Abstract
Background
Multiple factors impact subcutaneous insulin injection pain. Injection devices [e.g., syringe or pen needle (PN)] affect pain due to needle length, diameter, needle polishing and lubrication, and needle tip geometry.
Methods
We evaluated a modified 5-bevel PN tip in 32 G × 4 mm 31 G × 5 mm and 8 mm PNs vs the equivalent marketed 3-bevel PNs in laboratory penetration force testing, as well as in insulin-taking subjects for overall acceptability, comparative pain, and preference. The clinical tests were done in three ways: paired insertions with the subjects blinded to PN tip geometry, after brief at-home use of 5-bevel PNs, and again with subjects informed about each needle’s tip geometry in paired insertions.
Results
Average penetration force in a skin substitute was 23% lower with the 5-bevel PNs vs similar 3-bevel PNs (p ≤ 0.01). In blinded testing and after at-home use, patients rated the 5-bevel needle as acceptable. After short-term home use, patients rated the 5-bevel PN less painful and preferable to their usual PN (both p < 0.01). In paired, informed testing, the 5-bevel PN was less painful and preferred to subjects’ currently used needles (p ≤ 0.01) and to other marketed PNs (p < 0.01).
Conclusions
Needle tip geometry affects penetration force. When blinded, patients did not distinguish differences in PN tip geometry with fine-gauge PN insertions. A 5-bevel needle tip is perceived as less painful and is preferred by subjects following home use for usual injections. Similar results occurred when patients were informed that they were using a needle with a modified tip.
Keywords: bevel, injection, needle tip, pain, pen needle
Introduction
Multiple factors affect pain experienced with sub-cutaneous (SC) delivery of medication. Injection volume and the drug itself—including preservative and solvent—can affect pain perception.1,2 For injection delivery devices [e.g., syringes and pen needles (PNs)], important contributors include needle diameter (gauge), needle length, needle smoothness, and lubrication.3–7 Additionally, sharpness or bluntness of a needle directly affects pain.8
Needle tips vary in terms of bevel design, e.g., the number and angularity of the tip facets. Currently, all commercially available insulin syringes and PNs have 3-bevels (Figure 1A). When evaluated in a clinically relevant laboratory model, 5-bevel tips significantly reduced needle insertion force.9 Differences between insertion forces of these 3- and 5-bevel needle tips originally observed in the laboratory were also demonstrated in healthy volunteers and in patients’ subjective experience using the needles to inject interferon for multiple sclerosis.10 This study was under-taken to demonstrate the acceptability of the 5-bevel needle (Figure 1B) in patients injecting insulin and to compare perceived pain and preference between 3- and 5-bevel needles.
Figure 1.
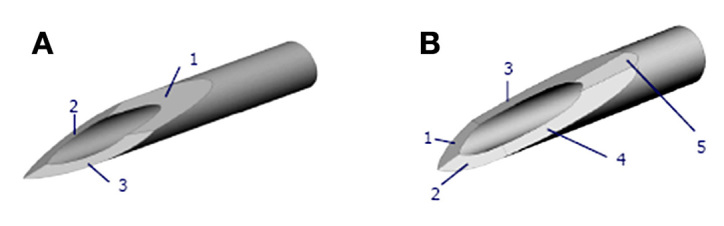
(A) 3-bevel tip. (B) 5-bevel tip.
Subjects and Methods
Preclinical Penetration Force Testing
An Instron® Universal Testing Machine (Instron, Norwood, MA) was used to evaluate the peak force applied to a needle point when piercing a human skin substitute in vitro that has been shown to correlate (R2 = 0.89) with penetration forces perceived by nurses.9 Insertions were done at a constant speed and maximum penetration force was recorded. Pen needles were segmented into three, 3-bevel vs 5-bevel groups: (1) 32 G × 4 mm and 6 mm, (2) 31 G × 5–6 mm, and (3) –31 G × 8 mm. Multiple lots were used, except with one manufacturer’s PN where only one lot was available. All comparator needles for preclinical and clinical testing were obtained through retail pharmacies and assumed to meet packaging specifications. The 5-bevel PNs were made with the same manufacturing process, including electro-etching, as production quality 3-bevel PNs, other than a modified tip-grinding process to produce 5-bevels.
Self-Injector Acceptability, Pain, and Preference Study
A prospective, three-part, two-center study compared PNs with the 5-bevel tip to the currently available 3-bevel PNs for acceptability, preference, comfort, ease of insertion, and perceived pain. This study was conducted in compliance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the Schulman Associates Institutional Review Board. All participants provided written informed consent.
Eligible subjects were male or female, 18–75 years with type 1 or 2 diabetes and were using insulin pens with a BD (Becton, Dickinson and Co., Franklin Lakes, NJ)4 mm, 5 mm, or 8 mm PN at least once daily for ≥2 months. On the basis of their currently used PN, subjects were assigned to the corresponding study group. (The 4 mm PN was launched recently and if unable to recruit enough subjects, additional 5 mm users could be assigned to the 4 mm study group). Exclusion criteria included significant skin conditions, neuropathy, physical conditions making them unable to perform study procedures, recent history of unstable diabetes, bleeding disorders, or pregnancy.
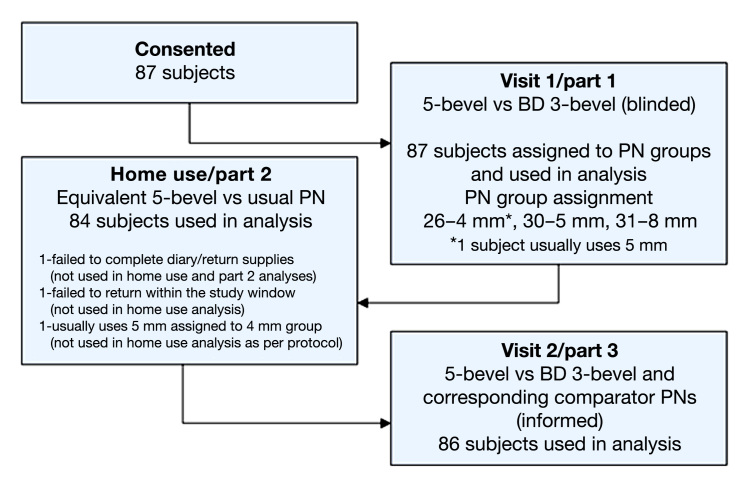
Study conduct is outlined in Figure 2. Following screening, consent, and qualification, subjects were enrolled into one of the three study groups, as described. The order of PN insertions was randomized in parts 1 and 3.
Figure 2.
Study flow/conduct diagram.
Study Conduct
At visit 1, each subject inserted four pairs of PNs attached to a pen device without any cartridge (dry insertions). Subjects were blinded and uninformed as to needle tip geometry. The abdomen was the preferred site, but whichever site was used, all insertions were done within that site approximately 2.5 cm apart. The assigned PN size was used in the first two insertion pairs, and the two other sizes in the remaining two pairs. The order of insertions (3- and 5-bevel) in the 1st pair was randomized and reversed in the 2nd pair. The 3rd and 4th pairs were similarly randomized. After each insertion, subjects were asked, “Was the pen needle acceptable—Yes or No?” After each pair of insertions, subjects answered the following questions as either “The 1st or the 2nd or no difference”:
“Which pen needle (1) was easier to insert? (2) was more comfortable? (3) did you prefer?”
On a 15-cm pain visual analog scale (VAS), subjects compared the perceived pain of the 2nd to the 1st insertion (Figure 3). A vertical line drawn to the left of the center mark indicated that the second PN was less painful than the first and vice versa if drawn to the right.
Figure 3.
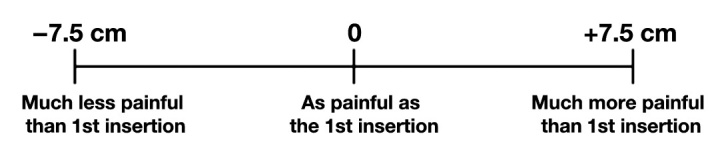
A 15-cm visual analog scale used for comparative pain perception. After each pair of insertions, subjects placed a vertical line indicating comparative pain. Pain scores ranged from –7.5 cm (2nd insertion much less painful than 1st insertion) to +7.5 cm (2nd insertion much more painful than 1st insertion), with a score of 0 cm representing no difference. Study scale did not have centimeter indicators.
Before home use, subjects were informed only that they were testing a PN with a new design. No other information about the PN was provided. Subjects performed at least five injections with the assigned 5-bevel PN and recorded information about dosage, adverse events, or product failures. Subjects returned for visit 2 within 1–7 days, and home use was assessed using the same questions as in Part 1, comparing the study PN to the subject’s usual PN. The comparison for the pain VAS was changed from “1st insertion” to “my usual needle.”
In part 3 (same visit), four pairs of PNs were inserted, similar to part 1. However, subjects were informed about the change in needle tip design and the reductions in penetration force of the 5-bevel needle and were unblinded to which PN they were inserting. The 1st two insertion pairs compared the 5-bevel and corresponding BD 3-bevel PNs of the same gauge and length, as in part 1. The 2nd two pairs compared the assigned 5-bevel to corresponding comparator PNs as shown in Table 1. After each pair of insertions, subjects answered the same questions and pain VAS as in part 1.
Table 1.
5-Bevel vs 3-Bevel Comparator (Marketed Non-BD) PNs Used in Visit 2/Part 3
| 5-Bevel PN | Comparator 3-Bevel PN |
|---|---|
| 8 mm × 31 G | 8 mm × 30 G |
| 5 mm × 31 G | 6 mm × 31 G |
| 4 mm × 32 G | 6 mm × 32 G tip |
Before insertions, injection instructions were provided based on PN length and subject’s weight (Table 2). For home use, subjects were instructed to use their usual injection sites and technique.
Table 2.
Injection Technique Instructions for Needle Lengths (Parts 1 and 2)
| Patient type | Angle | Skin pinch | ||
|---|---|---|---|---|
| 4 mm | All | Straight-in | No | |
| 5 mm | All | Straight-in | Either | |
| 6 mm | Normal weight | Straight-in | Yes—abdomen or thigh | |
| Obese | Straight-in | Yes—thigh | ||
| No—abdomen | ||||
| 8 mm | All | Straight-in | Yes | |
Note: since no children were in the study, 45° insertion angle for 6 mm was not instructed. All instructions are based on information provided on manufacturers’ Web sites.
Statistical Methods and Criteria
Preclinical Testing
A sample size of 60 for each PN in the preclinical penetration force testing provided at least 90% statistical power to show, at minimum, a 10% difference in peak penetration force.
Self-Injector Study
Seventy-five subjects, each evaluating four insertion pairs, provided 300 replicates per bevel type (all PN lengths and gauges pooled) with at least 90% statistical power to meet the primary acceptability objective of noninferiority of the 5-bevel compared to the 3-bevel PNs with 95% confidence.
Primary Objective (Part 1)
The 5-bevel would be considered noninferior (equivalent) if the acceptability rate is greater than, equal to, or no more than 20% less than the acceptability rate with the 3-bevel PNs, with 95% confidence. All completed insertions were included in this analysis. (Subjects who rated the 5-bevel as unacceptable were not segmented or analyzed differently.)
Secondary Objective (Part 1)
The 5-bevel would be considered preferred if the 95% lower bound for the percentage of insertions where subjects preferred the 5-bevel PN is greater than the 95% upper bound where they preferred the 3-bevel PN. Comfort and ease of insertion were compared similarly. The 5-bevel would be considered clinically less painful if the average VAS score was at least 10 mm less than for the 3-bevel PN.
Secondary Objective after Home Use
The percentage of users who found the 5-bevel acceptable must be ≥80% (with 95% confidence). The criteria for preference, comfort, ease of insertion, and pain were the same as for part 1, but with “percentage of users” replacing “insertions.” The home-use analysis included subjects who completed at least five injections at home. Results from subjects placed into the 4 mm group who usually use a 5 mm PN were excluded from the pooled home-use analysis.
Tertiary Objectives (Part 3)
Acceptance criteria for preference, comfort, ease of insertion, and pain were the same as in part 1.
Results
Preclinical Force Testing
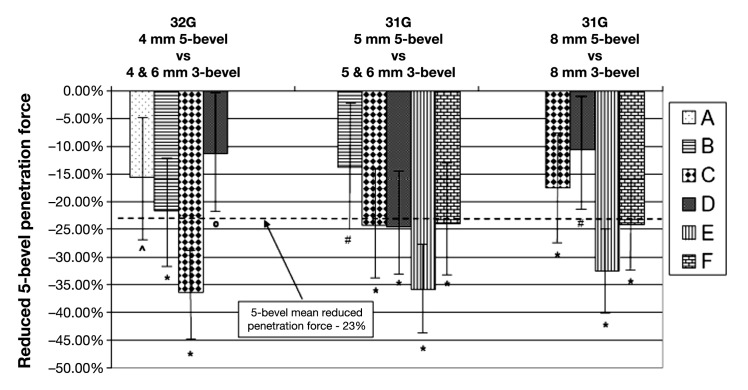
The 5-bevel PN had 23% less mean penetration force compared to similar sized 3-bevel PNs (p < 0.01). The penetration force for 32 G × 4 mm 5-bevel PNs was 11–36% less, the 31 G × 5 mm 5-bevel PNs was 14–36% less, and the 31 G × 8 mm 5-bevel PNs was reduced 11–33%. Performance of each comparator group (manufacturers “A–F”) is summarized in Figure 4.
Figure 4.
Mean percent (with 95% CI) reduced penetration force of 5-bevel compared to similar 3-bevel pen needles. * = p < 0.001, ^ = p < 0.01, # = p < 0.05, 0 = p ≥ 0.05 (NS).
Self-Injector Acceptability, Pain, and Preference Study
Eighty-six subjects completed all three parts of the study (used for analysis—visit 1, 87; home use, 84; visit 2, 86; see Figure 2). Age ranged from 19 to 74 years (mean 55.6 years), 54% were female, diabetes duration was 1–43.5 years (mean 13.8 years), 71% had type 2 diabetes, and 90% were white/Caucasian.
Primary Objective
In visit 1, with 348 insertions, the 5- and 3-bevel PNs were rated acceptable in 92.5% and 93.7% of insertions, respectively, with a difference of 1.1% [95% upper bound confidence interval (CI) 4.4%]. The 5-bevel PNs (pooled) were acceptable (equivalent to the 3-bevel) based on the 20% noninferiority criteria. Each length 5-bevel PN was also rated acceptable by ~90–94% of subjects. No subject consistently rated the 5-bevel (or 3-bevel) PNs as unacceptable: the 48 of 696 insertions in part 1 rated as unacceptable came from 31 different subjects—19 rated only 1 insertion out of 8 as unacceptable. The mostinsertions one subject rated as unacceptable was 5 (3 for 3-bevel and 2 for 5-bevel).
Secondary Objectives
In visit 1, when patients were blinded to the PN bevel designs, no differences were found for ease of insertion (37.1%, 36.8%), comfort (37.1%, 37.6%), or preference (38.2%, 37.6%), 3- vs 5-bevel, respectively, or pain (see Table 3).
Table 3.
Difference in Pain Ratings with 15 cm Visual Analog Scale (VAS)a
| N | Mean difference (mm) | 95% CI (mm) | p value | |
|---|---|---|---|---|
| 5-bevel vs same brand 3-bevel (visit 1, part 1) | 348 | 2 | −3, 7 | NS |
| After home-use (visit 2, part 2) | 83 | −36 | −44, −28 | <0.01 |
| 5-bevel vs same brand 3-bevel (visit 2, part 3) | 172 | −14 | −21, −8 | <0.01 |
| 5-bevel vs comparator 3-bevel (visit 2, part 3) | 172 | −13 | −19, −6 | <0.01 |
NS, not significant.
A negative result indicates the 5-bevel pen needle was rated less painful. An absolute mean pain difference of 10 mm or more indicates that the difference is clinically important.
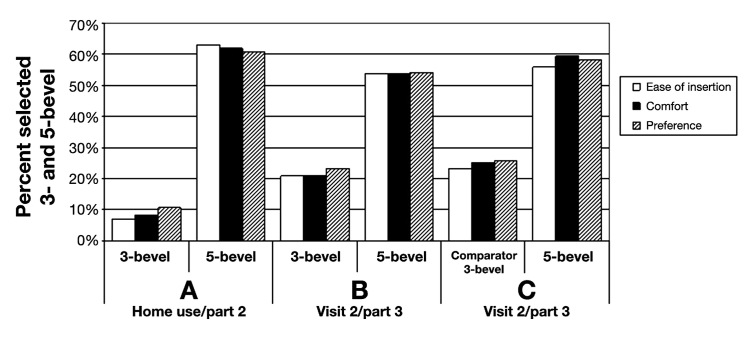
At home, 810 injections were completed; about 2/3 of subjects used the 5-bevel PN 5–10 times. After home use, 97.6% (95% lower confidence bound, 93.1%) of users found the 5-bevel acceptable, significantly higher than the 80% criterion. The 5-bevel PNs were rated higher than usual PNs for ease of insertion (63.1% vs 7.1%), comfort (61.9% vs 8.3%), and preference (60.7% vs 10.7%), each p < 0.01 (Figure 5A). Also, the mean VAS score was significantly less for 5-bevel vs usual 3-bevel PNs (Table 3). The number of injections completed was not related to pain (p = 0.99).
Figure 5.
Ease of insertion, comfort, and preference for 3- and 5-bevel pen needles; same manufacturer usual 3-bevel vs 5-bevel after home use (A), same manufacturer 3-bevel vs 5-bevel (B), and comparator 3-bevel vs 5-bevel (C).
Tertiary Objectives
In visit 2, after subjects were informed about the reduced penetration force of the 5-bevel design and which PN was being inserted, the 5-bevel PN was selected more often than the corresponding 3-bevel PNs for greater ease of insertion, comfort, and preference (p = 0.01), shown in Figure 5B and C. In addition, the VAS pain scores were less for the 5-bevel PN (Table 3).
Eighteen adverse events were reported, all nonserious and rated either unlikely or not related to study device. There were no reports of local site bleeding, bruising, infections, wounds, or excessive pain.
Discussion
We assessed the performance of a 5-bevel PN in both preclinical penetration force testing and in subjects who self-administer insulin. The 5-bevel needles evaluated were 4 mm × 32 G, and 5 and 8 mm × 31 G. In an accepted human skin substitute,9 the 5-bevel needle demonstrates a significant mean 23% lower penetration force. The primary objective for the self-injector study was met by demonstrating the acceptability of the 5-bevel PN.
Although the blinded paired comparison of 3- and 5-bevel PNs of identical length and diameter was the most objective method to validate the product design for acceptability, blinded insertions (in a study environment without actual insulin injections) do not reflect the full injection experience of self-injectors. The most realistic evaluation of the PN was accomplished when all self-injections were done in the subjects’ home environment, without knowledge of needle design change.
In the blinded comparison of 3- and 5-bevel PNs, nearly 93% and 94% of subjects, respectively, rated all PNs combined acceptable, demonstrating equivalence between the needle tip geometries. These findings were extended after home-use testing, in which subjects were told only that they were using a PN with a different design. After five or more injections at home, nearly 98% found the 5-bevel acceptable.
Additional outcome measures compared the different needle tip geometries for comfort, ease of insertion, preference, and pain. These parameters reflect the needle’s impact on the injection experience: pain and comfort have contrary implications, are measured differently, and complement each other; ease of insertion is specific to the initial needle penetration into skin (an important indicator for the changed needle tip geometry), and preference is an aggregate evaluation of the entire injection experience. In the blinded paired testing, subjects rated both needle tips similarly on these attributes. However, following home-use, subjects rated the 5-bevel needles significantly more comfortable, easier to insert, and more preferable to their usual (3-bevel) needles by ~6- to 9-fold. On VAS, the new needle tip design was significantly less painful by a substantial 36 mm (10 mm is considered a good indicator of a clinically meaningful difference in pain.11,12). Not unexpectedly, subjects also gave significantly higher ratings for the 5-bevel when they compared open-label needle insertions after being informed about design change: the 5-bevel was favorably rated by >50% of the subjects for comfort, insertion ease, and preference by ~ 2- to 2.5-fold over 3-bevel PNs.
These difference ratings, when assessed under different conditions, are not unexpected. Several prior studies have evaluated PNs of differing lengths and diameter—primarily for effect on glycemic control—but also for pain, ease of insertion, pain, and preference.5–7,13,14 The findings in open-label (not blinded) use are clear—patients almost universally prefer smaller, shorter needles. When patients compared 29 G × 12.7 mm to 31 G × 6 mm PNs in a two-period crossover trial, nearly 90% preferred the smaller PN and rated it less painful. However, when the subjects compared the two study PNs in blinded fashion at the study clinic, they could not detect a difference.5 This occurred even though the needles were substantially different from each other. By International Organization of Standardization standard 9626:1991/Amd.1:2001,15 29 G needles have a maximum external diameter of 0.351 mm and 31 G needles, 0.267 mm, a difference of approximately 30%, along with a 100% needle length difference (maximum external diameters for 30 G and 32 G needles are 0.320 mm and 0.241 mm, respectively15). In the current study, the 3- and 5-bevel PN lengths and diameters did not differ—hence the failure of subjects to detect differences in pain, preference, and such in blinded testing is not surprising. Other open-label crossover studies conducted in Japan16–18 have compared fine-gauge PNs in similar fashion as described earlier.5 These trials have found small, inconsistent differences or noninferiority between the BD 31 G × 5 mm, Terumo 33 G × 5 mm tapered tip, and Novo Nordisk 32 G tip × 6 mm PNs. Yet, in our published open-label crossover trial, the 4 mm × 32 G (3-bevel) PN was reported to be less painful, easier to use, and preferred when compared to other 3-bevel PNs, 31 G × 5 mm and 8 mm.7 Additional studies completed in Japan found similar positive findings for the same BD 4 mm × 32 G (3-bevel) PN vs comparator needles and have been presented in abstract form (Kizaki M, personal communication).
Our current study substantiates that blinded evaluations of insertions may not mirror the self-injection experience of patients. Real-life insulin injections require physical preparation (e.g., attaching the PN, dialing a dose), thus generating psychological anticipation for injections that are often considered painful and difficult.19,20 When aware of needle design differences, whether visible (length, gauge) or not (needle lubrication, tip sharpness), anticipation appears to contribute to a patient’s injection experience and subsequent subjective ratings. As a consequence, the home-use portion was likely the most revealing part of this study. Prior to using the 5-bevel PN for self-injection, subjects were informed only that they were testing a PN with a new design. No specific information about the design changes or laboratory testing was provided. Subjects injected their usual insulin doses without any change in injection technique, acting as their own controls between their usual PNs and study PNs. In this setting, there was an equal 1/3 chance that they would rate the study needle as better than, equal to, or worse than, their usual PN. However, the 5-bevel PN was rated significantly more comfortable, easier to insert, and preferred to the subjects’ usual (3-bevel) needles by large margins, ~61–63% vs ~7–11%.
Will these changes in needle tip geometry have longer-term benefits including reduced tissue trauma? Lipohypertrophy is a well-recognized, poorly understood complication of insulin therapy—both by injection and by infusion.20–25 Some factors associated with its development include lack of site rotation and needle reuse, suggesting a role for local tissue trauma. It is possible that the 5-bevel tip may translate into less adipose hypertrophy and SC scarring, but this remains speculative.
Limitations of this study include that the home-use portion was of limited duration. We attempted to balance an adequate number of injections against subjects’ ability to recall what their usual needles felt like. There is no agreement on the most appropriate number of injections or time span, but comparisons are often made on the basis of single injections. Also, while subject fatigue can have an impact on study results, it was minimized by using only four pairs of insertions in parts 1 and 3, on separate days. Paired insertions in part 3 evaluated the impact of providing detailed information about the 5-bevel needle tip and showed positive ratings for it. To fully assess the impact of different amounts of information—minimal vs detailed—additional insertions would have been required (potentially causing subject fatigue). We believe the home-use data with >800 injections addressed this question adequately. Psychological influences on the injection experience were not directly assessed, but subjects acted as their own controls in all parts of the study. Although nonphysical factors may play a role, the study design and randomization within each part provides reasonable control for other hard-to-measure influencers.
Conclusion
This study validated the design of the 5-bevel needle tip in subjects with diabetes. Preclinical testing demonstrated a significant 23% reduction in penetration force for the 5-bevel needle tip vs current 3-bevel PNs. Although this difference was not perceived with blinded insertions, when told only that it was a modified design, subjects did report significantly more comfort, insertion ease, preference, and less pain after home use, and when given detailed information. This 5-bevel needle tip may support better acceptance of self-injection therapy, but this will require additional study.
Acknowledgments
The authors would like to thank the following for their assistance in coordinating and conducting the study: Anna Valenti-Miccio, Kenneth Kassler-Taub, Ronald K. Mayfield, M.D., and John J. Shelmet.
Glossary
Abbreviations
- (CI)
confidence interval
- (PN)
pen needle
- (SC)
subcutaneous
- (VAS)
visual analog scale
Funding
This work was funded by BD (Becton, Dickinson and Company).
Disclosure
All authors are employees of BD.
References
- 1.Jørgensen JT. Improvement of patient convenience in treatment with growth hormone. J Pediatr Endocrinol. 1994;7(2):175–180. doi: 10.1515/jpem.1994.7.2.175. [DOI] [PubMed] [Google Scholar]
- 2.Jørgensen JT, Romsing J, Rasmussen M, Møller-Sonnergaard J, Vang L, Musaeus L. Pain assessment of subcutaneous injections. Ann Pharmacother. 1996;30(7–8):729–732. doi: 10.1177/106002809603000703. [DOI] [PubMed] [Google Scholar]
- 3.Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of skin following mechanical needle insertions. Eur J Pain. 1999;3(1):41–49. doi: 10.1053/eujp.1998.0099. [DOI] [PubMed] [Google Scholar]
- 4.Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23(1–2):37–43. doi: 10.1080/08990220600700925. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz S, Hassman D, Shelmet J, Sievers R, Weinstein R, Liang J, Lyness W. A multicenter, open-label, randomized, two-period crossover trial comparing glycemic control, satisfaction, and preference with a 31 gauge × 6 mm needle versus a 29 gauge × 12.7 mm needle in obese patients with diabetes mellitus. Clin Ther. 2004;26(1):1663–1678. doi: 10.1016/j.clinthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Kreugel G, Keers JC, Kerstens MN, Wolffenbuttel BHR. Randomized trial on the influence of the length of two insulin pen needles on glycemic control and patient preference in obese patients with diabetes. Diab Tech Ther. 2011;13(7):737–741. doi: 10.1089/dia.2011.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch LJ, Gibney MA, Albanese J, Qu S, Kassler-Taub K, Klaff LJ, Bailey TS. Comparative glycemic control, safety, and patient ratings for a new 4 mm × 32 G insulin pen needle in adults with diabetes. Curr Med Res Opin. 2010;26(6):1531–1541. doi: 10.1185/03007995.2010.482499. [DOI] [PubMed] [Google Scholar]
- 8.Chantelau E, Lee DM, Hemmann DM, Zipfel U, Echterhoff S. What makes insulin injections painful? BMJ. 1991;303(6793):26–27. doi: 10.1136/bmj.303.6793.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vedrine L, Prais W, Laurent PE, Raynal-Olive C. Improving needle-point sharpness in prefillable syringes. Med Device Tech. 2003;14(4):32–35. [PubMed] [Google Scholar]
- 10.Jaber A, Bozzato GB, Vedrine L, Prais WA, Berube J, Laurent PE. A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurol. 2008;8:38. doi: 10.1186/1471-2377-8-38. Doi: 10.1186/1471-2377-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med. 2001;37(1):28–31. doi: 10.1067/mem.2001.111517. [DOI] [PubMed] [Google Scholar]
- 12.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8(12):1153–1157. doi: 10.1111/j.1553-2712.2001.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 13.Ross SA, Jamal R, Leiter LA, Josse R, Parkes J, Qu S, Kerestan S, Ginsberg B. Evaluation of 8 mm insulin pen needles in people with type 1 and type 2 diabetes. Pract Diab Intl. 1999;16(5):145–148. [Google Scholar]
- 14.Strauss K, Hannet I, McGonigle J, Parkes JL, Ginsberg B, Jamal R, Frid A. Ultra-short (5 mm) insulin needles: trial results and clinical recommendations. Pract Diab Intl. 1999;16(7):218–222. [Google Scholar]
- 15. http://www.iso.org/iso/home.htm. Accessed August 22, 2011.
- 16.Miyakoshi M, Kamoi K, Iwanaga M, Hoshiyama A, Yamada A. Comparison of patient’s preference, pain perception, and usability between Micro Fine Plus® 31-gauge needle and microtapered Nanopass® 33-gauge needle for insulin therapy. J Diab Sci Technol. 2007;1(5):718–724. doi: 10.1177/193229680700100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwanaga M, Kanoi K. Patient perceptions of injection pain and anxiety: a comparison of NovoFine 32-gauge tip 6 mm and Micro Fine Plus 31-gauge 5 mm needles. Diab Tech Ther. 2009;11(2):81–86. doi: 10.1089/dia.2008.0027. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto R, Okeda T, Nobuyuki A. Study on penetration pain of pen needles for insulin injection. Practice. 2009;26:95–99. [Google Scholar]
- 19.Hunt LM, Vlaenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy. The basis of patient reluctance. Diabetes Care. 1997;20(3):292–298. doi: 10.2337/diacare.20.3.292. [DOI] [PubMed] [Google Scholar]
- 20.Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28(10):2543–2545. doi: 10.2337/diacare.28.10.2543. [DOI] [PubMed] [Google Scholar]
- 21.Vardar B, Kizilc S. Incidence of lipohypertrophy in diabetic patients and a study of influencing factors. Diab Res Clin Pract. 2007;77(2):231–236. doi: 10.1016/j.diabres.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Richardson T, Kerr D. Skin-related complications of insulin therapy: epidemiology and emerging management strategies. Am J Clin Dermatol. 2003;4(10):661–667. doi: 10.2165/00128071-200304100-00001. [DOI] [PubMed] [Google Scholar]
- 23.Strauss K, De Gols H, Hannet I, Partanen TM, Frid A. A pan-European epidemiologic study of insulin injection technique in patients with diabetes. Pract Diab Int. 2002;19(3):71–76. [Google Scholar]
- 24.Roper NA, Bilous RW. Resolution of lipohypertrophy following change of short-acting insulin to insulin lispro (Humalog) Diabet Med. 1998;15(12):1063–1064. doi: 10.1002/(SICI)1096-9136(1998120)15:12<1063::AID-DIA706>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 25.Radermecker RP, Piérard GE, Scheen AJ. Lipodystrophy reactions to insulin: effects of continuous insulin infusion and new insulin analogs. Am J Clin Dermatol. 2007;8(1):21–28. doi: 10.2165/00128071-200708010-00003. [DOI] [PubMed] [Google Scholar]