Abstract
Control of blood glucose (BG) in an acceptable range is a major therapy target for diabetes patients in both the hospital and outpatient environments. This review focuses on the state of point-of-care (POC) glucose monitoring and the accuracy of the measurement devices. The accuracy of the POC glucose monitor depends on device methodology and other factors, including sample source and collection and patient characteristics. Patient parameters capable of influencing measurements include variations in pH, blood oxygen, hematocrit, changes in microcirculation, and vasopressor therapy. These elements alone or when combined can significantly impact BG measurement accuracy with POC glucose monitoring devices (POCGMDs). In general, currently available POCGMDs exhibit the greatest accuracy within the range of physiological glucose levels but become less reliable at the lower and higher ranges of BG levels. This issue raises serious safety concerns and the importance of understanding the limitations of POCGMDs. This review will discuss potential interferences and shortcomings of the current POCGMDs and stress when these may impact the reliability of POCGMDs for clinical decision-making.
Keywords: blood glucose, glucose error, glucose measurement, hypoglycemia, laboratory, point-of-care device
Introduction
The prevalence of diabetes mellitus continues to increase with approximately 12.9% of the population in the United States diagnosed with diabetes and an even larger portion (29.5%) estimated to be living in a prediabetic state.1 Control of blood glucose (BG) in an acceptable range remains a target for diabetes patients in both the hospital and outpatient environments.2 Glycemic control using an insulin infusion in critically ill patients requires frequent and rapid BG monitoring with devices available for bedside use.3–7 The accuracy of the BG measurements plays an important role for treatment decisions when aiming for glycemic control. This article reviews the accuracy and limitations of current point-of-care glucose monitoring devices (POCGMDs).
The accuracy of glucose monitoring depends on many aspects, including the device measurement mechanism, sample source and collection, and patient attributes. This review will summarize the details of measurement techniques and potential interferences that may alter these measurements to provide background for the subsequent discussion of device accuracy.
Glucose Monitoring Techniques
Point-of-care Glucose Monitoring Devices
During the 1970s, POCGMDs were originally designed for home self-monitoring of blood glucose (SMBG) for diabetes patients to improve glucose control during regular life activities. However, ease of use of a POCGMD and its rapid reporting of BG information led to its utilization in the inpatient setting, recognizing that POCGMDs might have certain limitations with this application. Depending on the specific glucose measurement technique of a POCGMD, the measurements can be influenced by various circumstances. Therefore, technical data are listed in Table 1, and the glucose measurement methodology will be briefly discussed.
Table 1.
Summary of Frequently Used POCGMDs
| Company and devices | Method | Range (mg/dl) | Information | |
|---|---|---|---|---|
| Enzyme | Analysis | |||
| Roche (Basel, Switzerland) | ||||
| AccuChek II | GOR | Photo | Discontinued | |
| Accuchek Advantage | GDH | Amp | 10–600 | |
| AccuChek Compact Plus | GDH | Amp | 10–600 | |
| AccuChek Comfort | GDH | Amp | Discontinued | |
| AccuTrend | GDH | Amp | Glucose/lactate/triglycerides | |
| HemoCue (Cypress, California) | GDH | Photo | ||
| Abbott/MediSense (Alameda, California) | ||||
| Precision QID | GOX | Amp | 20–600 | |
| Precision PCX | GOX | Amp | 20–600 | |
| FreeStyleFlash | GDH | Amp | 20–500 | |
| Optium | GDH | Amp | 20–500 | |
| Optium Xceed | GDH | Amp | 20–500 | |
| Bayer (Leverkusen, Germany) | ||||
| Elite XL | GOX | Amp | 20–600 | |
| Ascensia Contour | GDH | Amp | 20–500 | |
| LifeScan (Milpitas, California) | ||||
| OneTouch II/Ultra | GOX | Photo | 0–600 | |
| SureStep Pro/Flexx | GOX | Photo | 0–500 | |
| DDI Prodigy (Charlotte, North Carolina) | GDH | Amp | Voice controlled | |
| Menarini GlucoMen PC (Berlin, Germany) | GOX | Amp | 20–600 | |
GOR, glucose dye oxidoreductase mediator reaction; photo, photometric; amp, amperometric.
Glucose Oxidase
This measurement technique uses glucose oxidase (GOX) as a catalyst for oxidation of glucose to gluconic acid and hydrogen peroxide; the amount of hydrogen peroxide produced is proportional to the glucose concentration in the blood sample. This change in the hydrogen peroxide concentration can be measured by using a color change as an indicator using a photometric technique or in newer devices, which rely on the production of an electrical current (amperometric technique).8 The basic chemical pathways for the GOX reactions are shown in Figure 1.
Figure 1.
Basic chemical pathways for the glucose oxidase and glucose dehydrogenase-based glucose measurement.H2O2, hydrogen peroxide; FAD, flavin adenine dinucleotide; MBTH, meta[3-methyl 2 benzothiazoline hydrazine]N-sulfonyl benzene sulfonic acid;HRP, horseradish peroxidase; DMAB, dimethylaminobenzoic acid.9
Glucose-1-dehydrogenase
This measurement technique uses glucose-1-dehydrogenase (GDH) to convert glucose to gluconolactone with older devices using a coenzyme to convert nicotinamide adenine dinucleotide (NAD) to NADH (reduced form of NAD). The NADH concentration is measured and is proportional to the BG concentration. The NADH concentration can be measured using a photometric or amperometric technique. Newer POCGMDs use the coenzyme pyrroloquinoline quinone (PQQ) because of less sensitivity to ambient oxygen and electrochemical interference. However, this coenzyme has introduced a dangerous situation, as described later. The outline for the GDH PQQ reaction is shown in Figure 1.
Both GOX and GDH measurement techniques present limitations. The GOX method is extremely specific for BG concentration. However, blood oxygen concentrations influence GOX devices, but not the GDH technique.9,10 The potential influence of physically dissolved oxygen for the GOX reaction is shown in Figure 1. When there are high levels of dissolved oxygen in the sample (e.g., hyperoxia), oxygen is readily available for the GOX reaction and can cause an underestimation of blood glucose; conversely, hypoxemia may falsely elevate GOX glucose measurements. The significance of the oxygen influence is relatively small compared to other potential interferences.9,11 The GDH technique using PQQ has limitations as well. This coenzyme reacts with other sugars (e.g., maltose, galactose, mannose, xylose, and ribose) and detects them as glucose; alternate techniques should be used when these other sugars are present.12,13 The Food and Drug Administration (FDA) issued a public health notification in 2009, secondary to a number of deaths, for the use of the GDH-PQQ glucose monitor because of the potential fatal error related to the interference of other sugars with this methodology.14 For example, icodextrin (a substance commonly used in peritoneal dialysis fluid) is broken down to maltose, which is reported as glucose with the GDH-PQQ POCGMDs. Because of this substance interference, the POCGMD overreports the BG level and, in some cases, can lead to critical treatment errors with significant consequences of hypoglycemia.
Drugs may interfere with both GOX and GDH glucose measurement methods, including but not limited to ascorbic acid and acetaminophen.15,16 The presence of high doses of ascorbic acid has the potential to read falsely low in GOX- and GDH-based devices. Acetaminophen, in therapeutic concentrations, results in lower and higher glucose measurements with the GOX and GDH POCGMD techniques, respectively.12
Central Laboratory Devices
One comparison used for glucose devices is the central laboratory device (CLD) because of its higher accuracy, and studies assessing POCGMD accuracy often employ CLD. Measurement techniques of CLDs vary by device type, and most frequently utilize either the GOX or, more commonly, the glucose hexokinase reaction to measure BG concentration. According to a proficiency report surveying U.S. laboratories, the majority of CLDs use a hexokinase-based method, and the remaining facilities use GOX-based assays.17 Glucose hexokinase phosphorylates glucose to glucose-6-phosphate which is then oxidized by glucose-6-phosphate dehydrogenase using NAD as a cofactor. This results in production of NADH, and the concentration is measured with a spectrophotometer (absorption 340 nm) to determine BG concentration.
The YSI (Yellow Springs Instruments, Yellow Springs, OH) has been used in many studies as a glucose reference. The YSI uses GOX to measure the hydrogen peroxide produced with an amperometric technique. However, in clinical practice, the YSI analysis has been replaced by multianalyte automated instrumentation. Rarely, a radioactive labeled isotope assay is used for instrument validation. Few laboratories still report YSI values.17,18
Sample Source and Collection Site
Methodologies involved with the collection site and storage can significantly impact BG measurements. At room temperature, glucose is metabolized by blood cells at a rate of 5–7% per hour.19 Glycolysis is typically not an issue for a POC measurement, but can cause falsely lower values in CLD results with delayed analysis. One should be cognizant of this when comparing results from both sources.
The CLD reports plasma glucose concentrations; POCGMD measurements typically involve whole blood samples with results usually internally converted to plasma values. Glucose concentrations are higher in the plasma than whole blood because the water content (and thus the glucose concentration) is higher in plasma than in erythrocytes.20 The water content of the plasma can be affected by the concentration of other components. Hypertriglyceridemia and paraproteinemias decrease the water concentration in the whole blood sample, potentially causing a “pseudohypoglycemia,” measured by POCGMDs.20
Sample source can also significantly impact glucose concentration measurements. Potential sampling sites include arterial, venous, or capillary (e.g., finger tips, ear lobes, etc.). As a general rule, the highest to lowest glucose concentration by sampling site is artery, capillary, and then venous.21 The difference in glucose concentrations between capillary and venous blood can be altered by the patient’s metabolic state, with insignificant differences in the absence of stress and fasting. In a critically ill patient, the presence of a hypermetabolic state and other stressors, including fasting, can cause significant differences between these values.21,22 As an example, a critically ill patient with circulatory shock may exhibit a significant difference caused by increased glucose extraction and poor tissue perfusion.
Blood oxygenation affects POCGMD glucose measure-ment techniques with GOX and not with GDH.9–11 Although values may not differ significantly within the normal oxygen range, errors with GOX measurement techniques can be 15% or more when PaO2 (blood oxygen content) exceeds 100 mm Hg or falls below 44 mm Hg depending on the type of test strip and measurement method.9,23 As shown in Figure 1, GOX test strips using peroxide/meta[3-methyl 2 benzothiazoline hydrazine]N-sulfonyl benzene sulfonic acid (MBTH) are less vulnerable to oxygen presence than the GOX/ferrocene method. On the basis of a comparative analysis of several POC test strips by Tang and colleagues,11 using ±15% of reference value from CLD as tolerated error, the GOX/ferrocene strips had the highest glucose measurements outside of the error tolerances (20.1–31.6%), while 14.3% of the GOX/MBTH measurements were outside of the set limits. The impact of oxygen tension on accuracy worsened when blood glucose concentration fell below 100 mg/dl and the oxygen tension was above 100 mm Hg. Only one study investigated the influence of extremely low oxygen tension on POC glucose measurements and found that GOX-based techniques might be inaccurate at extremely low oxygen tensions (PO2 less than 20 mm Hg).23 Although the impact of oxygen tension on the overall accuracy of POCGMD in the cited studies can be minimal, it is not negligible. Therefore, it has been recommended to minimize the oxygen tension effect on glucose testing variability by using oxygen-insensitive test methods in critically ill patients with PaO2 >100 mm Hg or patients with unpredictable blood PO2 levels.11
The impact of patient factors on POC glucose accuracy has been investigated by assessing POCGMDs during tight glycemic control for critically ill patients. The application of POCGMDs for glucose monitoring in critically ill patients is thus important with a detailed discussion covered later in this review. The FDA MAUDE (Food and Drug Administration Manufacturer and User Facility Device Experience) database has been searched for reports related to glucose monitors, revealing 189 records for the year 2011.24 An inquiry of the FDA recall database indicated 30 recalls related to glucose monitors in the time frame 2004–2011.24
Based on the review of the databases mentioned earlier, the POCGMD technology is not always the cause of inaccuracy. Additional effects can come from sample sources, collection sites, and patient factors, and may include the glucose meter cleaning solution or the disinfectant wipe interfering with the measurement.25,26
Point-of-Care Glucose Monitoring Devices Accuracy
There are two ways to assess the accuracy of glucose measurement techniques: technical or clinical. Technical accuracy assesses the agreement between the measured and reference glucose values. Clinical accuracy judges how the differences in the measurements impact clinical decision processes. Both have clinical implications.
A review by Krouwer and Cembrowski27 details the standards and statistical methods used to characterize accuracy of POCGMDs and highlight the different criteria acceptable for accuracy between standard organizations and professional societies (Table 2). In 1987, an American Diabetes Association (ADA) consensus statement recom-mended that the acceptable error for POCGMDs from all sources (user, analytical, etc.) should be less than 10% for glucoses ranging from 30 to 400 mg/dl at all times.28 This ADA consensus statement also recommended that glucose measurements should not differ more than 15% from values obtained by a laboratory reference method. The ADA decreased the maximum allowable analytical error to <5% in 1996.29,30
Table 2.
Acceptable Performance Criteria
| Glucose range | ADA (1987) | ADA (1996) | FDA (1988) | ISO 15197 (2001) |
|---|---|---|---|---|
| <100 mg/dl | <10% | ±5% | ±20 mg/dl | At <75 mg/dl, 95% of measurements should be ±15 mg/dl; at >75 mg/dl, 95% of measurements should be ±20% |
| ≥100 mg/dl | <10% | ±5% | ±20% | 95% of measurements should agree with the reference method; the regression slope can only deviate by ±5% |
| at 100% times | at 100% times | <100% of data | 95% of data | |
International Organization for Standardization (ISO) 15197 provided different recommendations in 2003.31 These state that 95% of the individual glucose measurements compared to the reference measurements are required to be in the range ±15 mg/dl for values less than or equal to 75 mg/dl and ±20% for glucose values greater than 75 mg/dl.30 This is the standard that the FDA normally uses as the goal for approval of POCGMDs. The standards set by the ADA (ADA 1987/1996), requiring all glucose measurements with POCGMDs to be within 5% of CLD values, were deemed technically unachievable by the International Federation of Clinical Chemistry and Laboratory Medicine.19,27 Additionally, the ranges of error set by the technical standards and allowable error do not address the possibility that these errors might provide safety concerns for patients by decision-making being based on inaccurate glucose values.19,31,32
Because of these concerns, publications have reviewed the technical and statistical aspects of POCGMD glucose monitoring.20,27,33,34 Several statistical and graphical options has been used to correlate POCGMD measurements with CLD values. One option to assess analytical error is to use bias plots, such as Bland-Altman (BA) plots. The BA plot graphs the difference between a candidate and a reference method plotted against the mean of the two measurements, where the candidate method is the method under validation. Therefore, the BA plot is a direct visualization of the difference between the two methods. Bias plots allow the analysis of bias and variation from reference of POCGMD measurement over a range of glucose concentrations.35 However, information about the clinical significance of the error is not included in this type of analysis. The error grid plots accuracy in terms of the effect on clinical decisions. The recognized need for a more clinically oriented approach to ensure patient safety for evaluation and regulation accuracy of devices was addressed by Clarke and colleagues36 in 1987, with these recommendations modified by Parkes and colleagues37 in 2000, which is commonly called the consensus grid. Clarke and colleagues36 developed the error grid to evaluate the accuracy of the clinical decision-making based on the measured glucose value. The grids define several zones: zone A, points with no clinical implication because of clinically accurate measurement; zone B, points still lead to accurate clinical decisions; zone C, misinterpretation of euglycemia as hyper- or hypoglycemia; and zone D/E, points lead to overestimation of hypo-glycemia or underestimation of hyperglycemia. Decisions and/or interventions that were clinically inappropriate due to errors in glucose measurements were illustrated by the points located in zones C, D, and E. Parkes and colleagues37 subsequently modified the Clarke error grid (shown in Figures 2 and 3) because of concerns of the proximity of the results in zone A (acceptable result) and zone D (dangerous result). Although the error grids of Clarke and Parkes have been used to explore the clinical accuracy and implications of POCGMDs, organizations responsible for establishing standards have yet to adopt this approach. The FDA commonly uses error grids in the approval process for a POCGMD.
Figure 2.
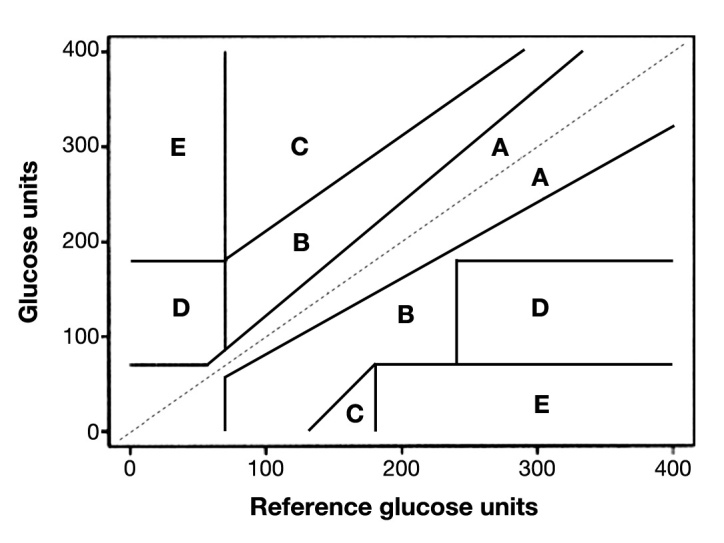
Clarke error grid.
Figure 3.

Parkes consensus error grid (printed with permission from the Journal of Diabetes Science and Technology).
On the basis of recent publications reviewing the difficulties of the technical and statistical aspect of POCGMD glucose monitoring, the currently available standards are not meeting the needs of clinicians in certain environments (hyper- or hypoglycemia, intensive insulin therapy), and clinicians are concerned with the lack of agreement between POCGMD results with serum/plasma laboratory results.20,27 With concerns that the current allowable errors could potentially harm patients, there has been a call for the development of more clinically relevant standards.20,27,33,34
Studies of Point-of-Care Glucose Monitoring Devices Accuracy
A Medline search was performed for the years 1990–2011 using the following key words: blood glucose, glucose meter, glucose error, glucose measurement, hypoglycemia, point-of-care device, tight glucose control, and insulin therapy. Review of this literature identified publications relevant to the evaluation of POCGMD accuracy. Characteristics of the relevant publications studying outpatients or hospitalized ward patients excluding intensive care unit (ICU) patients are listed in Table 3, with a study summary in Table 4. This search revealed publications that compared several POCGMDs to a reference CLD. Giordano and colleagues38 compared seven commercially available POCGMDs with a reference method, finding only three devices had acceptable measurement accuracy. The remaining four devices consistently underestimated blood glucose levels of less than 100 mg/dl, raising concerns about the potential implications of unrecognized and thus untreated hypo-glycemia. Chen and colleagues39 blindly evaluated four POCGMDs to prevent any potential bias created by commercial pressure. Only two out of four devices performed with acceptable accuracy according to ISO standards; none achieved the ADA 1996 recommendations for POCGMD accuracy. All four POCGMDs showed less reliability with lower glucose values compared to normal or higher values. Cohen and colleagues40 evaluated five POCGMD using the Clarke error grid; four of the five devices met criteria for accurate clinical decision-making. However, only one out of five devices met ADA 1996 accuracy standards.
Table 3.
Selection of Publications Evaluating POCGMD Accuracy Measuring Glucose Levels
| Author | Publication year | POCGMD(s) | Sample source | Study characteristics |
|---|---|---|---|---|
| Giordano et al.38 | 1989 | AccuChek II | Cap | n = 27; accuracy at high altitude |
| Diascan–S | ||||
| ExacTech | ||||
| Glucometer II | ||||
| Glucoscan 3000 | ||||
| OneTouch | ||||
| Tracer | ||||
| Ashworth et al.51,a | 1992 | HemoCue | Ven | n = 30; triglyceride influence; range 32.4–129.6 mg/dl |
| Wiener et al.52 | 1993 | HemoCue | Ven | Hematocrit influence |
| Larbig et al.53,a | 2003 | Prestige IQ | n = 61; outpatient setting | |
| Chen et al.39 | 2003 | 4 GOX brand POCGMD (meter A, B, C, D) | Ven | n = 461; range 10–600 mg/dl, hct 25–60%, normoxia |
| DIRECNET45,a | 2003 | OneTouch Ultra | Cap, ven | n = 91; children 3–17 years, outpatient setting |
| Singh et al.54 | 2004 | SureStepFlexx | Ven | Accuracy evaluation |
| Dai et al.55 | 2004 | EasyTouch | Cap | n = 516, range 42–555 mg/dl |
| Kendall et al.56 | 2005 | Ascensia Confirm 10 disk system | Cap | n = 100, patient vs health care provider, self-monitoring accuracy; range 41.4–352.8 mg/dl |
| Stork et al.46 | 2005 | HemoCue | n = 24 (500 measurements); hypoglycemic range (289 measurements) | |
| Rao et al.57 | 2005 | 3 GOX meters | Ven | 600 measurements, 50 at each hct level over Hct 30–60%; automatic hct correction |
| Meter 1: GOX/Ph-m | ||||
| Meter 2: GOX/Am-m | ||||
| Meter 3: GOX/Am-m with hct correction | ||||
| Hawkins et al.58,a | 2005 | AccuChek Go | n = 120; whole blood sample (AC) vs plasma glucose (Optium) | |
| Optium | ||||
| Cohen et al.40,a | 2006 | AccuChek Go | Cap | n = 49; clinic setting |
| AccuChek Advantage | ||||
| Optium | ||||
| CareSense | ||||
| GlucoMen PC | ||||
| Rosenthal et al.47 | 2006 | Accutrend | Cap | n = 110 (122 samples); postnatal monitoring |
| Khan et al.41 | 2006 | One Touch II | Cap, ven | n = 358; clinic setting |
| Precision QID | ||||
| Precision PCX | ||||
| Elite XL | ||||
| SureStepFlexx | ||||
| AccuChek Advantage | ||||
| AccuCheck Comfort C | ||||
| Rivers et al.48,a | 2006 | FreeStyleFlash | Cap, ven, finger vs forearm | n = 100; clinic setting; range 69–354 mg/dl |
| One Touch Ultra | ||||
| Lippi et al.49 | 2006 | Accuchek | Ven | n = 225 measurements; outpatient setting; range 2.2–22 mmol/liter |
| One Touch II | ||||
| Elite XL | ||||
| GlucoMen PC | ||||
| Bellini et al.50 | 2007 | HemoCue | Cap | n = 78; neonatal monitoring |
| Thomas et al.42,a | 2008 | FreeStyle Flash | Cap, ven | n = 202; clinic setting |
| AccuChek | ||||
| BD Logic | ||||
| AccuChek Compact Plus | ||||
| Ascensia Contour | ||||
| Karon et al.16,a | 2008 | Statstrip | Ven | n = 185 measurements; hematocrit influence; acetaminophen influence |
| AccuCheck II | ||||
| Precision PCx | ||||
| SureStepFlexx | ||||
| Sheffield et al.43,a | 2009 | Optium | Cap, ven | n = 125; clinic setting |
| DDI Prodigy | ||||
| HDI True TrackSmart System | ||||
| Hypoguard Assure | ||||
| Florkowski et al.46,a | 2009 | Roche Performa | Cap | n = 100; outpatient setting |
| Optium Xceed (5s and 10s) | ||||
Cap, capillary; Ven, venous
Industrial sponsorship.
Table 4.
Selection of POCGMD Accuracy Data
| Author | POCGMD(s) | Results | Conclusions |
|---|---|---|---|
| Giordano et al.38 | AccuChek II | R = 0.98, underestimates BGlu by 20.6% at levels <100 mg/dl | The four out of seven tested POCGMD underestimated BGlu at <100 mg/dl. AccuChek performed best, followed by OneTouch. Measurements at high altitude increased the BGlu underestimation. |
| OneTouch | R = 0.97, underestimates BGlu <100 mg/dl—overall reliable | ||
| Diascan–S | R = 0.93, underestimates BGlu at all levels—inconsistent measurements | ||
| Tracer | R = 0.94, overestimates BGlu <100 mg/dl, underestimates >250 mg/dl—scatter | ||
| ExacTech | R = 0.96, overestimates BGlu at all levels, especially <100 mg/dl | ||
| Glucometer II | R = 0.89, overestimates BGlu <100 mg/dl, underestimates >250 mg/dl | ||
| Glucoscan 3000 | R = 0.87, underestimates BGlu at all levels, scatter—not consistent | ||
| Ashworth et al.51 | HemoCue | R = 0.947 with all measurements <5% of RM | Reliable and accurate POCGMD |
| Triglyceride concentration affected glucose measurements | Correct for triglyceride concentration | ||
| Hct 35–65% did not affect glucose measurement with HemoCue | Hct range did not affect the accuracy | ||
| Wiener et al.52 | HemoCue | CV 1.8% | Hct range did not affect the accuracy |
| Larbig et al.53 | Prestige IQ | R = 0.972 with 95.9% in Clarke A or B area | Reliable and accurate POCGMD |
| Chen et al.39 | 4 GOX brand POCGMD | Tested 4 brand POCGMDs—covering 90% of the market at the time | Meter A overestimated; meter D underestimated; only meter B and C fit ISO accuracy criteria, none met ADA criteria for accuracy |
| A: R = 0.989; at high BGlu R = 0.977, at low BGlu R = 0.956; CV 2% | |||
| B: R = 0.988; at high BGlu R = 0.974, at low BGlu R = 0.952; CV 3.3% | |||
| C: R = 0.989; at high BGlu R = 0.982, at low BGlu R = 0.947; CV 3.5% | |||
| D: R = 0.975; at high BGlu R = 0.934, at low BGlu R = 0.900; CV 4.3% | |||
| DIRECNET45 | OneTouch Ultra | R = 0.97, 99% of measurements in Clarke A/B area. More variability at low BGlu; ISO criteria were met at 96% of venous samples but only at 84% of capillary samples | POCGMD less reliable at BGlu <70 mg/dl; ISO criteria were met with venous samples |
| Singh et al.54 | SureStepFlexx | When compared nurse operator vs lab tech, both had <5% measurement error when compared to RM. However, inconsistent BGlu measurements with total error variability for nurse 0–21% and for lab tech 4–13% | Inconsistent measurements but not operator dependent |
| Dai et al.55 | EasyTouch | R2 = 0.957, 100% of measurements are in Clarke A or B zone | Reliable POCGMD |
| Reading are consistent in all BGlu ranges: <100, 100–200, >200 | |||
| Kendall et al.56 | Ascensia Confirm | Device vs RM R = 0.97, 92.3% of BGlu <5% of RM, 93% of values are in Clarke A zone, 7% in Clarke B zone; SMBG vs HCP R = 0.96 | Reliable POCGMD, good accuracy in SMBG use |
| 10 disk system | |||
| Stork et al.46 | HemoCue | R in normoglycemia = 0.979 | Not reliable during severe hypoglycemia |
| R in hypoglycemia = 0.880, difference in BGlu between POCGMD and RM increased during hypoglycemia conditions | |||
| Rao et al.57 | 3 GOX meters | At hct >50%, all POCGMD underestimated BGlu | Automatic Hct correction did not improve POCGMD accuracy |
| Hawkins et al.58 | AccuChek Go | AC measures whole blood and has consistent BGlu bias +2.5%, 97% in Clarke A zone, 3% in Clarke B zone | Both devices perform satisfactory for clinical use, but AccuChek had better accuracy and consistency than Optium |
| Optium | Optium measure plasma glucose, showed concentration-dependent bias with positive bias at low BGlu and negative bias at high BGlu; 94% of values in Clarke A zone, 6% in Clarke B zone | ||
| Cohen et al.40 | AccuChek Go | R = 1.06x + 0.12; CV low 5.51%; CV high 3.26% | All POCGMD measured higher than RM. Only CareSense met ADA accuracy criteria (<5% error) |
| AccuChek Advantage | R = 1.03x + 0.29; CV low 3.64%; CV high 2.62%; error range 6.5% | ||
| Optium | R = 0.99x + 0.67; CV low 4.36%; CV high 3.71% | ||
| CareSense | R = 0.93x + 0.95; CV high 2.83%; error range 4% | ||
| GlucoMen PC | R = 1.15x + 0.03; CV NA (no standard provided); error 15.5% (All measurements were in Clarke A or B zone) | ||
| Rosenthal et al.47 | Accutrend | From 122 measurements, 39 overestimated BGlu, 81 underestimated BGlu. R = 0.68; 17% of measurements were outside the 95% CI | Not reliable for neonatal BGlu screening |
| Khan et al.41 | OneTouch II | Bias varied from -7.9% to 2.8% | AccuChek POCGMD showed lowest bias. However at hypoglycemia, BGlu differences >20% occurred in 57% of values, differences >10% in 61% |
| Precision QID | Bias varied from -10.4% to -0.7% | ||
| Precision PCX | Bias varied from -17.0% to -5.2% | ||
| Elite XL | Bias varied from -30.6% to -6.1% | ||
| SureStepFlexx | Bias varied from -2.7% to 12.7% | ||
| AccuChek Advantage | Bias varied from -15.5% to -5.8% | ||
| AccuCheck Comfort C | Bias varied from -5.1% to 0.8% | ||
| Rivers et al.48 | FreeStyleFlash | 72% of BGlu (finger), 64% (forearm) were within 10% RM value | FreeStyleFlash POCGMD measurements were more accurate than OneTouch. Finger capillary samples were more reliable than Forearm sampling |
| OneTouch Ultra | 57% of BGlu (finger), 36% (forearm) were within 10% RM value | ||
| Lippi et al.49 | AccuChek | Bias was -4.9 to 14.1%. GlucoMen and Elite POCGMD consistently overestimated BGlu and OneTouch consistently underestimated BGlu. OneTouch/AccuChek/Elite showed acceptable BGlu within 95% CI; GlucoMen BGlu 15% of measurements were outside of acceptable error tolerance level | No POCGMD met ADA accuracy criteria. AccuChek/OneTouch/Elite XL were in the ISO accuracy recommendations |
| OneTouch II | |||
| Elite XL | |||
| GlucoMen PC | |||
| Bellini et al.50 | HemoCue | R = 0.905, POCGMD overestimated BGlu by 16.7mg/dl (average) | Accuracy is dependent on birth weight. HemoCue cannot be used for neonatal BGlu screening |
| Thomas et al.42 | FreeStyle Flash | BGlu within 10% of RM = 70%, Clarke A 97, B 4, C 0, D 0 | Only FreeStyle Flash and Ascensia Contour measurements were within 20% accuracy criterias. Only FreeStyle flash fulfilled the <10% error tolerance |
| AccuChek Advantage | BGlu within 10% of RM = 30%, Clarke A 75, B 23, C 1, D 1 | ||
| AccuChek Compact Plus | BGlu within 10% of RM = 38%, Clarke A 70, B 29, C 1, D 1 | ||
| Ascensia Contour | BGlu within 10% of RM = 46%, Clarke A 88, B 10, C 0, D 2 | ||
| BD Logic | BGlu within 10% of RM = 48%, Clarke A 67, B 26, C 1, D 6 (All POCGMD had a tendency to read higher at low BGlu level) | ||
| Sheffield et al.43 | Optium | 42% of BGlu varied <5% of RM, precision 9 ± 10 mg/dl | Optium was found to be the most accurate POCGMD with 94% of BGlu were in <20% agreement with RM. Only Optium and DDI Prodigy met ISO accuracy criteria |
| DDI Prodigy | 24% of BGlu varied <5% of RM, precision 11 ± 10 mg/dl | ||
| HDI True TrackSmart | 13% of BGlu varied <5% of RM, precision 15 ± 18 mg/dl | ||
| Hypoguard Assure | 29% of BGlu varied <5% of RM, precision 11 ± 16 mg/dl | ||
| Florkow-ski et al.44 | Roche Performa | Bias 0.52%, 99% in Clarke A, 1% in Clarke B | <5% of POCGMD measurements were within 20% of RM values (ISO standard); ADA goals were not met |
| Optium Xceed (5s) | Bias -2.78%, 98% in Clarke A, 2% in Clarke B | ||
| Optium Xceed (10s) | Bias -1.36%, 96% in Clarke A, 4% in Clarke B | ||
BGlu, blood glucose concentration; RM, reference method; GOX, glucose oxidase method; HCP, health care provider; R, regression coefficient; CI, confidence interval; CV, coefficient of variation.
Seven POCGMDs involving four different manufacturers were compared to a reference method (YSI) by Khan and colleagues.41 Only one device met ADA 1996 performance requirements. Of major concern was the significant disagreement with reference values within the critical hypoglycemic range that could result in an adverse clinical decision. At the extremes of hyperglycemia and hypoglycemia, when compared to CLD, 61% of values differed by more than 10% from the reference method with an alarming 57% of measurements differing by more than 20% in the hypoglycemic range. This study emphasizes the shortcomings for accurate detection and thus treatment of hypoglycemia with POCGMDs.
In 2008, Thomas and colleagues42 evaluated several POCGMDs not available in the United States. Four of the five devices were deemed accurate enough to be used in a clinical setting based on a Clarke error grid. However, only two of the five devices provided measurements with less than 20% variation from the reference method, with one device having less than 10% error.
Although the ADA and ISO guidelines have been published for over a decade, few POCGMDs meet these accuracy standards. Two examples of evaluations include (1) Sheffield and colleagues43 studied four commercially available POCGMDs and reported that only two devices met ISO standard requirements and (2) Florkowski and colleagues44 evaluated two POCGMDs, and although both passed ISO requirements, they failed to meet ADA 1996 recommendations.
One specific concern with POCGMDs is errors in the hypoglycemic range and the potential impact on clinical decision-making. When errors occur in the lower glucose ranges, it most commonly entails a report of a higher than actual blood glucose value. This can lead to a misdiagnosis of euglycemia when in fact hypoglycemia exists, placing the patient at risk for neurological sequelae because of a failure of early recognition or aggressive treatment of hypoglycemia.38,39,41,45–50 Because of the importance of accurate glucose values in the hypoglycemic range, Stork and colleagues46 focused further evaluation of POCGMDs in these patients. While measurements in the euglycemic range were acceptable, measurement accuracy decreased significantly in the hypoglycemic range. In neonates, POCGMDs for hypoglycemia screening did not have the required accuracy.47,50
Although less frequent, POCGMDs can report a falsely low value. The error may result in treatment for hypo-glycemic when, in fact, euglycemia actually exists; the hypoglycemia treatment with additional glucose may lead to hyperglycemia. These examples illustrate some of the limitations of current POCGMDs. The accuracy of POCGMDs in these studies rarely met ADA accuracy recommendations in the hypoglycemia range. These devices were originally designed for outpatient SMBG, not to accurately reflect hypoglycemia in hospitalized ward and critically ill patients, whose condition may mask signs and symptoms of hypoglycemia.
Studies of Point-of-Care Glucose Monitoring Devices Accuracy in Intensive Care Unit Patients
Critically ill patients offer additional challenges for POC glucose monitoring. Microcirculatory abnormalities may affect capillary sampling or may be receiving other therapies, including vasoactive and additional pharmacological agents, that interfere with POCGMD. Other factors that may impact accuracy of testing include significant variation in hematocrit (Hct), pH, and blood oxygen or carbon dioxide level. Hyperglycemia treatment for many critically ill patients requires intravenous insulin infusion to control blood glucose.6,7,59,60 Accurate BG measurements are extremely important for patients on insulin infusions. Relevant publications of POCGMD evaluated in the critically ill patient are listed in Table 5.
Table 5.
Selection of Publications Evaluating POCGMD Accuracy Measuring Glucose Levels on Critically Ill Patients
| Author | Publication year | POCGMD (s) | Patient collective | Sample source | Findings |
|---|---|---|---|---|---|
| Maser et al.61 | 1994 | AccuChek II | n = 50, hematocrit correction | Ar, cap | Ar > cap samples by 30 mg/dl, decrease to 10 mg/dl if hematocrit corrected. Cap samples are up to 21 mg/dl from CLD values. |
| Potential for incorrect insulin dosing if both ar and cap samples are used in ICU patients | |||||
| Louie et al.62 | 2000 | SureStepPro | n = 247; influence of paO2, paCO2, pH, hct | Ar | POCGMD accuracy between 91% and 95% (Pre) and 98–100% (SSPro); hct influence on glucose measurement accuracy |
| Precision G | |||||
| Ray et al.75 | 2001 | OneTouch | n =10 | Ar | Study range 86.4–392.4 mg/dl. |
| POCGMD remained ±41.4 mg/dl of certainty | |||||
| Kanji et al.68 | 2005 | AccuChek | n = 30; influence of peripheral edema, vasopressor therapy | Ar, cap | POCGMD meet CLD measurement in 69.9% (ar) and 56.8% (cap), the reliability is less in hypotension. CLD agreement is achieved in <80% of samples, during hypotension <70% of samples |
| Finkielman et al.69 | 2005 | SureStepFlexx | n = 197; influence of hypotension (retrospective) | Ar, cap | Difference between CLD and POCGMD 8–9 mg/dl, no influence of MAP or vasopressor therapy |
| Karon et al.76 | 2007 | AccuChek | n = 20; s/p CABG under insulin therapy and inotropic medications | Ar, cap, ven | Cap measurements were more accurate than ar or ven samples. A total of 78/96 measurements were within 10% of CLD. More positive bias at glucose levels >160 mg/dl |
| Lacara et al.63 | 2007 | SureStep Pro | n = 49; influence of Hct, paCO2, MAP 56–130 mmHg | Ar, ven | Ar and ven samples less bias than cap sampling. POCGMD did not differ from CLD measurements at range 52–281 mg/dl. |
| No influence of Hct, paCO2, or MAP | |||||
| Critchell et al.77 | 2007 | AccuChek | n = 80; MICU pt | Cap | Cap measurements were not reliable with variation of 8.6 ± 18.6 mg/dl from CLD, 19% of values were >5% from CLD level |
| Hoedemaekers et al.78 | 2008 | AccuChek | Critically ill pt under insulin therapy vs non-ICU pt | Ar (ICU), ar, ven (non-ICU) | All three POCGMD are less accurate in ICU pt than in non-ICU pt. HemoCue more accurate than AccuChek or Precision |
| Precision | |||||
| HemoCue | |||||
| Petersen et al.79,a | 2008 | AccuChek | n = 144; MICU pt | Ar, cap, ven | POCGMD had a positive bias 12.6–16.2 mg/dl compared to CLD, ar and ven are less variable than cap. Cap sample 3/144 severely underestimated CLD glucose. |
| Slater-MacLean et al.70 | 2008 | SureStepFlexx | n = 60; influence of vasopressor and insulin therapy | Ar, cap | AccuChek POCGMD with higher level of bias. In all three POCGMD ar samples more reliable than ven or cap measurements |
| AccuChek | |||||
| FreeStyle | |||||
| Desachy et al.71 | 2008 | AccuChek | n = 85; critically ill pt in shock | Cap | Low tissue perfusion correlates with value discrepancy between POCGMD and CLD. A total of 7% discordant values—cap samples not accurate |
| Cook et al.64 | 2009 | SureStepFlexx | N = 67; critically ill pt | Cap, ven (CVL) | Range 62–247 mg/dl, CVL samples 15% differ >20% from CLD, cap samples 21% differ from CLD. Discrepancies improved with hct correction |
| Meynaar et al.80 | 2009 | AccuChek | n = 32; critically ill pt | Ar | Average 11 mg/dl difference between POCGMD and CLD, POCGMD accuracy better at high glucose levels. Mostly POCGMD is underestimating glucose level |
| Fekih-Hassen et al.72 | 2010 | AccuChek | n = 43; influence of catecholamine therapy | Cap, ven | POCGMD and CLD difference >40 mg/dl in 29% without catecholamine therapy and 40% in patients with catecholamine therapy. Cap glucose monitoring not reliable during catecholamine therapy |
MAP, mean arterial blood pressure; ar, arterial; cap, capillary; ven, venous; pt, patients; vs, versus; CVL, central venous catheter.
Industrial sponsor for study.
The impact of Hct on the POCGMD accuracy in critically ill patients has received attention. Some studies documented the influence16,51,52,57,61–64 of Hct variation, especially when acute anemia develops in certain patient populations (e.g., cardiac surgery),61,62,64 while others report accuracy over a wide range of Hcts.51,52,63 Newer POCGMDs correct for Hct variation with several amperometric methods, involving a new technique called dynamic electrochemistry.65,66 There are certainly numerous possible interferences with these techniques, which may be similar to those recently documented for Hct POC measurements, which use similar methodology.67
Blood oxygen content (PaO2), carbon dioxide tension (PaCO2), and blood pH changes all can impact glucose measurement accuracy. Dissolved blood oxygen can interfere with the GOX assay as previously detailed.9,11,23 All POCGMD methods rely on enzymatic activity with function potentially affected by changes in blood pH and PaCO2. Louie and colleagues62 studied the influence of PaO2, PaCO2, and pH with POCGMDs using GOX and GDH technology. Neither POCGMD using GOX or GDH technology were affected by PaCO2 or pH, and the POCGMD using GDH was unaffected by PaO2. However, the GOX POCGMD was sensitive to oxygen tension, consistently reporting lower than actual BG value when PaO2 levels exceeded 150 mm Hg. However, Lacara and colleagues,63 using a multifactorial regression model in a small patient population (n = 42 patients), found that plasma PaCO2 contributed to the difference between CLD and POC glucoses in critically ill patients after Hct correction. In summary, the influence of carbon dioxide tension on the POCGMD accuracy remains controversial and is most likely minimal.
The influences of peripheral edema, hypotension, vaso-pressor, or catecholamine therapy on POCGMD accuracy in critically ill patients had been the subject of several investigations.21,63,68–73 Kanji and colleagues68 investigated the influences of peripheral edema and vasopressor therapy on POC glucose monitoring. Disagreement was defined when CLD and POCGMD results led to different treatment decisions based on an institutional insulin therapy protocol. Compared to arterial sampling, capillary sampling was less accurate for glucose measurements in critically ill patients, especially in the hypoglycemia range.68,70 With fast changing BG values, the time constant for capillary blood may be quite long and could be a contributor to inaccuracy under these circumstances.74 Fewer than 80% of the capillary samples were in agreement with the CLD measurement. When hypotension was present, this agree-ment further decreased to less than 70%. With the presence of edema or when patients were receiving vasopressor medications and BG levels were <80 mg/dl, only 25% of the capillary POCGMD measurements and less than 55% of arterial POCGMD measurements were accurate when compared with CLD results (Figure 4).68 Focusing on patients with low perfusion indices and requiring vasopressor therapy, Desachy and colleagues71 noted disagreement between POCGMD and CLD in more than 15% of capillary samples. These measurement discrepancies correlated with low tissue perfusion index, generalized mottling, and hypotension. These influences were noted in older investigations by Atkin et al.22 and Sylvain et al.,73 where hypotensive patients’ capillary blood glucose values were inaccurate. The time constant of the capillary blood space may contribute to this discrepancy, as lower perfusion may lengthen the time constant. Catecholamine administration to critically ill patients also influences POC glucose. Fekih-Hassen and colleagues72 investigated the accuracy of two capillary sampling sites in critically ill patients being administered catecholamines compared to hemodynamically stable patients not receiving these drugs. In critically ill patients receiving catecholamines, the POCGMD and CLD measurements differed from 29% to 40%. Even in hemodynamically stable patients, there was less than acceptable POCGMD accuracy with capillary sampling.72 When comparing the performance of three POCGMDs in critically ill patients compared with noncritically ill patients, Hoedemaekers and colleagues78 found all three devices to be less accurate in the critically ill patients. When inaccurate, the POC glucose levels were most often falsely elevated, with the potential for inappropriate insulin administration and/or masking “true hypoglycemia.” The use of insulin infusions to manage BG levels in critically ill patients demands monitoring to be precise, reliable, and frequent.
Figure 4.
POCGMD glucose measurement agreement with reference method during hypoglycemia versus nonhypoglycemia in critically ill patients. The POCGMD glucose measurement were said to agree with the reference method (CLD) if both measurements resulted in a similar clinical intervention. A total of 118 paired observations were analyzed (all), divided into three groups: vasopressor-dependent (n = 36: hypoglycemia n = 8, nonhypoglycemia n = 28), edematous (n = 43: hypoglycemia n = 21, nonhypoglycemia n = 22), and postsurgical (n = 39: hypoglycemia n = 9, nonhypoglycemia n = 30). Data are presented as mean with 95% confidence interval. For the statistical analysis, please review the original data source.68
Several studies have investigated POCGMDs during intensive insulin therapy in critically ill patients.70,76,78 Capillary samples in one study showed acceptable accuracy during intensive insulin therapy in normotensive and euglycemic patients after cardiac surgery.76 Two other studies found POCGMDs to have significant inaccuracies in critically ill patients.70,78 Hoedemaekers and colleagues78 tested three POCGMDs in ICU patients; all devices failed to meet ISO standards in hypoglycemic samples. Using simulation modeling, Karon and colleagues81 defined performance criteria for using glucose meter technology for tight glucose control with insulin infusions, stating that POCGMDs that operate within a 15% total allowable error tolerance would be acceptable. The current criteria allow 20%. With these limitations, these authors concluded that POCGMDs were ill-suited to monitor glucose during intensive insulin infusion in critically ill patients. Sampling arterial blood rather than capillary blood may reduce measurement variability and inaccuracies70 because of the variable time constant in the capillary sample, and the use of different sites might explain some discrepancies in POCGMD compared to CLD. When initiating intensive insulin therapy, close attention should be paid to potential sources of error, including sample source, measurement techniques, and patient factors. All these factors should be taken into consideration when making treatment decisions. Blood glucose concentration should be measured frequently (at minimum hourly) during intensive insulin therapy in critically ill patients, and when values obtained from POCGMD pose a patient safety risk, those values should be confirmed by CLD.
Conclusion
Accuracy can be defined as the variation from the reference value. When assessing laboratory values for glucose, the testing method is accurate if the measurement is within acceptable error compared to the reference method. Within the range of hypoglycemia, if the values reported by the POCGMD are inaccurate (e.g., reported higher than actual values), this inaccuracy could lead to failure to recognize and treat life-threatening values or even more worrisome result in a different treatment (e.g., increasing insulin infusions) that could pose a serious patient safety risk. The importance of accuracy for clinical treatment assesses whether the measurement value is within a range close enough to the actual value that the clinical approach to therapy remains the same. The current ADA device recommendations for SMBG with POCGMDs include the following: (a) achieve and maintain glycemic control, (b) prevent and detect hypo-glycemia, (c) avoid severe hyperglycemia, and (d) facilitate diabetes therapy adjustment to lifestyle changes (activity, diet changes, etc.). The accuracy requirements set by the professional organizations are still rarely met by POCGMDs. With outpatients and other hospitalized noncritically ill patients, most clinicians appear satisfied with POCGMD accuracy when glucose values avoid the extremes of hypoglycemia and hyperglycemia. This is because, in the range of normal glucose, the accuracy in this range is typically acceptable for clinical decision-making. For the care of critically ill patients, accuracy becomes more important as some of the early signs present with hypoglycemia and hyperglycemia may be difficult to detect in this patient population due to decreased mental status, sedatives, and other patient conditions. For optimal glucose control in high-demand states in critically ill patients, POCGMD technology has yet to provide a high enough degree of accuracy and reliability that leads to appropriate clinical decision-making. Continuous glucose monitoring devices based on invasive, minimal invasive, or noninvasive methodology are being developed to improve blood glucose monitoring.82 Available technology, including future advances and current limitations, has been reviewed by Vaddiraju and colleagues.83 Development of a meter with accuracy equal to CLDs should continue to be the industry goal.
Acknowledgments
The authors would like to thank Pamela Knight for her assistance with manuscript preparation.
Glossary
Abbreviations
- (ADA)
American Diabetes Association
- (BA)
Bland-Altman
- (BG)
blood glucose
- (CLD)
central laboratory devices
- (FDA)
Food and Drug Administration
- (GDH)
glucose-1-dehydrogenase
- (GOX)
glucose oxidase
- (Hct)
hematocrit
- (ICU)
intensive care unit
- (ISO)
International Organization for Standardization
- (MBTH)
meta[3-methyl 2 benzothiazoline hydrazine]N-sulfonyl benzene sulfonic acid
- (NAD)
nicotinamide adenine dinucleotide
- (NADH)
nicotinamide adenine dinucleotide (reduced form)
- (PaO2)
blood oxygen content
- (PaCO2)
carbon dioxide tension
- (PO2)
oxygen tension
- (POC)
point of care
- (POCGMD)
point-of-care glucose monitoring device
- (PQQ)
pyrroloquinoline quinone
- (SMBG)
self-monitoring of blood glucose
- (YSI)
Yellow Springs Instruments
References
- 1.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacks DB, Bruns DE, Goldstein DE, MacLaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436–472. [PubMed] [Google Scholar]
- 3.Alter D, Deines G. Tight glycemic control and point-of-care testing. Clin Lab Med. 2009;29(3):511–522. doi: 10.1016/j.cll.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Fahy BG, Sheehy AM, Coursin DB. Glucose control in the intensive care unit. Crit Care Med. 2009;37(5):1769–1776. doi: 10.1097/CCM.0b013e3181a19ceb. [DOI] [PubMed] [Google Scholar]
- 5.Pitkin AD, Rice MJ. Challenges to glycemic measurement in the perioperative and critically ill patient: a review. J Diabetes Sci Technol. 2009;3(6):1270–1281. doi: 10.1177/193229680900300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 8.Bergel A, Souppe J, Comtat M. Enzymatic amplification for spectro- photometric and electrochemical assays of NAD+ and NADH. Anal Biochem. 1989;179(2):382–388. doi: 10.1016/0003-2697(89)90149-8. [DOI] [PubMed] [Google Scholar]
- 9.Tang Z, Louie RF, Payes M, Chang KC, Kost GJ. Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther. 2000;2(3):349–362. doi: 10.1089/15209150050194215. [DOI] [PubMed] [Google Scholar]
- 10.Tang Z, Du X, Louie RF, Kost GJ. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124(4):577–582. doi: 10.5858/2000-124-0577-EOPOGM. [DOI] [PubMed] [Google Scholar]
- 11.Tang Z, Louie RF, Lee JH, Lee DM, Miller EE, Kost GJ. Oxygen effects on glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062–1070. doi: 10.1097/00003246-200105000-00038. [DOI] [PubMed] [Google Scholar]
- 12.Rice MJ, Pitkin AD, Coursin DB. Glucose measurement in the operating room: more complicated than it seems. Anesth Analg. 2010;110(4):1056–1065. doi: 10.1213/ANE.0b013e3181cc07de. [DOI] [PubMed] [Google Scholar]
- 13.Weitgasser R, Gappmeyer B, Pichler M. Newer portable glucose meters—analytical improvement compared with previous generation devices. Clin Chem. 1999;45(10):1821–1825. [PubMed] [Google Scholar]
- 14.Schultz DG. FDA public health notification: potentially fatal errors with GDH-PQQ* glucose monitoring technology. http://www.fda.gov/medicaldevices/safety/alertsandnotices/publichealthnotifications/ucm176992.htm Accessed August 13, 2009.
- 15.Tang Z, Du X, Louie RF, Kost GJ. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol. 2000;113(1):75–86. doi: 10.1309/QAW1-X5XW-BVRQ-5LKQ. [DOI] [PubMed] [Google Scholar]
- 16.Karon BS, Griesmann L, Scott R, Bryant SC, Dubois JA, Shirey TL, Presti S, Santrach PJ. Evaluation of the impact of hematocrit and other interference on the accuracy of hospital-based glucose meters. Diabetes Technol Ther. 2008;10(2):111–120. doi: 10.1089/dia.2007.0257. [DOI] [PubMed] [Google Scholar]
- 17.Howerton D, Krolak JM, Manasterski A, Handsfield JH. Proficiency testing performance in US laboratories: results reported to the Center for Medicare and Medicaid Services, 1994 through 2006. Arch Pathol Lab Med. 2010;134(5):751–758. doi: 10.5858/134.5.751. [DOI] [PubMed] [Google Scholar]
- 18.Miller WG, Myres GL, Ashwood ER, Killeen AA, Wang E, Ehlers GW, Hassemer D, Lo SF, Seccombe D, Siekmann L, Thienpont LM, Toth A. State of the art in trueness and interlaboratory harmonization for 10 analytes in general clinical chemistry. Arch Pathol Lab Med. 2008;132(5):838–846. doi: 10.5858/2008-132-838-SOTAIT. [DOI] [PubMed] [Google Scholar]
- 19.Arabadjief D, Nichols JH. Assessing glucose meter accuracy. Curr Med Res Opin. 2006;22(11):2167–2174. doi: 10.1185/030079906X148274. [DOI] [PubMed] [Google Scholar]
- 20.Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3(4):971–980. doi: 10.1177/193229680900300446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Orazio P, Burnett RW, Fogh-Anderson N, Jacobs E, Kuwa K, Külpmann WR, Larsson L, Lewenstam A, Maas AH, Mager G, Naskalski JW, Okorodudu AO IFCC-SD-WG-SEPOCT. Approved IFCC recommendation on reporting results for blood glucose: International Federation of Clinical Chemistry and Laboratory Medicine Scientific Division, Working Group on Selective Electrodes and Point-of-Care Testing (IFCC-SD-WG-SEPOCT) Clin Chem Lab Med. 2006;44(12):1486–1490. doi: 10.1515/CCLM.2006.275. [DOI] [PubMed] [Google Scholar]
- 22.Atkin SH, Dasmahapatra A, Jaker M, Chorost MI, Reddy S. Fingerstick glucose determination in shock. Ann Intern Med. 1991;114(12):1020–1024. doi: 10.7326/0003-4819-114-12-1020. [DOI] [PubMed] [Google Scholar]
- 23.Kilpatrick ES, Rumley AG, Smith EA. Variation in sample pH and pO2 affect ExacTech meter glucose measurements. Diabet Med. 1994;11(5):506–509. doi: 10.1111/j.1464-5491.1994.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.cfm MAUDE database (updated December 12, 2011) [Google Scholar]
- 25.Larsen CL, Jackson C, Lyon ME. Interference of Accel® wipes with LifeScan SureStep® Flexx glucose meters. Clin Biochem. 2006;39(4):414–416. doi: 10.1016/j.clinbiochem.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Desmeules P, Ethier J, Allard P. Disinfectant wipes containing hydrogen peroxide induce overestimation of glucose results obtained with LifeScan SureStep® Flexx glucose meter. Clin Biochem. 2010;43(18):1472–1474. doi: 10.1016/j.clinbiochem.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Krouwer JS, Cembrowski GS. A review of standards and statistics used to describe blood glucose monitor performance. J Diabetes Sci Technol. 2010;4(1):75–83. doi: 10.1177/193229681000400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes Association (ADA) Consensus statement on self-monitoring of blood glucose. Diabetes Care. 1987;10(1):93–99. [PubMed] [Google Scholar]
- 29.American Diabetes Association. Self-monitoring of blood glucose: clinical practice recommendations. Diabetes Care. 1996;19:S62–6. [Google Scholar]
- 30.American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;(31 Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 31.International Organization for Standardization: ISO 15197. Geneva: International Organization for Standardization; 2003. In vitro diagnostic test systems—requirements for blood glucose monitoring systems for self-testing in managing diabetes mellitus. [Google Scholar]
- 32.U.S. Food and Drug Administration. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm094134.htm Accessed November 24, 2011.
- 33.Cox DJ, Gonder-Frederick LA, Kovatchev BP, Julian DM, Clarke WL. Understanding error grid analysis. Diabetes Care. 1997;20(6):911–912. doi: 10.2337/diacare.20.6.911. [DOI] [PubMed] [Google Scholar]
- 34.Solnica B, Naskalski JW. Quality control of SMBG in clinical practice. Scand J Clin Lab Invest Suppl. 2005;240:80–85. doi: 10.1080/00365510500235996. [DOI] [PubMed] [Google Scholar]
- 35.Morey TE, Gravenstein N, Rice MJ. Assessing point-of-care hemoglobin measurement. Anesth Analg. 2011;113(6):1289–1291. doi: 10.1213/ANE.0b013e31822906b2. [DOI] [PubMed] [Google Scholar]
- 36.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 37.Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143–1148. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 38.Giordano BP, Hodges C, Trash W, Dube WP, Hodges C, Swain A, Banion CR, Klingensmith GJ. Performance of seven blood glucose testing systems at high altitude. Diabetes Educ. 1989;15(5):444–448. doi: 10.1177/014572178901500515. [DOI] [PubMed] [Google Scholar]
- 39.Chen ET, Nichols JH, Duh SH, Hortin G. Performance evaluation of blood glucose monitoring devices. Diabetes Technol Ther. 2003;5(5):749–768. doi: 10.1089/152091503322526969. [DOI] [PubMed] [Google Scholar]
- 40.Cohen M, Boyle E, Delaney C, Shaw J. A comparison of blood glucose meters in Australia. Diabetes Res Clin Pract. 2006;71(2):113–118. doi: 10.1016/j.diabres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Khan AI, Vasquez Y, Gray J, Wians FH Jr, Kroll MH. The variability of the results between point-of-care testing glucose meters and the central laboratory analyzer. Arch Pathol Lab Med. 2006;130(10):1527–1532. doi: 10.5858/2006-130-1527-TVORBP. [DOI] [PubMed] [Google Scholar]
- 42.Thomas LE, Kane MP, Bakst G, Busch RS, Hamilton RA, Abelseth JM. A glucose meter accuracy and precision comparison: the FreeStyle Flash versus the Accu-Chek Advantage, Accu-Chek Compact Plus, Ascensia Contour, and the BD Logic. Diabetes Technol Ther. 2008;10(2):102–110. doi: 10.1089/dia.2007.0244. [DOI] [PubMed] [Google Scholar]
- 43.Sheffield CA, Kane MP, Bakst G, Busch RS, Abelseth JM, Hamilton RA. Accuracy and precision of four value-added blood glucose meters: the Abbott Optium, the DDI Prodigy, the HDI True Track, and the HypoGuard Assure Pro. Diabetes Technol Ther. 2009;11(9):587–592. doi: 10.1089/dia.2008.0143. [DOI] [PubMed] [Google Scholar]
- 44.Florkowski C, Budgen C, Kendall D, Lunt H, Moore MP. Comparison of blood glucose meters in a New Zealand diabetes centre. Ann Clin Biochem. 2009;46(Pt 4):302–305. doi: 10.1258/acb.2009.008193. [DOI] [PubMed] [Google Scholar]
- 45.The Diabetes Research in Children Network (DIRECNET) Research Group. A multicenter study of the accuracy of the One Touch Ultra home glucose meter in children with type I diabetes. Diabetes Technol Ther. 2003;5(6):933–941. doi: 10.1089/152091503322640971. [DOI] [PubMed] [Google Scholar]
- 46.Stork AD, Kemperman H, Erkelens DW, Veneman TF. Comparison of the accuracy of the HemoCue glucose analyzer with the Yellow Springs Instrument glucose oxidase analyzer, particularly in hypoglycemia. Eur J Endocrinol. 2005;153(2):275–281. doi: 10.1530/eje.1.01952. [DOI] [PubMed] [Google Scholar]
- 47.Rosenthal M, Ugele B, Lipowsky G, Küster H. The Accutrend sensor glucose analyzer may not be adequate in bedside testing for neonatal hypoglycemia. Eur J Pediatr. 2006;165(2):99–107. doi: 10.1007/s00431-005-0013-z. [DOI] [PubMed] [Google Scholar]
- 48.Rivers SM, Kane MP, Bakst G, Busch RS, Hamilton RA. Precision and accuracy of two glucose meters: FreeStyleFlash versus OneTouch Ultra. Am J Health-Syst Pharm. 2006;63(15):1411–1416. doi: 10.2146/ajhp050473. [DOI] [PubMed] [Google Scholar]
- 49.Lippi G, Salvagno GL, Guidi GC, Negri M, Rizzati P. Evaluation of four portable self-monitoring blood glucose meters. Ann Clin Biochem. 2006;43(Pt 5):408–413. doi: 10.1258/000456306778520007. [DOI] [PubMed] [Google Scholar]
- 50.Bellini C, Serra G, Risso D, Mazzella M, Bonioli E. Reliability assessment of glucose measurement by HemoCue analyser in a neonatal intensive care unit. Clin Chem Lab Med. 2007;45(11):1549–1554. doi: 10.1515/CCLM.2007.302. [DOI] [PubMed] [Google Scholar]
- 51.Ashworth L, Gibb I, Alberti KG. HemoCue: evaluation of a portable photometric system for determining glucose in whole blood. Clin Chem. 1992;38(8 Pt 1):1479–1482. [PubMed] [Google Scholar]
- 52.Wiener K. An assessment of the effect of haematocrit on the HemoCue blood glucose analyzer. Ann Clin Biochem. 1993;30(Pt 1):90–93. doi: 10.1177/000456329303000116. [DOI] [PubMed] [Google Scholar]
- 53.Larbig M, Forst T, Mondok A, Forst S, Pfützner A. Investigation on the accuracy of the blood glucose monitoring device Prestige IQ. Diabetes Nutr Metab. 2003;16(4):257–261. [PubMed] [Google Scholar]
- 54.Singh DG, Agarwal M, Bishawi B. Evaluation of a glucose meter against analytical quality specifications for hospital use. Clin Chim Acta. 2004;343(1–2):217–221. doi: 10.1016/j.cccn.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Dai KS, Tai DY, Ho P, Chen CC, Peng WC, Chen ST, Hsu CC, Liu YP, Hsieh HC, Yang CC, Tsai MC, Mao SJ. Accuracy of the EasyTouch blood glucose self-monitoring system: a study of 516 cases. Clin Chim Acta. 2004;349(1–2):135–141. doi: 10.1016/j.cccn.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Kendall DM, Kaplan RA, Paulson CF, Parks JL, Tideman AM. Accuracy and utility of a 10-test disk blood glucose meter. Diabetes Res Clin Pract. 2005;67(1):29–35. doi: 10.1016/j.diabres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Rao LV, Jakubiak F, Sidwell JS, Winkleman JW, Snyder ML. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clin Chim Acta. 2005;356(1–2):178–183. doi: 10.1016/j.cccn.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 58.Hawkins RC. Evaluation of Roche Accu-Chek Go and Medisense Optium blood glucose meters. Clin Chim Acta. 2005;353(1–2):127–131. doi: 10.1016/j.cccn.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 59.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 60.Moghissi ES, Korytowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE, American Association of Clinical Endocrinologists; American Diabetes Association American Association of Clinical Endocrino-logists and American Diabetes Association Consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 61.Maser RE, Butler MA, DeCherney GS. Use of arterial blood with bedside glucose reflectance meters in an intensive care unit: are they accurate? Crit Care Med. 1994;22(4):595–599. doi: 10.1097/00003246-199404000-00014. [DOI] [PubMed] [Google Scholar]
- 62.Louie RF, Tang Z, Sutton DV, Lee JH, Kost GJ. Point-of-care glucose testing: effects of critical care variables, influence of reference instruments, and a modular glucose meter design. Arch Pathol Lab Med. 2000;124(2):257–266. doi: 10.5858/2000-124-0257-POCGT. [DOI] [PubMed] [Google Scholar]
- 63.Lacara T, Domagtoy C, Lickliter D, Quattrocchi K, Snipes L, Kuszaj J, Prasnikar M. Comparison of point-of-care and laboratory glucose analysis in critically ill patients. Am J Crit Care. 2007;16(4):336–346. [PubMed] [Google Scholar]
- 64.Cook A, Laughlin D, Moore M, North D, Wilkins K, Wong G, Wallace-Scroggs A, Halvorsen L. Differences in glucose values obtained from point-of-care glucose meters and laboratory analysis in critically ill patients. Am J Crit Care. 2009;18(1):65–72. doi: 10.4037/ajcc2009626. [DOI] [PubMed] [Google Scholar]
- 65.Mushholt PB, Schipper C, Thomé N, Ramljak S, Schmidt M, Forst T, Pfützner A. Dynamic electrochemistry corrects for hematocrit interference on blood glucose determinations with patient self-measurement devices. J Diabetes Sci Technol. 2011;5(5):1167–1175. doi: 10.1177/193229681100500520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rice MJ. Dynamic electrochemistry: a step in the right direction. J Diabetes Sci Technol. 2011;5(5):1176–1178. doi: 10.1177/193229681100500521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu P, Morey TE, Harris NS, Gravenstein N, Rice MJ. Intravenous fluids cause systemic bias in a conductivity-based point-of-care hematocrit meter. Anesth Analg. 2011 doi: 10.1213/ANE.0b013e31823fecbd. Epub, PMID 22156329. [DOI] [PubMed] [Google Scholar]
- 68.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33(12):2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]
- 69.Finkielman JD, Oyen LJ, Afessa B. Agreement between bedside blood and plasma glucose measurement in the ICU setting. Chest. 2005;127(5):49–51. doi: 10.1378/chest.127.5.1749. [DOI] [PubMed] [Google Scholar]
- 70.Slater-Maclean L, Cembrowski G, Shalapay C, Binette T, Hegadoren K, Newburn-Cook C. Accuracy of glycemic measurements in the critically ill. Diabetes Technol Ther. 2008;10(3):169–177. doi: 10.1089/dia.2008.0263. [DOI] [PubMed] [Google Scholar]
- 71.Desachy A, Vuagnat AC, Ghazali AD, Baudin OT, Longuet OH, Calvat SN, Gissot V. Accuracy of bedside glucometry in critically ill patients: influence of clinical characteristics and perfusion index. Mayo Clin Proc. 2008;83(4):400–405. doi: 10.4065/83.4.400. [DOI] [PubMed] [Google Scholar]
- 72.Fekih Hassen M, Ayed S, Gharbi R, Ben Sik Ali H, Marghli S, Elatrous S. Bedside capillary blood glucose measurements in critically ill patients: influence of catecholamine therapy. Diabetes Res Clin Pract. 2010;87(1):87–91. doi: 10.1016/j.diabres.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 73.Sylvain HF, Pokorny ME, English SM, Benson NH, Whitley TW, Ferenczy CJ, Harrison JG. Accuracy of fingerstick glucose values in shock patients. Am J Crit Care. 1995;4(1):44–48. [PubMed] [Google Scholar]
- 74.Stout PJ, Peled N, Erickson BJ, Hilgers ME, Racchini JR, Hoegh TB. Comparison of glucose levels in dermal interstitial fluid and finger capillary blood. Diabetes Technol Ther. 2001;3(1):81–90. doi: 10.1089/152091501750220046. [DOI] [PubMed] [Google Scholar]
- 75.Ray JG, Hamielec C, Mastracci T. Pilot study of the accuracy of bedside glucometry in the intensive care unit. Crit Care Med. 2001;29(11):2205–2207. doi: 10.1097/00003246-200111000-00025. [DOI] [PubMed] [Google Scholar]
- 76.Karon BS, Gandhi GY, Nuttall GA, Bryant SC, Schaff HV, McMahon MM, Santrach PJ. Accuracy of Roche Accu-Chek inform whole blood capillary, arterial, and venous glucose values in patients receiving intensive intravenous insulin therapy after cardiac surgery. Am J Clin Pathol. 2007;127(6):919–926. doi: 10.1309/6RFQCKAAJGKWB8M4. [DOI] [PubMed] [Google Scholar]
- 77.Critchell CD, Savarese V, Callahan A, Aboud C, Jabbour S, Marik P. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med. 2007;33(12):2076–2084. doi: 10.1007/s00134-007-0835-4. [DOI] [PubMed] [Google Scholar]
- 78.Hoedemaekers CW, Klein Gunnewiek JM, Prinsen MA, Willems JL, Van der Hoeven JG. Accuracy of bedside glucose measurement from three glucometers in critically ill patients. Crit Care Med. 2008;36(11):3062–3066. doi: 10.1097/CCM.0b013e318186ffe6. [DOI] [PubMed] [Google Scholar]
- 79.Petersen JR, Graves DF, Tacker DH, Okorodudu AO, Mohammad AA, Cardenas VJ., Jr Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clin Chim Acta. 2008;396(1–2):10–13. doi: 10.1016/j.cca.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 80.Meynaar IA, van Spreuwel M, Tangkau PL, Dawson L, Sleeswijk Visser S, Rijks L, Vlieland TV. Accuracy of AccuChek glucose measurement in intensive care patients. Crit Care Med. 2009;37(10):2691–2696. doi: 10.1097/ccm.0b013e3181a564fe. [DOI] [PubMed] [Google Scholar]
- 81.Karon BS, Boyd JC, Klee GG. Glucose meter performance criteria for tight glycemic control estimated by simulation modeling. Clin Chem. 2010;56(7):1091–1097. doi: 10.1373/clinchem.2010.145367. [DOI] [PubMed] [Google Scholar]
- 82.Jax T, Heise T, Nosek L, Gable J, Lim G, Calentine C. Automated near-continuous glucose monitoring measured in plasma using mid-infrared spectroscopy. J Diabetes Sci Technol. 2011;5(2):345–352. doi: 10.1177/193229681100500222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technology for continuous glucose monitoring: current problems and future promises. J Diabetes Sci Technol. 2010;4(6):1540–1562. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]




