Two limitations shared by all continuous glucose monitors (CGMs) are poor accuracy in the hypoglycemic range1 and poor accuracy when glycemia is changing rapidly.2 This raises the issue of whether aspects of the calibration procedure could improve sensor accuracy in the hypoglycemic range. Retrospective analysis of CGM readings using in silico (i.e., computer-based) modeling suggest that the glucose levels at which calibrations are performed affect the performance of CGMs.3–5 The purpose of this study was to determine whether the intrinsic accuracy per se of CGMs also improves in the low glucose range if multiple calibrations are performed at different and low glucose concentrations in vitro. Twelve sensors (Sof-sensor®; Medtronic, Northridge, CA) were exposed to three calibration conditions using Paradigm® 722 REAL-Time CGMs (Medtronic, Northridge, CA) following a randomized counterbalanced design. Each CGM was calibrated and tested in glucose solutions prepared in a Krebs physiological bicarbonate buffer at 37 °C and pH of 7.4. Since Paradigm 722 REAL-Time CGM units must be calibrated four times daily, with several calibration points rather than only the most recent calibration value being used for calibration, each unit was calibrated four times in a 144 mg/dl solution (144/144/144/144 mg/dl) or calibrated four times in 72, 144, or 216 mg/dl solutions following the sequence 144/72/216/144 mg/dl or 144/216/72/144 mg/dl, with a 40 min period between each calibration. Then, the units were tested by transferring the sensors to a 40-mg/dl solution and data compared when glucose readings reached stable levels. A one-way analysis of variance with repeated measures and Bonferroni post-hoc tests were adopted to compare calibration protocols (results expressed as mean ± standard error of the mean, p < 0.05). All sensors significantly overestimated the low glucose solution to a similar extent, irrespective of whether multiple calibrations were performed at the same or different glucose levels including one of the calibrations entered at a low glucose concentration (72 mg/dl; Figure 1; p > 0.05). There were also large mismatches between the CGM and reference glucose associated with the CGM’s lag, most notably during the initial rapid fall in glucose levels (5–15 min; Figure 1) but no differences in the magnitude of these mismatches between calibration protocols at 5, 10, and 15 min as well as with the lag time defined as the time required for CGM glucose readings to decrease by half of the absolute change in reference glucose concentration (Figure 1; p > 0.05). However, since the glucose level of the last calibration solution was matched for all calibration protocols, we examined whether different glucose levels at the last calibration step affect accuracy and found that CGMs calibrated at 72 mg/dl misread the 40 mg/dl solution to a lesser extent (3.6 ± 1.8 mg/dl) compared to CGMs calibrated at 144 mg/dl (12.6 ± 1.8 mg/dl; p < 0.05) and 216 mg/dl (16.2 ± 1.8 mg/dl; p < 0.05). This indicates that the accuracy of the CGM tested here was influenced by the glucose level at which the last calibration was performed, thus corroborating, in part, the findings based on computer-based modeling.3–5 However, given the evidence that CGM performance in vivo is poorer than in vitro,6 it remains to be tested whether calibration at different glucose levels might be even more beneficial in vivo than reported here in vitro.
Figure 1.
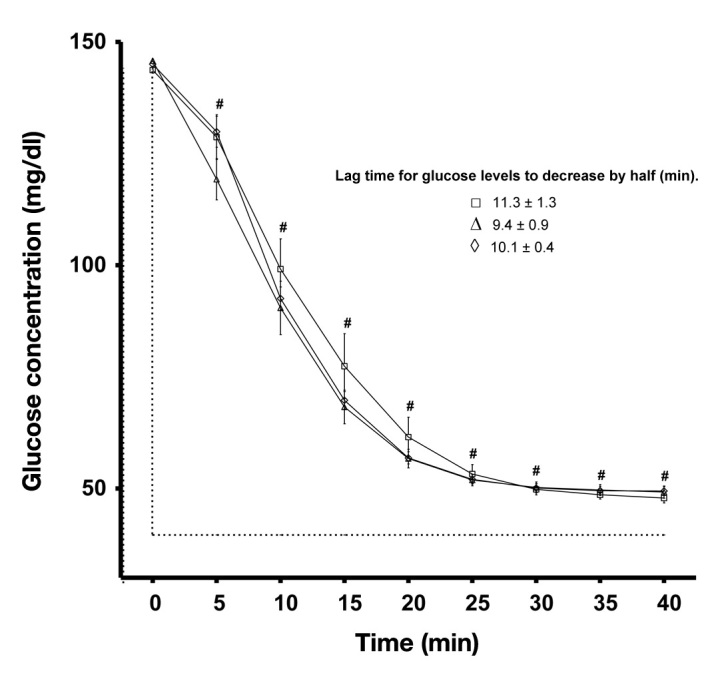
Effect of calibration protocol on CGM glucose measurements during an immediate fall in glucose concentration (from a 144 to 40 mg/dl solution). Repeated calibration at 144/144/144/144 mg/dl (□), 144/72/216/144 mg/dl (Δ), or 144/216/72/144 mg/dl (⋄).
Acknowledgments
The authors acknowledge the lending of CGMs from Medtronic Australasia for the completion of this study.
Glossary
Abbreviations
- (CGM)
continuous glucose monitor
Disclosures
Katherine Iscoe has received speaker’s fees from Medtronic and Medtronic Australasia.
References
- 1.McGowan K, Thomas W, Moran A. Spurious reporting of nocturnal hypoglycemia by CGMS in patients with tightly controlled type 1 diabetes. Diabetes Care. 2002;25(9):1499–1503. doi: 10.2337/diacare.25.9.1499. [DOI] [PubMed] [Google Scholar]
- 2.Davey RJ, Low C, Jones TW, Fournier PA. Contribution of an intrinsic lag of continuous glucose monitoring systems to differences in measured and actual glucose concentrations changing at variable rates in vitro. J Diabetes Sci Technol. 2010;4(6):1393–1399. doi: 10.1177/193229681000400614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monsod TP, Flanagan DE, Rife F, Saenz R, Caprio S, Sherwin RS, Tamborlane WV. Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycemia and hyperinsulinemia? Diabetes Care. 2002;25(5):889–893. doi: 10.2337/diacare.25.5.889. [DOI] [PubMed] [Google Scholar]
- 4.King C, Anderson SM, Breton M, Clarke WL, Kovatchev BP. Modeling of calibration effectiveness and blood-to-interstitial glucose dynamics as potential confounders of the accuracy of continuous glucose sensors during hyperinsulinemic clamp. J Diabetes Sci Technol. 2007;1(3):317–322. doi: 10.1901/jaba.2007.1-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes Research In Children Network (Direcnet) Study Group, Buckingham BA, Kollman C, Beck R, Kalajian A, Fiallo-Scharer R, Tansey MJ, Fox LA, Wilson DM, Weinzimer SA, Ruedy KJ, Tamborlane WV. Evaluation of factors affecting CGMS calibration. Diabetes Technol Ther. 2006;8(3):318–325. doi: 10.1089/dia.2006.8.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman B, Brazg R, Schwartz S, Weinstein R. A continuous glucose sensor based on wired enzyme technology—results from a 3-day trial in patients with type 1 diabetes. Diabetes Technol Ther. 2003;5(5):769–779. doi: 10.1089/152091503322526978. [DOI] [PubMed] [Google Scholar]


