Abstract
The use of U-500 regular insulin (U-500R) to treat diabetic patients with severe insulin resistance has increased. In this review, we performed a meta-analysis of PubMed studies reporting the use of U-500R to evaluate the effects of U-500R on hemoglobin A1c (HbA1c), body weight, and total daily insulin dose (TDD). These studies included 310 patients using U-500R as multiple daily injections (MDI) and 55 patients using U-500R via continuous subcutaneous insulin infusion (CSII). Overall, the use of U-500R as MDI resulted in a significant HbA1c reduction of 1.59%, a significant weight gain of 4.38 kg, and a significant increase in TDD by 51.9 units. The use of U-500R via CSII resulted in a similarly significant HbA1c reduction of 1.64% but a nonsignificant weight gain and a nonsignificant change in TDD.
The use of U-500 regular insulin both as MDI and via CSII was not reported to be associated with severe hypoglycemia but was associated with an increase in patient satisfaction as well as in cost savings. Suggestions in initiating U-500R in the outpatient setting using U-500R in hospitalized patients are reviewed. In addition, precautions for avoiding prescription and patient errors are discussed.
Keywords: continuous subcutaneous insulin infusion, insulin resistance, multiple daily injections, U-500 regular insulin
Introduction
Tight glycemic control has been shown to reduce microvascular complications in patients with type 1 (T1DM) and type 2 diabetes mellitus (T2DM).1,2 There are at least 10 classes of medications available for treating T2DM and eight different types of insulin. While these treatments are successful for most, a subset of diabetic patients with severe insulin resistance (IR), who require large amounts of insulin, presents a challenge in achieving glycemic control.
Patients who require more than 200 units of insulin per day are considered to have severe IR. Besides obesity, other causes of severe IR include severe infections, steroid use, pregnancy, genetic defects of the insulin receptor gene (type A IR syndromes, Leprechaunism, and Rabson-Mendenhall syndrome), insulin receptor antibodies (type B IR syndrome), and lipodystrophy.3–6 These patients often have to inject more than 100 units or 1.0 ml of U-100 insulin at a time, which can cause injection site discomfort. The largest insulin syringes hold only 100 units, and insulin pens can deliver only 60–80 units per injection. Thus, these patients may require 4–8 injections per day, which can be burdensome and may lead to poor compliance.7–9 In addition, a large subcutaneous insulin depot may impede absorption, and hypoglycemic effects may be delayed.10,11 Patients with severe IR using U-100 insulin via continuous subcutaneous insulin infusion (CSII) have to change insulin cartridges frequently, as the largest reservoir holds only 300 units of insulin. This is inconvenient and increases the cost of pump supplies.
U-500 regular insulin (U-500R) is five-fold concentrated, such that each 1 ml contains 500 units of insulin. Therefore, the volume of insulin injected is reduced by 80%, resulting in fewer injections and less discomfort, as well as potentially improved insulin absorption. U-500 insulin beef regular was first available in 1952, and later U-500 insulin pork regular entered the market in 1980.12 In 1997, human U-500R became commercially available [Humulin R U-500 in the United States, Eli Lilly and Company; Actrapid U-500 in the United Kingdom, Novo Nordisk (voluntary withdrawn in 2008)]. The use of U-500R has since received more attention. Prescriptions have increased 137% from June 2007 to June 2009.12 The purpose of this article is to review efficacy, safety, and guidelines in the clinical use of U-500R.
Pharmacokinetics and Pharmacodymanics
Earlier studies10,13 have demonstrated a decreased absorption rate with increasing concentration or volume of subcutaneously injected insulin. In 1981, Galloway and colleagues14 compared pork regular insulin injections at a dose of 0.25 units/kg and found no differences in serum insulin concentration between U-40, U-100, or U-500. However, the time to peak blood glucose response was significantly delayed with U-500 insulin.14
Studies of human U-500R injections at a dose of 0.2 units/kg in lean, healthy volunteers demonstrated an onset of action within 30 min, peak action between 1.75 and 4 h, and duration of 6 to >10 h.15 Using the same dose of U-500R in healthy obese subjects, the onset of action was found to be 45 min, time to peak action 7–8.5 h, and duration of action of 11.5 h.16 A study performed in severely insulin resistant T2DM patients after 100 units of U-500R injections revealed that serum insulin concentration rose briskly in 30 min and remained elevated for at least 7 h, giving an onset time similar to U-100 regular insulin (U-100R) and a duration similar to U-100 neutral protamine Hagedorn.15,17 Interestingly, a study in healthy obese subjects comparing equivalent doses of U-500R and U-100R, 50- and 100-unit doses, demonstrated similar overall insulin exposure and overall effect. However, for U-500R, peaks of concentration and action profiles were blunted, and the effect after the peak was prolonged.18
Review of Studies with U-500 Regular Insulin in Type 1 and 2 Diabetes
Methods
We searched PubMed and performed a meta-analysis of studies reporting U-500R use by multiple daily injections (MDI) and via CSII in nonpregnant T1DM and T2DM patients. We evaluated the effects of the treatment on the changes in hemoglobin A1c (HbA1c), body weight, and total daily insulin dose (TDD). The meta-analysis was done by fitting a DerSimonian-Laird random-effects model for each outcome, treating study as a random effect. The importance (i.e., weighting) of each study was inversely proportional to the standard error. If only the standard deviation/error was available for the pre- and posttreatment with U-500R, the standard error for the change was estimated assuming a correlation between pre- and posttreatment values of 0.50 for TDD, 0.90 for body weight, and 0.15 for HbA1c. These correlations were estimated from studies where raw data were made available.
Results
Use of U-500R via Multiple Daily Injections
We found 10 studies reporting a use of U-500R by MDI.17,19–27 We excluded 1 study because part of the data was included in a subsequent study.17 Therefore, we analyzed 9 studies with a total of 310 patients (Table 1). Of note, this included data of 4 patients using U-500R via CSII, as they were not separately reported in the original studies.21,23 All studies but one17 were retrospective. Mean duration of follow-up ranges between 6 and 36 months. Most patients had T2DM, with few having T1DM.21,24,25
Table 1.
Studies of U-500R Use
| Study | Subjects (n) | Mean duration (months) | Mean baseline HbA1c (%) | Mean HbA1c reduction (%) | Mean baseline TDD (units) | Mean TDD change (units) | Mean weight change (kg) |
|---|---|---|---|---|---|---|---|
| Studies using MDI (n = 310) | |||||||
| Garg et al. (24) | 16 | 24 | 11.34 | 3.29 | 313 | −31 | 6.5 |
| Neal et al. (22) | 20 | 6 | 9.59 | 1.76 | 221 | −7 | 3.2 |
| Wafa et al. (20) | 15 | 12 | 9.8 | 2.2 | 219 | 116 | 2.02 |
| Nayyar et al.a (21) | 81 | 30 | 10.0 | 1.1 | 311 | 57 | 5.1 |
| Davidson et al.b (17) | 11 | 26 | 9.9 | 2.5 | 304 | 99.9 | 8.16 |
| Dailey et al.a (23) | 40 | 12 | 9.4 | 1.4 | 240 | 97 | 5.7 |
| Boldo et al.b (25) | 53 | 36 | 10.1 | 1.5 | 225 | 69 | 6.8 |
| Ziesmer et al. (27) | 53 | 20 | 9.1 | 1.0 | 391 | 24 | 2.0 |
| Quinn et al.c (26) | 21 | 12 | 9.51 | 1.8 | 297.5 | 57.5 | −0.35 |
| Overall effectd (95% CI) | 1.59 (1.26–1.92) | 51.9 (19.6–84.1) | 4.38 (2.35–6.41) | ||||
| Studies using CSII (n = 55) | |||||||
| Knee et al. (31) | 4 | 3 | 10.8 | 3.5 | 334 | −120 | n/a |
| Schwartz et al. (32) | 5 | n/a | 10.24 | 2.49 | 410 | n/ae | n/af |
| Lane et al.b (28) | 9 | 3 | 8.8 | 1.13 | 172 | −5.3 | 1.9 |
| Bulchandani et al. (29) | 6 | 6 | 9.1 | 2.2 | 391 | −95 | −2.8 |
| Lane et al. (30) | 21 | 12 | 8.6 | 1.23 | 197 | 12 | 5.4 |
| Reutrakul et al. (33) | 10 | 30 | 9.0 | 1.6 | 234 | 1.7 | 8.9 |
| Overall effectd (95% CI) | 1.64 (1.14–2.14) | −13.6 (−42.4–15.2) | 2.99 (−1.83–7.81) | ||||
Two patients in each study used CSII
Additional information obtained through personal communications with authors
Analysis done according to individual patient’s data published
From DerSimonian-Laird random-effects models
Change could not be calculated because final TDD was not reported
Mean weight change was 4.5 kg, but the standard deviation of this change could not be estimated, so it was excluded from the meta-analysis
Patients in these studies had uncontrolled diabetes with severe IR. Mean baseline HbA1c was 9.1–11.3%, and TDD was 219–391 units. After U-500R initiation, there was a significant HbA1c reduction reported in all studies, ranging 1.0–3.29%, with an overall reduction of 1.59% [95% confidence interval (CI), 1.26–1.92] based on the meta-analysis. There was a significant weight gain of 4.38 kg (95% CI, 2.35–6.41). Total daily insulin dose increased significantly by 51.9 units (95% CI, 19.6–84.1) (Figure 1, Table 1).
Figure 1.
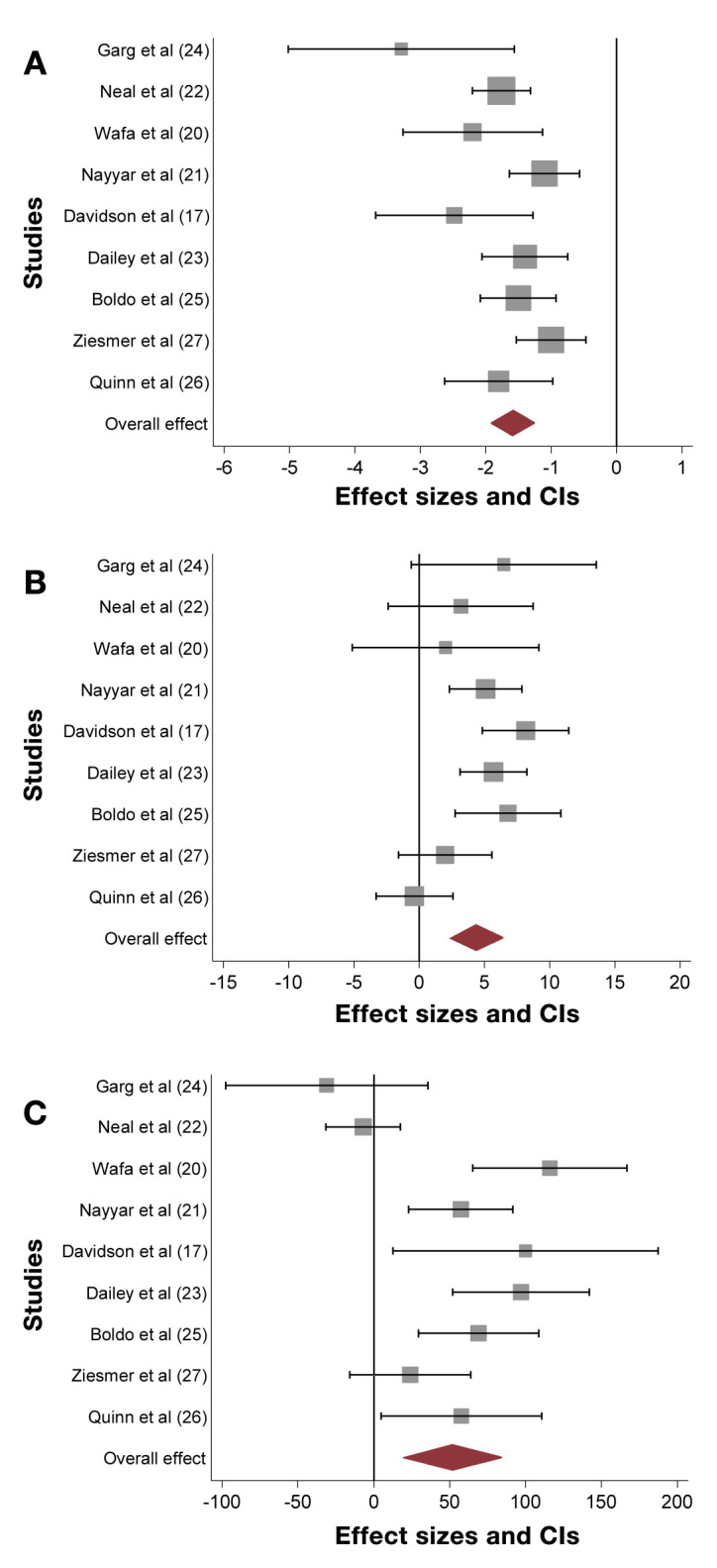
Studies using U-500R MDI; (A) Changes in HbA1c with an overall significant reduction of 1.59% (95% CI, 1.26–1.92); (B) Changes in weight with an overall significant increase of 4.38 kg (95% CI, 2.35–6.41); (C) Changes in TDD with an overall significant increase of 51.9 units (95% CI, 19.6–84.1)
In most studies, severe hypoglycemia was either not reported to be a problem or was no more frequent than with U-100R use. There was a slight increase in mild hypoglycemia within the first few months, but this usually declined afterwards.23 One study, however, did find a significant increase in mild hypoglycemia from 13% to 42%. This was one of the most common reasons for U-500R discontinuation in that study.25
Two studies reported that the number of insulin injections significantly decreased.25,26 In addition, there were significantly fewer problems with insulin leakage from injection sites.23
Three studies (total 137 patients) found no changes in systolic and diastolic blood pressure.21,23,24 Four studies (total 190 patients) examined changes in lipid levels.21,23,24,27 Total cholesterol and triglycerides levels decreased in two studies21,27 but were unchanged in the others. High-density lipoprotein levels decreased in one study27 but were unchanged in the other two.21,23 Low-density lipoprotein levels were unchanged.21,23
Use of U-500R via Continuous Subcutaneous Insulin Infusion
Six case series have reported the use of U-500R via CSII, with a total of 55 patients and mean duration of follow up between 3 and 30 months.28–33 All but one of the studies30 were retrospective. Prior to the start of U-500R via CSII, the patients were on U-100 MDI, U-500R MDI, or U-100 via CSII. Mean baseline HbA1c ranged 8.6–10.8% and TDD 172–410 units.
After U-500R via CSII initiation, there was a significant HbA1c reduction of 1.64% (95% CI, 1.14–2.14). Weight increased overall by 2.99 kg (95% CI, -1.83–7.81) but this did not reach statistical significance, perhaps due to the small number of studies on which it was based. There was a nonsignificant decrease in TDD by 13.6 units (95% CI, -42.4–15.2 units) (Figure 2, Table 1).
Figure 2.
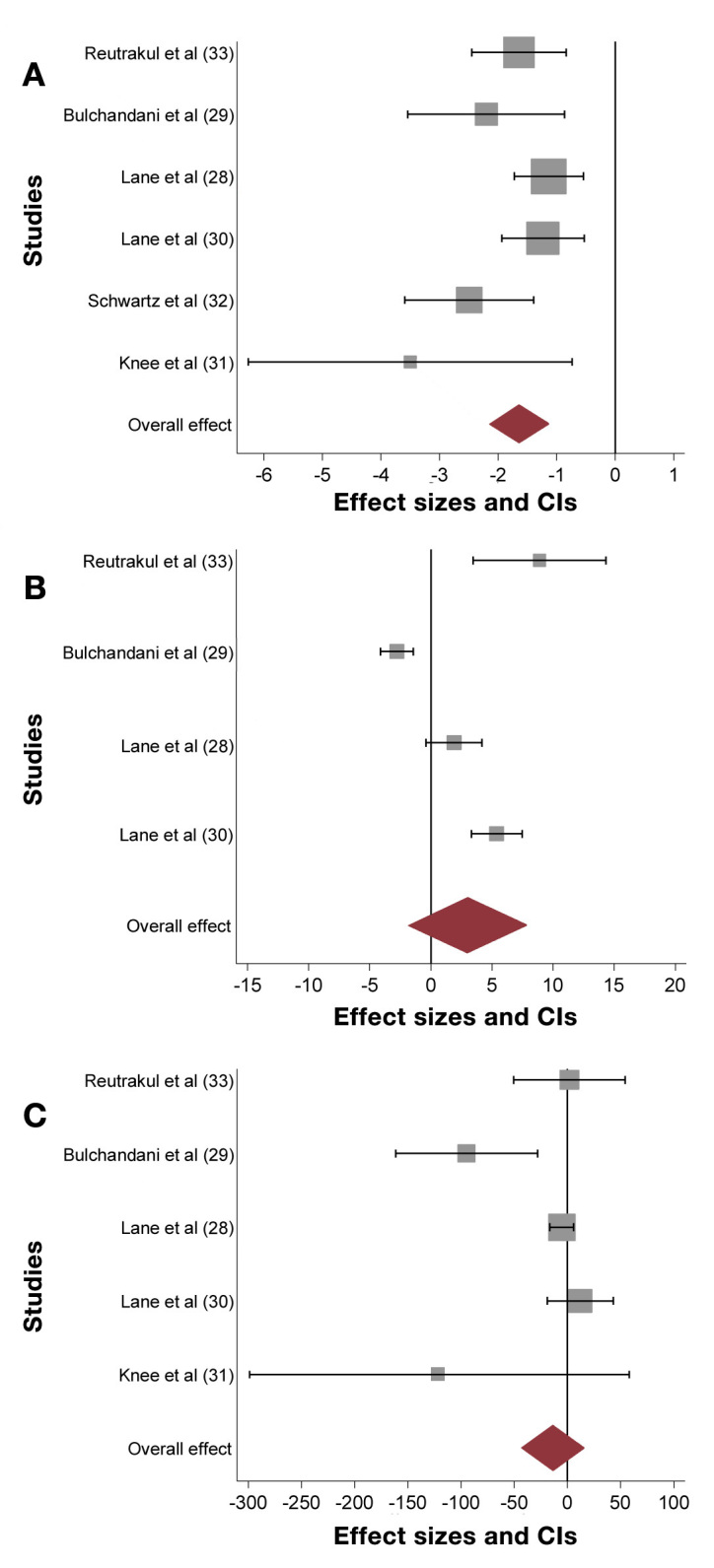
Studies using U-500R via CSII; (A) Changes in HbA1c with an overall significant reduction of 1.64% (95% CI, 1.14–2.14); (B) Changes in weight with an overall nonsignificant increase of 2.99 kg (95% CI, -1.83–7.81); (C) Changes in TDD with an overall nonsignificant decrease of 13.6 units (95% CI, -42.4–15.2)
Similar to U-500R by MDI, hypoglycemia was not common after U-500R via CSII use, although a few patients had to suspend overnight infusion to avoid nighttime and early morning hypoglycemia.32,33 Interestingly, a 12-month prospective study of U-500R delivered by OmniPod® (Insulet, Bedford MA) found a 70% increase in time spent in the target blood glucose range (70–180 mg/dl) without a significant increase in hypoglycemia as assessed by 72 h glucose monitoring.30
Overall, use of U-500R MDI and U-500R via CSII appears to be equally effective in achieving glycemic control with HbA1c reduction of approximately 1.6% without significant increase in severe hypoglycemia. Use of U-500R MDI was associated with significant increase in body weight and TDD, while no significant changes were found in U-500R use via CSII. It is unclear whether lack of significance was due to the small number of patients and/or a relatively shorter follow-up period in CSII patients. Further investigation is required.
Other Clinical Use: Pregnancy and Syndromic Forms of Insulin Resistance
U-500R has been used to treat diabetes in pregnancy. It is labeled by the Food and Drug Administration (FDA) as category B for pregnancy use. Use of U-500R for both MDI and via CSII has been reported in a few pregnant patients with severe IR.3,33–35 Similarly for nonpregnant patients, this seems to be safe and effective. Due to the importance of postprandial glucose control during pregnancy and the slower onset of action of U-500R, meal bolus timing is crucial.
U-500R has been used in patients with extreme IR, including type A and type B IR syndrome, congenital and acquired generalized lipodystrophy, Rabson-Mendenhall syndrome, and HAIR-AN (hyperandrogenism, IR, acanthosis nigricans) syndrome.36 Insulin requirements of these patients range from 1.6 units/kg/day to >566 units/kg/day. U-500R dosing is similar to that of other diabetes patients (see later), although delivery via CSII is preferred once TDD exceeds 2000 units.36 In patients with insulin receptor abnormalities, the duration of U-500R is more prolonged because of a deficiency in insulin degradation.36
Combination with Other Antidiabetic Medications
U-500R can be combined with metformin provided there is no contraindication to metformin. As with other types of insulin, combination with thiazolidinediones can lead to significant weight gain and fluid retention. Interestingly, a study by Lane and colleagues37 reported that an addition of liraglutide in patients using U-500R for 12 weeks led to a 1.4% HbA1c reduction, along with 28% insulin dose reduction, and average weight loss of 5.1 kg.
Patient Satisfaction
Quality of life and patient satisfaction, including perceived clinical efficacy, time spent in diabetes management, and psychological well-being, have been shown to improve after U-500R initiation in several studies.23,29,30 The rate of discontinuation in studies with mean follow up of ≥1 year ranged 12–34%.21,25,30,33 Besides personal reasons and patient compliance, other reasons for discontinuation included improved glycemic control and decreased insulin requirement (hence no need for U-500R), increased frequency of mild hypoglycemia, and lack of insurance coverage.21,24,25 Bariatric surgery led to resolution of diabetes in some patients.21,33
Treatment Durability
The longest mean follow-up period reported was 30 to 36 months, with some patients followed for longer than 90 months.21,25,33 Nayyar and colleagues21 reported that subjects who were treated for longer than 36 months had a greater HbA1c reduction than those who were treated for a shorter period (1.88% vs 0.99%). In contrast, some studies showed that HbA1c trended up after an initial improvement during the first few months but was still lower than baseline.17,33 Boldo and colleagues25 found that HbA1c reduction was comparable in patients using U-500R for less than versus more than 1.5 years. Available data suggest that only 8–25% of the patients achieved a goal HbA1c of <7%,21,23,25,28,33 reflecting ongoing difficulties in diabetes management in this population. There is no information on U-500R use on long-term outcomes such as microvascular complications. However, they are expected to be prevented or delayed should glycemic control be maintained over long periods of time.
Cost Considerations
U-500R is supplied only in a 20 ml vial containing 10,000 units. Although the price per vial is higher than U-100R, the cost per unit is considerably less.12 U-500R has a shelf life of 28 days after opening; therefore, patients requiring less than 300 units per day might have some insulin wastage. Knee and colleagues31 calculated potential savings for insulin of up to $2600 per year and for pump supplies of up to $3400 per year when U-500R was used via CSII. However, the real potential savings are related to the reduction of diabetes complications with improved glycemic control.9
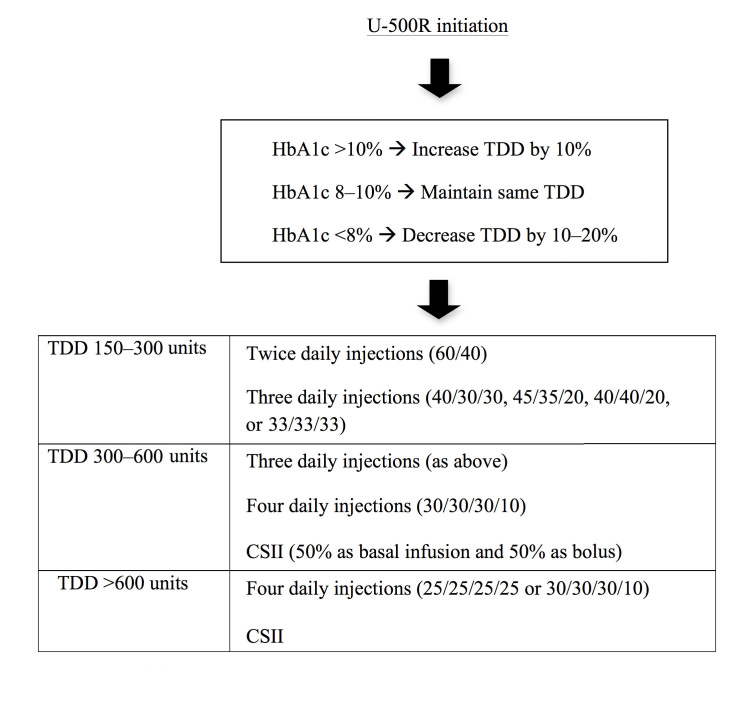
Suggestions in Initiating U-500 Regular Insulin
U-500R should be considered in patients with TDD >200 units and especially when TDD exceeds 300 units. Although there are no formal guidelines, we summarize suggestions proposed by several authors in transitioning patients from U-100 to U-500R—both as MDI and via CSII—taking into account HbA1c levels and TDD9,12,30,36 (Figure 3). For TDD, one should consider increasing by 10–20% for HbA1c levels >10% and decreasing by 10–20% if HbA1c is <8%. If HbA1c is 8–10%, unit per unit conversion is suggested.
Figure 3.
Suggestions for U-500R initiation. Adapted from Lane et al.9 and Segal et al.12
For TDD between 150 and 200 units, twice daily injections are proposed, with 60% dose given before breakfast and 40% before dinner (60/40). If three daily injections are preferred, then the dose can be distributed before meals at the proportion of 40/30/30, 45/35/30, 40/40/20, or 33/33/33. The patients should inject at least 30 min before meals. For severe premeal hyperglycemia and postprandial hyperglycemia not corrected by premeal U-500R, rapid acting insulin analogs can be added.9,17,25 As U-500R can be used either as a bolus or basal insulin, it can be combined with basal insulin such as detemir or glargine. In this case, the patients should maintain basal-bolus ratio used before the transition, with U-500R distributed as bolus insulin. Basal insulin can be adjusted according to fasting glucose values.
For TDD between 300 and 600 units, three daily injections should be considered. If morning hyperglycemia is experienced, a fourth injection can be added at bedtime at 10%, with close monitoring of 3 a.m. glucose to detect nighttime hypoglycemia. Combination with basal insulin, if needed, can be done as described earlier.
For TDD above 600 units, one can consider four daily injections at the proportion 25/25/25/25 or 30/30/30/10. Combination with basal insulin is not practical at this point because the dose is large and patients will likely require more than one injection per day.
In patients whose TDD is between 300 and 600 units and who require multiple injections daily, as well as patients with TDD >600 units, therapy via CSII should be considered (note that this is not an approved indication by the FDA). Lane and colleagues9 proposed transitioning to CSII from U-100 insulin as follows. The hourly basal rate is 50% of previous TDD divided by 24 and divided by 5 into “pump units.” The other 50% is distributed as premeal boluses. If there is a concern for hypoglycemia or if HbA1c is near normal, 20% dose reduction is suggested. For patients who are counting carbohydrates, the carbohydrate factor of U-100 insulin is multiplied by 5 (i.e., 1 unit of U-100 insulin per 4 g of carbohydrates becomes 1 “pump unit” of U-500R per 20 g of carbohydrates). Meal boluses should be taken at least 30 min before meals. Basal rate adjustment should be made 2 h prior to the desired time of glycemic effects. Insulin action time is typically set between 4 and 6 h to avoid hypoglycemia from insulin stacking.
Safety and Prescription Considerations
As U-500R is five times as concentrated as U-100R, precautions are needed to ensure correct dispensing and administration. Using postmarketing experience and clinical studies, a review of U-500R safety by the manufacturer in October 2008 found 22 cases of medication errors.12 Administration and dispensing were the most common adverse events (82%), with hypoglycemia occurring in 36.3% of the cases. Four patients were given U-100 instead of U-500R. One patient received U-500R instead of U-100 insulin, resulting in fatality.12 The calculated reporting rate of medication errors associated with U-500R was 0.06%—considered to be a rarely reported event.
Lane and colleagues9 provided recommendations for writing U-500R prescriptions suggesting that U-500R should be prescribed by both volume and actual units. The volume can be calculated by dividing U-100 dose by 5. Use of tuberculin syringes, which are marked in volume, is advocated by some authors. However, these may not be widely available, and the needle size is larger than insulin syringes (smallest 27 g). The prescription should indicate as follows: “U-500 insulin 50 units (0.1 ml or 10 insulin syringe units) inject twice daily.” In March 2011, the FDA issued safety label changes stating that “the prescribed dose of Humulin R U-500 should always be expressed in actual units of Humulin R U-500, along with corresponding markings on the syringe the patient is using,” and that “a conversion chart is provided and should always be used when administering Humulin R U-500 doses with U-100 insulin syringes or tuberculin syringes.”38 (Table 2).
Table 2.
Example of U-500 Insulin Conversion Charta
| U-500 actual unit dose | U-100 syringe unit marking | Volume of tuberculin syringe (ml) |
|---|---|---|
| 25 | 5 | 0.05 |
| 50 | 10 | 0.1 |
| 75 | 15 | 0.15 |
| 100 | 20 | 0.2 |
Adapted from Segal and colleagues.12
For use via CSII, insulin dosing should be instructed in “pump units,” as stated earlier. Patients need to ensure that the pump is disconnected from the body during the priming to prevent erroneous insulin administration.39
Hospital Use of U-500 Regular Insulin
U-500R is considered a high-alert medication for in-hospital use due to potential medication errors.12 While some advocate that patients already using U-500R at home should continue to do so when hospitalized,9 some institutions do not allow or do not have the insulin on formulary. When using U-500R in the hospital, an order sheet or a computer order entry screen specifically designed for U-500R should be used. This should include the actual number of units and either volume needed (for tuberculin syringe) or “unit markings” on U-100 insulin syringes. It is advised that U-500R not be stored on the hospital floors and each individual dose be prepared and dispensed by the pharmacy.12,40,41 These interventions have been shown to significantly reduce hypoglycemic incidences.40
Discussion
Although U-500R insulin may improve glycemic control in appropriate patients, a number of important issues remain unanswered. Treatment with U-500R MDI results in significant weight gain. Whether morbidity from weight gain (i.e., sleep apnea, osteoarthritis, cardio-vascular disease) negates the benefits of glycemic control over the long-term is unknown. In addition, hypoglycemia resulting from U-500R has not been rigorously evaluated. Almost all studies rely on self-reported hypoglycemia; the incidence of asymptomatic hypoglycemia is unclear. More extensive studies using continuous glucose monitoring are needed to better understand hypoglycemia risk. Whether U-500R prevents diabetes complications has also not been examined. Prospective evaluation of U-500R both via MDI and CSII is critical to better understand issues of efficacy and safety.
Conclusion
U-500R, both via CSII and MDI, is effective in improving glycemic control, increases satisfaction and quality of life, as well as offers potential cost savings in patients who require high doses of insulin. While retrospective data may be promising, prospective studies are necessary to assess long-term effectiveness and adverse effects of this insulin.
Glossary
Abbreviations
- (CI)
confidence interval
- (CSII)
continuous subcutaneous insulin infusion
- (FDA)
Food and Drug Administration
- (HbA1c)
hemoglobin A1c
- (IR)
insulin resistance
- (MDI)
multiple daily injections
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
- (TDD)
total daily insulin dose
- (U-100R)
U-100 regular insulin
- (U-500R)
U-500 regular insulin
Funding
Kristen Wroblewski was supported by Grant #UL1RR024999 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
References
- 1.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–843. [PubMed] [Google Scholar]
- 2.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Dolberg BK, Lenhard MJ. Successful outcome of pregnancy in a patient with generalized lipoatrophic diabetes mellitus. Endocr Pract. 2000;6(1):34–36. doi: 10.4158/EP.6.1.34. [DOI] [PubMed] [Google Scholar]
- 4.Arioglu E, Andewelt A, Diabo C, Bell M, Taylor SI, Gorden P. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore) 2002;81(2):87–100. doi: 10.1097/00005792-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- 6.Musso C, Cochran E, Moran SA, Skarulis MC, Oral EA, Taylor S, Gorden P. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore) 2004;83(4):209–222. doi: 10.1097/01.md.0000133625.73570.54. [DOI] [PubMed] [Google Scholar]
- 7.Crasto W, Jarvis J, Hackett E, Nayyar V, McNally PG, Davies MJ, Lawrence IG. Insulin U-500 in severe insulin resistance in type 2 diabetes mellitus. Postgrad Med J. 2009;85(1002):219–222. doi: 10.1136/pgmj.2008.073379. [DOI] [PubMed] [Google Scholar]
- 8.Dailey AM, Tannock LR. Extreme Insulin Resistance: indications and approaches to the use of U-500 insulin in type 2 diabetes mellitus. Curr Diab Rep. 2011;11(2):77–82. doi: 10.1007/s11892-010-0167-6. [DOI] [PubMed] [Google Scholar]
- 9.Lane WS, Cochran EK, Jackson JA, Scism-Bacon JL, Corey IB, Hirsch IB, Skyler JS. High-dose insulin therapy: is it time for U-500 insulin? Endocr Pract. 2009;15(1):71–79. doi: 10.4158/EP.15.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Binder C. Absorption of injected insulin. A clinical-pharmacological study. Acta Pharmacol Toxicol (Copenh) 1969;(27 Suppl 2):1–84. doi: 10.1111/j.1600-0773.1969.tb03069.x. [DOI] [PubMed] [Google Scholar]
- 11.Gagnon-Auger M, du Souich P, Baillargeon JP, Martin E, Brassard P, Ménard J, Ardilouze JL. Dose-dependent delay of the hypoglycemic effect of short-acting insulin analogs in obese subjects with type 2 diabetes: a pharmacokinetic and pharmacodynamic study. Diabetes Care. 2010;33(12):2502–2507. doi: 10.2337/dc10-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segal AR, Brunner JE, Burch FT, Jackson JA. Use of concentrated insulin human regular (U-500) for patients with diabetes. Am J Health Syst Pharm. 2010;67(18):1526–1535. doi: 10.2146/ajhp090554. [DOI] [PubMed] [Google Scholar]
- 13.Binder C, Lauritzen T, Faber O, Pramming S. Insulin pharmaco-kinetics. Diabetes Care. 1984;7(2):188–199. doi: 10.2337/diacare.7.2.188. [DOI] [PubMed] [Google Scholar]
- 14.Galloway JA, Spradlin CT, Nelson RL, Wentworth SM, Davidson JA, Swarner JL. Factors influencing the absorption, serum insulin concentration, and blood glucose responses after injections of regular insulin and various insulin mixtures. Diabetes Care. 1981;4(3):366–376. doi: 10.2337/diacare.4.3.366. [DOI] [PubMed] [Google Scholar]
- 15.Khan M, Lee YY. The pharmacokinetic and pharmacodynamic properties of regular U-500 insulin in healthy non-obese subjects. (Abstract) Diabetes. 2007;569(suppl):P-1294. [Google Scholar]
- 16.Khan M, Sarabu B. The pharmacokinetics and pharmacodynamic properties of regular U-500 insulin in healthy obese subjects (Abstract) Diabetes. 2009;58(suppl):PO-2333. [Google Scholar]
- 17.Davidson MB, Navar MD, Echeverry D, Duran P. U-500 regular insulin: clinical experience and pharmacokinetics in obese, severely insulin-resistant type 2 diabetic patients. Diabetes Care. 2010;33(2):281–283. doi: 10.2337/dc09-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De la Peña A, Riddle M, Morrow LA, Jiang HH, Linnebjerg H, Scott A, Win KM, Hompesch M, Mace KF, Jacobson JG, Jackson JA. Pharmacokinetics and pharmacodynamics of high-dose human regular U-500 insulin versus human regular U-100 insulin in healthy obese subjects. Diabetes Care. 2011;34(12):2496–2501. doi: 10.2337/dc11-0721. Epub 2011 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballani P, Tran MT, Navar MD, Davidson MB. Clinical experience with U-500 regular insulin in obese, markedly insulin-resistant type 2 diabetic patients. Diabetes Care. 2006;29(11):2504–2505. doi: 10.2337/dc06-1478. [DOI] [PubMed] [Google Scholar]
- 20.Wafa WS, Khan MI. Use of U-500 regular insulin in type 2 diabetes. Diabetes Care. 2006;29(9):2175–2176. doi: 10.2337/dc06-1148. [DOI] [PubMed] [Google Scholar]
- 21.Nayyar V, Lawrence IG, Kong MF, Gallagher A., Gregory R, Hiles S, Jackson S, McNally P, Davies MJ. Long-term follow-up of patients on U-500 Human Actrapid. (Abstract) Diabetologia. 2007;50(Suppl 1):S538. [Google Scholar]
- 22.Neal JM. Analysis of effectiveness of human U-500 insulin in patients unresponsive to conventional insulin therapy. Endocr Pract. 2005;11(5):305–307. doi: 10.4158/EP.11.5.305. [DOI] [PubMed] [Google Scholar]
- 23.Dailey AM, Williams S, Taneja D, Tannock LR. Clinical efficacy and patient satisfaction with U-500 insulin use. Diabetes Res Clin Pract. 2010;88(3):259–264. doi: 10.1016/j.diabres.2010.02.012. Epub 2010 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg R, Lawrence IG, Akinsola MO, Davies MJ, McNally PG. Improved glycaemic control in severely insulin resistant, insulin treated diabetic patients with U500 Human Actrapid over two year follow-up. (Abstract) Diabetologia. 2004;47(Suppl 1):A58. [Google Scholar]
- 25.Boldo A, Comi RJ. Clinical Experience with U-500 Insulin: Risks and Benefits. Endocr Pract. 2011:1–17. doi: 10.4158/EP11163.OR. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Quinn SL, Lansang MC, Mina D. Safety and effectiveness of U-500 insulin therapy in patients with insulin-resistant type 2 diabetes mellitus. Pharmacotherapy. 2011;31(7):695–702. doi: 10.1592/phco.31.7.695. [DOI] [PubMed] [Google Scholar]
- 27.Ziesmer AE, Kelly KC, Guerra PA, George KG, Dunn FL. U-500 Regular Insulin Use In Insulin Resistant Type 2 Diabetic Veteran Patients. Endocr Pract. 2011:1–15. doi: 10.4158/EP11043.OR. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Lane WS. Use of U-500 regular insulin by continuous subcutaneous insulin infusion in patients with type 2 diabetes and severe insulin resistance. Endocr Pract. 2006;12(3):251–256. doi: 10.4158/EP.12.3.251. [DOI] [PubMed] [Google Scholar]
- 29.Bulchandani DG, Konrady T, Hamburg MS. Clinical efficacy and patient satisfaction with U-500 insulin pump therapy in patients with type 2 diabetes. Endocr Pract. 2007;13(7):721–725. doi: 10.4158/EP.13.7.721. [DOI] [PubMed] [Google Scholar]
- 30.Lane WS, Weinrib SL, Rappaport JM, Przestrzelski T. A prospective trial of U500 insulin delivered by Omnipod in patients with type 2 diabetes mellitus and severe insulin resistance. Endocr Pract. 2010;16(5):778–784. doi: 10.4158/EP10014.OR. [DOI] [PubMed] [Google Scholar]
- 31.Knee TS, Seidensticker DF, Walton JL, Solberg LM, Lasseter DH. A novel use of U-500 insulin for continuous subcutaneous insulin infusion in patients with insulin resistance: a case series. Endocr Pract. 2003;9(3):181–186. doi: 10.4158/EP.9.3.181. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz FL. A caution regarding U-500 insulin by continuous subcutaneous infusion. Endocr Pract. 2004;10(2):163–164. doi: 10.4158/EP.10.2.163. [DOI] [PubMed] [Google Scholar]
- 33.Reutrakul S, Brown RL, Koh CK, Hor TK, Baldwin D. Use of U-500 regular insulin via continuous subcutaneous insulin infusion: clinical practice experience. J Diabetes Sci Technol. 2011;5(4):1025–1026. doi: 10.1177/193229681100500429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatipoglu B, Soni S, Espinosa V. Glycemic control with continuous subcutaneous insulin infusion with use of U-500 insulin in a pregnant patient. Endocr Pract. 2006;12(5):542–544. doi: 10.4158/EP.12.5.542. [DOI] [PubMed] [Google Scholar]
- 35.Wong VW, Pape AV. Extreme insulin resistance in a patient with pre-existing diabetes during pregnancy: role of U-500 insulin. Med J Aust. 2009;190(11):650–651. doi: 10.5694/j.1326-5377.2009.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 36.Cochran E, Musso C, Gorden P. The use of U-500 in patients with extreme insulin resistance. Diabetes Care. 2005;28(5):1240–1244. doi: 10.2337/diacare.28.5.1240. [DOI] [PubMed] [Google Scholar]
- 37.Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13(5):592–595. doi: 10.1089/dia.2010.0221. Epub 2011 Mar 15. [DOI] [PubMed] [Google Scholar]
- 38.Humulin R (insulin human [rDNA origin] injection), U-500. Safety Labeling Changes Approved By FDA Center for Drug Evaluation and Research (CDER) March 2011. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm250517.htmAccessed January 12, 2012.
- 39.U.S. Food and Drug Administration. MAUDE Adverse Event Report. U.S. Department of Health and Human Services. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__ID=1912699 Accessed January 12, 2012. [Google Scholar]
- 40.Deal EN, Tobin GS. Policy implementation for inpatient management of U-500 insulin resulting in lower incidence of hypoglycemia. Endocr Pract. 2011;17(3):521. [PubMed] [Google Scholar]
- 41.Samaan KH, Dahlke M, Stover J. Addressing safety concerns about U-500 insulin in a hospital setting. Am J Health Syst Pharm. 2011;68(1):63–68. doi: 10.2146/ajhp100224. [DOI] [PubMed] [Google Scholar]