Abstract
Nowadays, almost all persons with diabetes—at least those using antidiabetic drug therapy—use one of a plethora of meters commercially available for self-monitoring of blood glucose. The accuracy of blood glucose (BG) measurement using these meters has been presumed to be adequate; that is, the accuracy of these devices was not usually questioned until recently. Health authorities in the United States (Food and Drug Administration) and in other countries are currently endeavoring to tighten the requirements for the accuracy of these meters above the level that is currently stated in the standard ISO 15197. At first glance, this does not appear to be a problem and is hardly worth further consideration, but a closer look reveals a considerable range of critical aspects that will be discussed in this commentary. In summary, one could say that as a result of modern production methods and ongoing technical advances, the demands placed on the quality of measurement results obtained with BG meters can be increased to a certain degree. One should also take into consideration that the system accuracy (which covers many more aspects as the analytical accuracy) required to make correct therapeutical decisions certainly varies for different types of therapy. At the end, in addition to analytical accuracy, thorough and systematic training of patients and regular refresher training is important to minimize errors. Only under such circumstances will patients make appropriate therapeutic interventions to optimize and maintain metabolic control.
Keywords: accuracy, blood glucose measurement, self-monitoring, SMBG, system accuracy
Introduction
Meters for self-monitoring of blood glucose (SMBG) have undergone significant developments since the 1980s. While these devices were originally quite large and clumsy tabletop devices that required large volumes of blood (>20 ml) and took several minutes to carry out the full measurement, nowadays they are about the size of a small cell phone and can measure blood glucose (BG) concentrations within a few seconds using tiny blood samples (<1 ml).1 In addition, these devices have continued to become more intuitive for users and many sources of error have been eliminated. The costs of individual measurements have also been significantly reduced over time (after correcting for inflation).
Not only do different groups of patients with diabetes use these devices every day for SMBG, but they are also used regularly in many other areas of health care, such as in physician’s offices, in intensive care units (ICUs) and other hospital wards, in emergency response units, during dialysis, in aged care facilities, and by rescue services.
Surprisingly, the accuracy and precision with which these meters are able to determine the concentration of glucose in blood samples were not a widely discussed topic for a number of years, e.g., in advertisements for these devices, the accuracy of results was barely mentioned. At the same time, there is an erroneous widespread belief that approval requirements for these devices, such as the Conformité Européenne (CE) certification process in Europe, ensure adequate accuracy of glucose meters. Appropriate clinical–experimental evaluation indicates that a considerable number of devices available on the market do not meet such regulatory requirements.2 As manufacturers focus primarily on increasing the user-friendliness and cost-effectiveness of their devices, it is quite obvious that such meters are unable, in principle, to measure BG concentrations as accurately as laboratory devices, which are larger and maintained regularly. In addition, one has to take into consideration that the price of such laboratory device is by far different from that of a glucose meter.
The aim of this commentary is to consider various issues that are important for accurate measurement of BG using modern meters and to discuss the consequences of regulatory requirements being tightened [we presume that the Food and Drug Administration (FDA) will demand this soon, discussed later]. Our considerations are focused on the analytical accuracy of the meters and not on their overall performance, which is also known as total system accuracy (TSA). Total system accuracy depends on a range of further aspects such as interference with other drugs/sugars/substances and system limitations (dependence on temperature, height above sea level). Other aspects included in TSA are safety features of the system, labeling of the devices, quality assurance, associated support for patients, and training. Our discussion of SMBG and meter accuracy will focus on the combination of meter and test strips (the monitoring system) because errors in test strip storage or handling can reduce the accuracy of the TSA even though the actual device is functioning reliably. As a result of a range of pre- and postanalytical sources of error (e.g., cleanliness of hands, size of the blood drop), the results obtained by patients during daily practice can be significantly worse than one would assume from the analytical accuracy of the meter per se. Therefore, the analytical accuracy of a device has to be considered separately from TSA when in the hands of patients.
Principles of Analytical Accuracy
The definition of analytical accuracy of meters, i.e., the precision and accuracy with which glucose concentration in blood samples is measured, is described in the basic requirements for such medical devices, including the specific standard for blood monitoring meters DIN EN ISO 15197, which has been in force since 2003.3 The standard specifies that the difference (D) between the BG meter (B) and the reference measurement (R; D = B - R) is used if the BG concentration is <75 mg/dl (4.2 mmol/liter) and the percentage difference (PD) is used if the BG concentration is >75 mg/dl (4.2 mmol/liter; PD = (B - R)/ R × 100; Figure 1). According to these standards for analytical accuracy, deviations of up to ±15 mg/dl (0.83 mmol/liter) from the manufacturer’s reference are allowed in the lower measurement range and up to ±20% in the upper range. In concrete terms, the allowed deviations mean that for a BG concentration of 200 mg/dl, as measured using a reference method, results ranging from 160 to 240 mg/dl measured by a given meter are considered as sufficiently accurate. From a clinical point of view, the reliability with which low BG levels (hypo-glycemia) can be measured is probably the most relevant criterion for the quality of results: using the International Organization for Standardization (ISO) standards, for a true BG value of 50 mg/dl, the value shown on the meter may range from 35 to 65 mg/dl—these differences are relevant for subsequent therapeutic responses.
Figure 1.
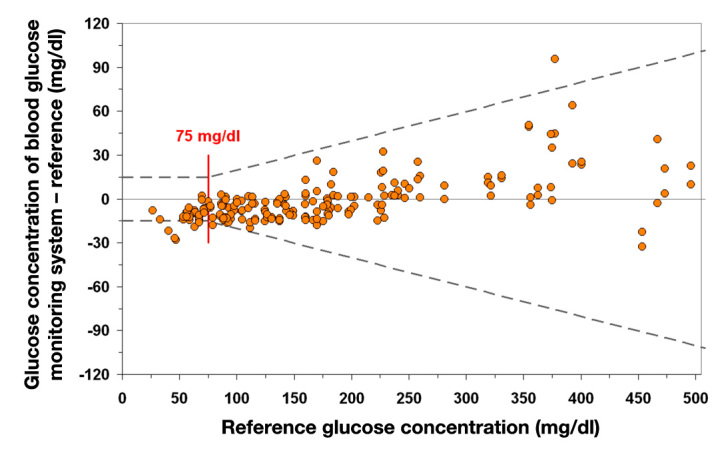
Illustration of the system accuracy test in accordance with DIN EN ISO 15197, 2003. At least 95% of the glucose measurements (n = 200) must be within the stipulated limit relative to the manufacturer’s reference: ±15 mg/dl (±0.83 mmol/liter) with glucose concentrations <75 mg/dl (<4.2 mmol/liter) and ±20% for glucose concentrations ≥75 mg/dl (≥4.2 mmol/liter). In this example, 96% of the values are within the limits specified in the ISO standard; the specifications in the standard are thus met. Example: plot for system accuracy presentation according to ISO.
It must be noted that this specification applies to glucose measurements over the full clinically relevant range and no separate specifications are made for three different clinically relevant ranges (hypoglycemic range 40–80 mg/dl, euglycemic range 80–180 mg/dl, and hyperglycemic range 180–400 mg/dl). Such a separation into different clinically relevant ranges has been proposed before for continuous glucose monitoring (CGM).4 It has also been mentioned that the ISO standard describes a distribution for different production lots of a given meter and test strips, different environmental conditions, and blood matrices. In a given patient, considering the maximum observable error for a given glucose meter, test strip lot, etc., the error observed in practice will be significantly lower than the specified ±20%.
Clinical studies to evaluate the accuracy of BG meters, which are carried out in accordance with these standards, compare the results of measurements of capillary blood samples from patients with diabetes using such meters with measurements made in parallel using reference methods indicated by the manufacturer (laboratory device). It has to be acknowledged that, e.g., the YSI glucose analyzer, which is widely used as the FDA regards it as a reliable reference method, is not a reference method in the true sense of the word. Not only is it cumbersome to use—the concordance of this method with true reference methods is mediocre. However, this procedure makes sense because, ultimately, therapeutic decisions depend on the glucose values measured.5 Of 200 results verified (standard guideline), it is expected that the deviations are below the limits for ≥95% of the results. However, this also means that 5% of the results may deviate to a larger extent. In plain terms, this means that these measurement results can also be completely wrong! In these cases, the patient has no means of recognizing incorrect results and the size of any deviations. From a purely statistical point of view, a theoretical patient who measures his or her BG four times daily will experience such a situation once every 5 days. For a patient who always uses the same meter with good accuracy in the same manner and who is well trained in the monitoring of BG, the deviations can be considered to be relatively small in practice, i.e., the intraindividual variability of glucose measurement is relatively small and the number of outliers is lower than 5%. Nevertheless, environmental factors such as extremely low or high temperatures and high altitude can also lead to significant measurement errors in patients’ hands. However, not too many adequate studies of these exceptions have been carried out.
The BG monitoring systems available today are calibrated using various reference methods such as the hexokinase method or the glucose oxidase method. Because the results obtained with both reference methods (see earlier) can differ by about 6–8%, there are differences in the results obtained by glucose meters calibrated using either of these methods.6 Furthermore, it must be noted that the reference measurements also shows measurement errors. Nevertheless, their measurement quality is better, i.e., modern laboratory systems have a coefficient of variation (CV) of <2% when measuring glucose under ideal conditions; the CV under real life conditions, including preanalytical factors and maintenance of the system, should be determined in a clinical trial.
One difficulty when using randomly obtained patient blood samples is that these samples are most often in the euglycemic range. For patients with diabetes, evaluating the quality of the measurement in the hypoglycemic and hyperglycemic ranges is also important, which is why, in principle, the quality of the accuracy for these three BG ranges mentioned earlier should also be indicated separately. Guidelines for evaluating glucose meters prescribe a distribution of BG data and describe how a full range of BG values can be covered by spiking blood samples with glucose to achieve high glucose values and by allowing glycolysis to have low values in any evaluation. Such a procedure has to be considered carefully as there is the risk of altering the matrix of the sample and the partial oxygen pressure. Such considerations are of relevance for all meters but especially for those using an oxygen-dependent enzyme reaction.
Another possibility for systematically and reproducibly evaluating the accuracy of glucose monitoring in the different BG ranges is the glucose clamp technique.7 The clamp technique also allows evaluation of the precision of the measurement when fresh samples are measured repeatedly; the ISO guideline describes an approach in which a venous blood sample is measured several times in a row (to separate accuracy and precision).
Both high accuracy and precision are extremely relevant in clinical practice, for example, in the diagnosis of gestational diabetes. This diagnosis (for which BG meters have no approval) requires that BG values of 122 mg/dl can be reliably differentiated from 115 mg/dl. This requires not only that the meters, which are used in practice, measure BG with high accuracy and precision, but also that the handling of the blood samples is performed with great care.
Historical Developments
It is interesting to see how the requirements of diabetes associations and standards boards for the quality of glucose measurement have changed and become more stringent over time. As early as 1987, the American Diabetes Association demanded that the total error be <10% in 100% of cases and further tightened this goal in 1993 to an analytical accuracy of <5%. Other organizations have proposed significantly less rigid requirements. For example, in 1993, the recommendation of a standards development organization in the United States (CLSI) was <20% in 95% of cases for glucose concentrations >100 mg/dl and <15 mg/dl below this concentration. This corresponds to the ISO standard apart from the different concentration limit for the switch from absolute to a percentage (75 vs 100 mg/dl). Both requirements of the ISO and CLSI are also being revised and a tightening of the requirements can be expected. The 2011 draft of the new ISO 15197 standard allows the system accuracy in the range <100 mg/dl to be within ±15 mg/dl (0.83 mmol/liter) of the manufacturer’s reference and >100 mg/dl within ±15% for 95% of measurements.8 This should ensure that the allowed range above 75 mg/dl is narrower than in the past and a new range threshold (previously 75 mg/dl) of 100 mg/dl will be introduced (Figure 2).
Figure 2.
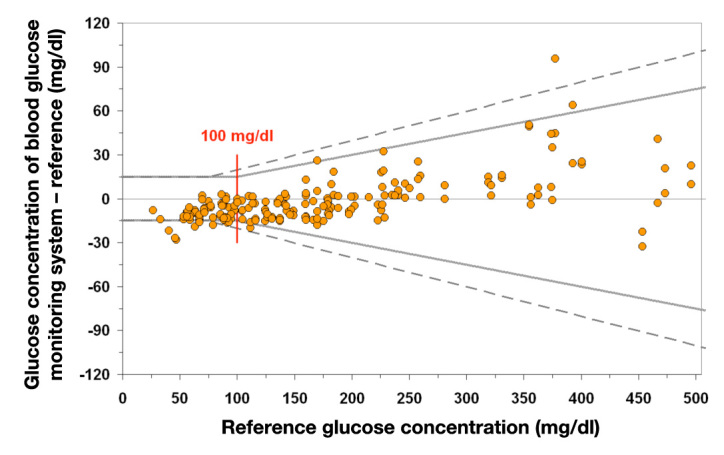
Current draft of DIN EN ISO 15197, 2011. A minimum of 95% of glucose measurements (n = 200) must be within the stipulated limit relative to the manufacturer’s reference: ±15 mg/dl (±0.83 mmol/liter) with glucose concentrations <100 mg/dl (<5.55 mmol/liter); ±15% for glucose concentrations ≥100 mg/dl (≥5.55 mmol/liter). If the same data as in Figure 1 are used, only 92.5% of the values are within the limits specified in the ISO standard, thus the new specifications in the standard are not met. Example: plot for system accuracy presentation according to ISO.
Affordable systems for CGM may also be available for sustained use in the future, provided that the development of CGM systems continues to progress as rapidly as in recent decades. If these systems no longer need to be calibrated using an SMBG measurement—or at least require calibration with an SMBG measurement less often—the issue of accuracy (and also of BG meters) resolves itself. Until then, it must be noted that current CGM systems rely heavily on a highly accurate glucose measurement during calibration because their own accuracy is critically dependent on this calibration.
Tightening the Requirements for Measurement Accuracy
During a 2-day public meeting in March 2010 organized by the FDA, a number of issues dealing with the accuracy of BG meters were discussed in detail in a series of presentations by experts from academia and industry; the measurement accuracy of point-of-care devices was a predominant issue during the meeting as these devices are primarily used in ICUs (all presentations are available at http://diabetestechnology.org/press_releases.shtml, a transcript is available at http://www.fda.gov/MedicalDevices/NewsEvents/WorkshopsConferences/ucm187406.htm, and an editorial on this meeting was published9). However, patients treated in ICUs differ considerably from healthy patients with diabetes: many of these hospitalized patients have considerable shifts in their electrolyte balance, they are being treated simultaneously with a wide range of drugs, and some receive special infusion solutions containing maltose, which interferes with the measurement technique of some BG meters. The aim of BG monitoring in these different environments is also rather different; it could also be said that meters intended for patients with diabetes do not belong in the ICU at all. This is also true for different user groups when it comes to patients with diabetes: patients on oral antidiabetic therapy using SMBG, e.g., structured testing, have different require-ments for accuracy compared with patients on intensified insulin therapy. As mentioned, this is also true for the special situation of ICUs. In the latter case, the disadvantages of limited accuracy of SMBG meters compared to laboratory systems has to be weighted against the advantage of immediate bed site testing (tight glycemic control).
From a regulatory point of view, BG meters are considered in vitro diagnostic devices and are classified in the United States as class II devices with a moderate risk. For all new meters, it need only be verified that they are as good as previously approved devices but not that they are an improvement. Each combination of meter and test strips is considered an independent system that is evaluated separately from a regulatory point of view.
Interestingly, the FDA has obviously not become active of its own accord in revising the requirements but as a response to written requests by the American Association of Clinical Endocrinologists, among others. Currently, it is believed that the FDA will lower the percentage limit for measurement quality. Improved quality of measurements is intended to reduce the risk of errors, e.g., during intensified insulin therapy aiming at tight metabolic control. It is still open if this will be the same regulation for hospital-used meters as for SMBG meters. Fortunately, the 2-day meeting did not deteriorate into a clash between regulatory authorities and industry with patients and physicians caught in the middle, but rather became a joint search for solutions.
More specifically, the FDA seems to be planning to tighten the specifications for the accuracy of BG monitoring from 20% to 15% for values >75 mg/dl and to 10 mg/dl for values <75 mg/dl. It is also possible that the specification that 5% of the values may deviate in practical terms may also be lowered to 2% or 3% and/or the limit up to which an absolute difference rather than a PD should not be exceeded may be increased from 75 to 100 mg/dl. Perhaps there will be different specifications depending on the target BG range or separated for patients with type 1 or type 2 diabetes. As stated earlier, such a change to the specifications will occur at least for professional use (ICUs, physicians’ offices, etc.), but it is unclear if and when this will also apply to SMBG meters used by patients with diabetes. Hopefully, the FDA will follow the new ISO guideline in establishing global requirements. Assuming that the FDA will revise the requirements for quality of glucose meters in general, it is imperative to consider the complex consequences arising from such a shift for all those involved—such a tightening would be a burden for patients and manufacturers alike. If the manufacturers increase the accuracy of their meters, then it would be safe to assume that the price of their test strips/meters would also increase. This in turn would increase the SMBG costs for both the patient and health insurance providers. In addition, glucose meters that are more “lab-like” would require more intensive training of patients to likewise reduce the risk of preanalytical error and to ensure the correct handling of the meters. One can imagine that patients would have to obtain a “driver’s license” to use glucose meters. For sure, using meters with different brands simultaneously—which is what many patients do in practice according to their actual needs—will result in a decrease of the glucose measurement quality. Patients should be instructed to avoid this at all times.
In real terms, there would probably be a transitional period so that it would most likely be several years before these specifications would have to be met. This would give manufacturers time to deal with the complex issues involved in optimization of the measuring technology. The next question is whether the regulatory authorities in Europe will fall into line with the changes made to the specifications by the FDA.
Which Blood Glucose Meters Meet These Requirements?
A comparative clinical study evaluated the measurement quality of 27 BG meters for SMBG use.2 If the tightened requirements being discussed (lowering from 20% to 15%) were applied, then only 6 of the 27 devices tested in this study would meet these specifications. This would mean that many manufacturers would have to either improve the quality of their meters significantly (if they are able to at all) or no longer be allowed to market their devices.
In a comment addressing the FDA meeting discussed earlier, it was suggested that ISO standard limits be retained but that BG meters be subjected to standardized testing (see later) and that the actual system accuracy of a particular device be indicated on the label.10 In this context, a separate evaluation of the quality of the measurement in the hypoglycemic range would be worth considering, as many devices perform poorly in this important range.11
Do More Stringent Requirements Prevent Practical Use of SMBG in Certain Patient Groups?
This initially surprising idea is based on the following question: If only good (i.e., expensive) devices meet the requirements, or the systems become more expensive as a result of meeting the tougher requirements, will practical implementation of SMBG be possible only for those patients who can afford to pay for the better devices themselves and/or to have the costs reimbursed by their health insurance provider? This could lead to an ironic situation where the quality of the devices has been improved, but they are used less often because the individual measurements have become more expensive. Because the frequency of SMBG has a direct effect on the overall quality of metabolic control, the ultimate consequences of tougher regulations must be considered: Could the end result of improving the quality of the actual glucose measurement be a deterioration in the overall metabolic control (due to higher costs), which is correlated with an increase in the risk of developing diabetes-related late complications that are expensive to treat? This result would be the opposite effect of the original intention! The requirement must therefore specify that improved quality of glucose measurement should not be associated with an increase in the costs of SMBG, ensuring that patients can continue to afford SMBG.
Are companies interested in toughening the requirements for measurement accuracy in order to have an advantage over other companies? If yes, which companies? The effort by health insurance companies in Germany to reduce their costs for SMBG in a concerted campaign with pharmacies in which patients are supplied primarily with low-priced devices, provided physicians have not checked the aut idem (exactly identical or brand substitution not permitted) box on the prescription, makes it clear that issues such as measurement accuracy are of little interest to insurance providers; for them, meters are interchangeable without further consideration.
Such a switch from one meter to the other (or using different meters concurrently at different locations/activities) should not be done without serious reasons. At the very least, patients should measure several times with their different meters in order to evaluate the differences in the measurement results. In other words, switching from one meter to the other (for whatever reason) should be done carefully, as the consequences might be clinically relevant. Also, the types of strips used should not be switched without a thorough check of their performance. It is also important to note that the treating diabetologist/diabetes team most often is not informed about such a switch from one meter to the other.
Another aspect is that improving the accuracy of a meter per se without reducing preanalytical and handling errors does not make sense. Therefore, intensive training of patients is needed. When patients participate in theoretical and practical training sessions, they should be able to pass an examination to obtain a SMBG User (= Driver) License. In such training sessions, the patients will also have to learn how to interpret the measurement results and choose adequate therapeutic interventions.
Systematic and Independent Evaluation of Blood Glucose Meters
It is sensible and desirable to devote greater attention to quality assessment, quality control of BG monitoring, and also to the precision of the measurements.12 A regular quality review of BG meters by independent institutes would be very helpful in ensuring adherence to quality standards.10 For these reviews, the performance of the combination of meter and test strips should be verified not just once but repeatedly (and randomly) as long as they are on the market. A public–private partnership between the manufacturers’ association and governmental institutions might be an elegant way to realize such a proposal.
Clinical Accuracy
When looking at the quality of glucose measurement, the primary application of the test must be considered. For patients with diabetes, the primary application of the SMBG test is measurement of preprandial glycemia. For intensified insulin therapy, this value is key in determining prandial insulin dose, which affects postprandial glycemic excursions and, consequently, the overall level of metabolic control (HbA1c value). Other factors that are important in this context include the following:
Estimation of the carbohydrate fraction of the meal to be consumed and the speed with which this can increase the BG (glycemic index)
Accuracy with which the estimated insulin dose (or the dose calculated using a bolus calculator) is drawn up/applied
Variability of the metabolic effect of this insulin
Quantity of insulin still circulating in the blood from the last insulin application
Amount of physical activity after the meal.
Considering the errors or variability of these factors, the errors/variability associated with BG monitoring are certainly not fundamentally greater than that of these factors. However, in this context, what is important is the law of propagation of errors (see http://en.wikipedia.org/wiki/Propagation_of_uncertainty). If the first step in this chain of factors that affects postprandial glycemic excursion already contains a considerable error, then this error will be amplified by subsequent errors. Therefore, it is sensible to aim for the most accurate BG monitoring possible as this is one of the first steps in the chain. The Diabetes Error Test Model is an approach that enables evaluation of various uncertainties in BG monitoring, estimation of carbohydrate quantities, and their effects on postprandial glycemic excursion, among others.13
The demand for a high-quality clinical measurement (in reference to the particular application or patient group) can thus be formulated as follows: Is the accuracy of the meter sufficient to answer clinical questions? There is no clear consensus on this question but there are many expert opinions. Clinical accuracy in this sense is understood as monitoring that provides information that enables appropriate therapeutic decisions to be made for a particular patient. In this context, error-grid analysis helps, e.g., in establishing whether or not deviations in measurements result in therapeutically relevant errors. As discussed earlier, the question of how relevant deviations are depends on the particular patient group or application in question.14 This raises the idea of revising the error-grid analysis to make the results more patient-group specific. With many devices offering poor quality of monitoring in the hypoglycemic range and hypoglycemia being the most threatening factor from the patient’s perspective, the requirements for the hypoglycemic range should be especially stringent.11
Although one can adopt the attitude that this is all one, small part of TSA (discussed earlier) of BG meters, which is made up of clinical and analytical accuracy, it must always be remembered, however, that is the patients with diabetes who ultimately experience the effect of the TSA with regard to their therapeutic decisions.
Measurement Quality That Can Be Permanently Achieved by the Manufacturer
Considering the different methodological approaches used in BG monitoring and the reluctance of manufacturers to provide detailed information about these approaches, it is not easy to estimate their true performance in terms of accuracy. Given the intense rivalry between manufacturers, it can be assumed that with appropriate regulatory requirements for accuracy, high-quality manufacturers will be able to comply fairly quickly and not only initially, when comparative measurements are necessary for approval, but also over time to guarantee accuracy in all devices and test strips. If the limit of what is technologically feasible with reasonable costs is reached during manufacture, this will certainly be a challenge; by the same token, it could also affect the ability to ensure a reliable supply. This could cause considerable problems in terms of consistency of production, particularly with the test strips.
Variability in Batch-to-Batch Measurement Accuracy
Test strips for BG monitoring are not produced continuously but rather in batches and in different quantities per batch. In the past, there were fairly significant differences between individual batches as a result of, e.g., variability in the glucose-specific enzymes or dyes that were produced or made up fresh for each batch. The measurement properties of each batch were therefore determined and communicated by a code on the device, using a batch number. This would ensure adequate measurement accuracy from batch to batch. On the whole, batch-to-batch variability was not widely publicized and has been reduced in recent years by optimizing production processes to the point where coding can be omitted. However, there is no systematic independent evaluation of batch-to-batch quality. This evaluation should be easy to include as part of the evaluation of BG meters in that multiple batches can be concurrently tested within a study (see public–private partnership earlier). This would enable comparable evaluation of different batches similar to a head-to-head comparison.
Clinical Evidence of the Significance of High Measurement Accuracy
For the purposes of achieving good metabolic control with few fluctuations, it is logical that the quality of BG monitoring should be high in terms of the accuracy of the results and the precision of the systems used to support the application of intensified insulin therapy. Accurate BG monitoring using quality BG meters certainly helps to reduce errors in insulin dosage.15 However, other sources of errors that have a significant effect on metabolic control (such as errors in estimating the carbohydrate fraction of a meal, see earlier) remain unaffected. Nevertheless, one has to state that, to date, there is no clear proof that increasing the requirements for the accuracy of BG meters leads to an actual clinical benefit, and until it is verified by a randomized controlled longitudinal study, there is insufficient evidence to support doing this. There is also no clear proof that patients who use a poor monitoring system have worse metabolic control or higher rates of hypoglycemia than patients who use a good monitoring system. Such evaluations should be performed for different patient groups, as the requirements might be different depending on the type of diabetes treatment involved.
It will not be easy to conduct good, evidence-based studies of accuracy, particularly under normal conditions, because various other factors are significant for the outcome of such studies. Longitudinal studies with large numbers of patients (from different patients groups) and suitable study design and study aims must be carried out to verify whether accurate monitoring actually has a positive effect on the long-term course of patients with diabetes with a reduction in associated morbidity and mortality. When such studies do not aim at truly optimizing metabolic control, different error limits will not manifest themselves in differences in the metabolic control/acute metabolic fluctuations.
What Are Realistic Recommendations for Accuracy of Blood Glucose Meters?
Possibly, a realistic scenario would be to stipulate that the quality of monitoring be improved to ±15% within a defined transition period. It remains to be seen whether this will be reduced further to ±10% (for which there is sufficient support and demand) in the near future. If the improvements in the first 30 years of BG meters are taken into consideration, then further improvement in coming decades is also plausible. However, the question remains at what cost will this be achieved? More important in this context is the question of how many of the values in the evaluation will be permitted to be outside this range? The following scenarios are conceivable: 1% of the values at ±15% or 5% at ±10%. Because there will always be individual outliers, a 0% deviation will probably never be achieved. It is also debatable whether different requirements will be specified for the different types of diabetes and therapy (= different BG meters for different prices). Until data exist from clinical trials that clearly support doing so, this will certainly continue to be the case. Another aspect to be considered is the question of accuracy requirements for proper insulin dosing. Currently, we have only modeling studies to address this question, they are the closest we have to real data on what is required.16,17
It is correct that the concept of one size fits all has certain limitations, but the question is whether patients will be classified if meters of varying quality are available for different patients groups.
In summary, our recommendations are as follows:
The accuracy of BG monitoring has greatly improved since the 1980s; however, information on this subject has not been—but deserves to be—more clearly and effectively communicated.
Appropriate labeling of devices (e.g., by indicating a quality class) or information provided during patient education is also conceivable.
The intended use and the indication for use must be kept firmly in mind for every patient.
Placing the data on accuracy and precision on a solid and trustable base, an independent institute financed with public–private partnership might be a good way to satisfy patients, industry, as well as regulatory agencies requirements.
If a given patient does not make the correct therapeutic decisions based on the measurement, an accurate measurement does not help whatsoever. Therefore, as there are no good or bad numbers, but rather they are simply a source of information, patients must be trained appropriately to interpret them.
We believe that setting patient-group specific require-ments for accuracy with a sense of proportion is justified (see new ISO standard that is currently under consideration).
Glossary
Abbreviations
- (BG)
blood glucose
- (CGM)
continuous glucose monitoring
- (FDA)
Food and Drug Administration
- (ICU)
intensive care unit
- (ISO)
International Organization for Standardization
- (PD)
percentage difference
- (SMBG)
self-monitoring of blood glucose
- (TSA)
total system accuracy
Disclosures
Lutz Heinemann hold shares in the Profil Institute for Metabolic Research, Neuss, Germany and the Profil Institute for Clinical Research, San Diego, California, and is a consultant for a range of companies that develop new diagnostic and therapeutic options for the treatment of diabetes. Volker Lodwig is an employee of Roche Diagnostics, Mannheim, Germany. Guido Freckmann is the Director of the Institute for Diabetes Technology Research and Development GmbH at the University of Ulm (IDT), which carries out studies testing blood glucose meters on behalf of various companies.
References
- 1.Hönes J, Müller P, Surridge N. The technology behind glucose meters: test strips. Diabetes Technol Ther. 2008;10:S-10–S-26. [Google Scholar]
- 2.Freckmann G, Baumstark A, Jendrike N, Zschornack E, Kocher S, Tshiananga J, Heister F, Haug C. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther. 2010;2:221–231. doi: 10.1089/dia.2009.0128. [DOI] [PubMed] [Google Scholar]
- 3.International Organization for Standardization: ISO 15197. Geneva: International Organization for Standardization; 2003. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. [Google Scholar]
- 4.Klonoff DC. The need for separate performance goals for glucose sensors in the hypoglycemic, normoglycemic, and hyperglycemic ranges. Diabetes Care. 2004;27:834–836. doi: 10.2337/diacare.27.3.834. [DOI] [PubMed] [Google Scholar]
- 5.Kristensen GB, Monsen G, Skeie S, Sandberg S. Standardized evaluation of nine instruments for self-monitoring of blood glucose. Diabetes Technol Ther. 2008;10:467–477. doi: 10.1089/dia.2008.0034. [DOI] [PubMed] [Google Scholar]
- 6.Twomey PJ. Plasma glucose measurement with the Yellow Springs Glucose 2300 STAT and the Olympus AU640. J Clin Pathol. 2004;57:752–754. doi: 10.1136/jcp.2003.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinemann L, Anderson JH. Measurement of insulin absorption and insulin action. Diabetes Technol Therap. 2004;6:698–718. doi: 10.1089/dia.2004.6.698. [DOI] [PubMed] [Google Scholar]
- 8.International Organization for Standardization: ISO 15197. Geneva: International Organization for Standardization; 2011. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (draft) [Google Scholar]
- 9.Klonoff DC. The Food and Drug Administration is now preparing to establish tighter performance requirements for blood glucose monitors. J Diabetes Sci Technol. 2010;4:499–504. doi: 10.1177/193229681000400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginsberg BH. We need tighter regulatory standards for blood glucose monitoring, but they should be for accuracy disclosure. J Diabetes Sci Technol. 2010;4:1265–1268. doi: 10.1177/193229681000400528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonmez A, Yilmaz Z, Uckaya G, Kilic S, Tapan S, Taslipinar A, Aydogdu A, Yazici M, Yilmaz MI, Serdar M, Erbil MK, Kutlu M. The accuracy of home glucose meters in hypoglycemia. Diabetes Technol Ther. 2010;12:619–626. doi: 10.1089/dia.2009.0183. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen GB, Sandberg S. Self-monitoring of blood glucose with a focus on analytical quality: an overview. Clin Chem Lab Med. 2010;48:963–972. doi: 10.1515/CCLM.2010.186. [DOI] [PubMed] [Google Scholar]
- 13.Koschinsky T, Heckermann S, Heinemann L. Parameters affecting postprandial blood glucose: effects of blood glucose measurement errors. J Diabetes Sci Technol. 2008;2:58–66. doi: 10.1177/193229680800200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10:622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3:903–913. doi: 10.1177/193229680900300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd JC, Bruns DE. Quality specifications for glucose meters: assessment by simulation modeling of errors in insulin dose. Clin Chem. 2001;47:209–214. [PubMed] [Google Scholar]
- 17.Karon BS, Boyd JC, Klee GG. Glucose meter performance criteria for tight glycemic control estimated by simulation modeling. Clin Chem. 2010;56:1091–1097. doi: 10.1373/clinchem.2010.145367. [DOI] [PubMed] [Google Scholar]


