes. Examples of aerobic exercises are brisk walking, bicycling, swimming, rowing, and jogging. | |
| 4 | Regular exercise will help you to lose weight or maintain your healthy weight by burning extra calories much faster. With every 5 pounds of body fat that you lose, your blood sugar levels will improve significantly. | |
| 5 | If you were to burn an extra 100 calories a day by increasing your physical activity, you could lose up to 10 pounds a year. Here are a few tips for increasing your physical activity: get off the subway or bus one stop earlier and walk the extra distance; go for a 15-minute walk on your lunch break; take your kids out for a bike ride after dinner; and set your alarm for 15 minutes earlier and go out for a walk. | |
| Medications | 1 | Take the time to make a list of your medications, including those for your diabetes and other medications as well. For each pill, write the name, dose, when and how often you are supposed to take it, and the reason for each medication. Show the list to your pharmacist and talk with him/her about the side effects of your medications and whether or not they can be taken together. Remember to always carry the list with you, especially when you go to any of your healthcare appointments. |
| 2 | Some diabetes oral medications can cause your blood sugar to go low. Talk with your health care provider or pharmacist about which—if any—of your medications can have this effect, and be sure to check your blood sugar before taking them. | |
| 3 | When you go to your appointments with your health care providers, bring all of the medicines you are taking. This will help him/her determine more accurately the date of prescription, dose, prescriber, pharmacy used, and other details that can help you. | |
| 4 | If you have problems using your hands and your health care provider has prescribed insulin for you, ask your provider about injection aids, such as an insulin pen device. | |
| 5 | People with diabetes have a 2- to 4-fold increased risk for cardiovascular disease. Thus, your health care provider has or will prioritize treating any risk factors for cardiovascular disease that you might have. This often means prescribing medications for treating your cholesterol levels and blood pressure. So don’t be surprised if your health care provider prescribes multiple medications—some for your blood sugar and some for your cardiovascular risk factors. | |
| Monitoring and reducing risks | 1 | In general, target blood sugar levels are 80–120 when you wake up in the morning, 80–120 before meals, 80–140 2 hours after meals, and 100–140 at bedtime. Those are good targets to aim for. However, depending on your individual situation, you and your provider may have set different goals, and you need to continue using those goals. |
| 2 | Check your blood sugar levels according to the plan you talked about with your provider. The more you test your blood sugar, the more you will know how you are taking care of your diabetes. Be sure to bring in your test results to your next appointment so that you and your health care provider can review them and set other goals if needed. | |
| 3 | The day-to-day blood sugar testing tells you what your blood sugar is at the time you test it and can help you fine tune things like your eating plan and your exercise plan. The A1C test gives you an idea about your average blood sugar levels over the previous 3 months. It basically tells you what your average blood sugar level has been, not what it is at this point in time. So you need both results to have a better idea of your overall diabetes control. | |
| 4 | Regularly monitoring your blood sugar, cholesterol, and blood pressure—and keeping them at or below target levels—along with regular eye and food exams and kidney function tests—help to prevent or slow diabetes complications. So be aware of your test results to help manage your diabetes better. | |
| 5 | Because high blood pressure is a silent killer, it’s important to have it checked at every appointment and at least twice a year. It should be less than 130/80. If high blood pressure is left untreated, it can lead to blood vessel damage, heart disease, stroke, and kidney and eye problems. Keep an eye on your blood pressure. | |
| Problem-solving and coping | 1 | Researchers have found that some people who get too little sleep or not good quality sleep end up with the worst overall blood sugar control. If you’re having trouble getting a good night’s sleep, talk to your doctor. Here are a few better-sleep tips: keep a regular bedtime and wake-up time—even on weekends; relax with a before-bed routine, such as reading, listening to soothing music, or taking a warm bath; and invest in a comfortable mattress. |
| 2 | You don’t have to be perfect to manage your diabetes successfully, however, you will need to make the best effort to understand how to take good care of yourself. In order to learn how to manage your diabetes and what your goals are, be prepared to make several visits to see your health care provider. Also be sure to register for and attend the diabetes classes if you haven’t already done so. | |
| 3 | Did you know that your success at managing your diabetes will depend on: (1) How knowledgeable you are about management of diabetes. (2) Whether you believe that you will be successful at managing your diabetes. (3) Whether you have made a conscious decision to take control of your diabetes. | |
| 4 | Living with diabetes can cause a lot of uncomfortable and changing emotions, including denial, anger, anxiety, and fear. If these or other feelings are making it difficult for you to take care of yourself and enjoy your life, consider talking to someone you love or trust who understands diabetes or just understands you. Sharing your emotions can help you to manage them. | |
| 5 | Depression makes it harder to initiate and stick to health behaviors for your diabetes self-care. Depression is also at least twice as common among people with diabetes. This application provides a module to help you track your mood. However, if you have often felt depressed, down, or hopeless in the past month, perhaps you should talk to your provider. Depression is a health problem for which there are many effective treatments. | |
Table 2.
Metrics from Self-Monitoring of Blood Glucose Logs, By Treatment Group and Viewership Group
| Usual care group | Video messages group | Early cessation group | Intermittent viewer group | Persistent viewer group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–6 months | 0–12 months | 0–6 months | 0–12 months | 0–6 months | 0–12 months | 0–6 months | 0–12 months | 0–6 months | 0–12 months | |
| # of subjects with data/total (%) | 23/33 (70) | 23/33 (70) | 17/31 (55) | 17/31 (55) | 4/11 (36) | 4/11 (36) | 7/10 (70) | 7/10 (70) | 6/10 (60) | 6/10 (60) |
| Mean (SD) glucose mg/dl | 193 (63) | 192 (64) | 194 (48) | 190 (37) | 195 (32) | 195 (32) | 208 (65) | 201 (44) | 177 (33) | 175 (31) |
| Mean (SD) % readings <70 mg/dl | 2 (2)a | 3 (3)a | 5 (6)a | 5 (6)a | 9 (9) | 9 (9) | 4 (4) | 4 (4) | 4 (5) | 4 (5) |
| Mean (SD) % readings >180 mg/dl | 47 (25) | 45 25) | 48 (21) | 47 (20) | 51 (24) | 51 (24) | 53 (22) | 52 (20) | 41 (21) | 39 (20) |
| Mean (SD) % readings >240 mg/dl | 22 (25) | 22 (25) | 26 (19) | 24 (16) | 28 (14) | 28 (14) | 30 (26) | 28 (20) | 19 (13) | 19 (13) |
Group differences for 0–6 months data were statistically significant (p = .05). No other comparisons found significant differences, so the notation is not shown.
Funding
This project was made possible by grants from the Telemedicine and Advanced Technology Research Center of the United States Army Medical Research and Materiel Command. The views, opinions, and/or findings contained in this publication are those of the authors and do not necessarily reflect the views of the Department of Defense (DoD) and should not be construed as an official DoD/Army position, policy, or decision unless so designated by other documentation. No official endorsement should be made.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 3.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31(1):81–86. doi: 10.2337/dc07-1572. Epub 2007 Oct 12. [DOI] [PubMed] [Google Scholar]
- 4.Wagner J, Malchoff C, Abbott G. Invasiveness as a barrier to self-monitoring of blood glucose in diabetes. Diabetes Technol Ther. 2005;7(4):612–619. doi: 10.1089/dia.2005.7.612. [DOI] [PubMed] [Google Scholar]
- 5.Vincze G, Barner JC, Lopez D. Factors associated with adherence to self-monitoring of blood glucose among persons with diabetes. Diabetes Educ. 2004;30(1):112–125. doi: 10.1177/014572170403000119. [DOI] [PubMed] [Google Scholar]
- 6.Karter AJ, Ferrara A, Darbinian JA, Ackerson LM, Selby JV. Self-monitoring of blood glucose: language and financial barriers in a managed care population with diabetes. Diabetes Care. 2000;23(4):477–483. doi: 10.2337/diacare.23.4.477. [DOI] [PubMed] [Google Scholar]
- 7.Nichol MB, Knight TK, Priest JL, Wu J, Cantrell CR. Nonadherence to clinical practice guidelines and medications for multiple chronic conditions in a California Medicaid population. J Am Pharm Assoc (2003) 2010;50(4):496–507. doi: 10.1331/JAPhA.2010.09123. [DOI] [PubMed] [Google Scholar]
- 8.Kramer H, Cao G, Dugas L, Luke A, Cooper R, Durazo-Arvizu R. Increasing BMI and waist circumference and prevalence of obesity among adults with Type 2 diabetes: the National Health and Nutrition Examination Surveys. J Diabetes Complications. 2010;24(6):368–374. doi: 10.1016/j.jdiacomp.2009.10.001. Epub 2009 Nov 14. [DOI] [PubMed] [Google Scholar]
- 9.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemed J E Health. 2009;15(3):231–240. doi: 10.1089/tmj.2008.0099. [DOI] [PubMed] [Google Scholar]
- 10.Istepanian RS, Zitouni K, Harry D, Moutosammy N, Sungoor A, Tang B, Earle KA. Evaluation of a mobile phone telemonitoring system for glycaemic control in patients with diabetes. J Telemed Telecare. 2009;15(3):125–128. doi: 10.1258/jtt.2009.003006. [DOI] [PubMed] [Google Scholar]
- 11.Krishna S, Boren SA. Diabetes self-management care via cell phone: a systematic review. J Diabetes Sci Technol. 2008;2(3):509–517. doi: 10.1177/193229680800200324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HS, Jeong HS. A nurse short message service by cellular phone in type-2 diabetic patients for six months. J Clin Nurs. 2007;16(6):1082–1087. doi: 10.1111/j.1365-2702.2007.01698.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim HS, Kim NC, Ahn SH. Impact of a nurse short message service intervention for patients with diabetes. J Nurs Care Qual. 2006;21(3):266–271. doi: 10.1097/00001786-200607000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008;10(3):160–168. doi: 10.1089/dia.2008.0283. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal S, Lau CT. Remote health monitoring using mobile phones and Web services. Telemed J E Health. 2010;16(5):603–607. doi: 10.1089/tmj.2009.0165. [DOI] [PubMed] [Google Scholar]
- 16.Mensing C. Chicago, IL: American Association of Diabetes Educators; 2006. The Art and Science of Diabetes Self-Management Education: A Desk Reference for Healthcare Professionals. [Google Scholar]
- 17.Cho JH, Lee HC, Lim DJ, Kwon HS, Yoon KH. Mobile communication using a mobile phone with a glucometer for glucose control in Type 2 patients with diabetes: as effective as an Internet-based glucose monitoring system. J Telemed Telecare. 2009;15(2):77–82. doi: 10.1258/jtt.2008.080412. [DOI] [PubMed] [Google Scholar]
- 18.Hanauer DA, Wentzell K, Laffel N, Laffel LM. Computerized Automated Reminder Diabetes System (CARDS): e-mail and SMS cell phone text messaging reminders to support diabetes management. Diabetes Technol Ther. 2009;11(2):99–106. doi: 10.1089/dia.2008.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arsand E, Tatara N, Østengen G, Hartvigsen G. Mobile phone-based self-management tools for type 2 diabetes: the few touch application. J Diabetes Sci Technol. 2010;4(2):328–336. doi: 10.1177/193229681000400213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36(2):165–173. doi: 10.1016/j.amepre.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34(9):1934–1942. doi: 10.2337/dc11-0366. Epub 2011 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]