Abstract
The effects of practice on the functional anatomy observed in two different tasks, a verbal and a motor task, are reviewed in this paper. In the first, people practiced a verbal production task, generating an appropriate verb in response to a visually presented noun. Both practiced and unpracticed conditions utilized common regions such as visual and motor cortex. However, there was a set of regions that was affected by practice. Practice produced a shift in activity from left frontal, anterior cingulate, and right cerebellar hemisphere to activity in Sylvian-insular cortex. Similar changes were also observed in the second task, a task in a very different domain, namely the tracing of a maze. Some areas were significantly more activated during initial unskilled performance (right premotor and parietal cortex and left cerebellar hemisphere); a different region (medial frontal cortex, “supplementary motor area”) showed greater activity during skilled performance conditions. Activations were also found in regions that most likely control movement execution irrespective of skill level (e.g., primary motor cortex was related to velocity of movement). One way of interpreting these results is in a “scaffolding-storage” framework. For unskilled, effortful performance, a scaffolding set of regions is used to cope with novel task demands. Following practice, a different set of regions is used, possibly representing storage of particular associations or capabilities that allow for skilled performance. The specific regions used for scaffolding and storage appear to be task dependent.
This paper describes practice-related changes in cerebral blood flow observed during procedural learning or skill acquisition. This type of learning is often gradual or iterative; in other words, skill is acquired over many trials, slowly moving toward a particular goal (1, 2). In some cases, the learning can take place with little attention directed to the learning events (3). It is “nondeclarative” in the sense that the person may not be able to explicate what has been learned or how, even though behavior has clearly changed over time. Neurobiologically, medial temporal lobe damage leaves this kind of learning essentially intact, but damage to other regions, such as the basal ganglia (4) and the cerebellum (5, 6), have been implicated in deficits in specific instances of nondeclarative learning.
There are at least two different, but nonexclusive, ideas related to skill acquisition mechanisms. The first emphasizes that learning can take place through more efficient use of specific “neuronal circuits.” The idea of learning at a Hebbian synapse would be an example of this type of mechanism, where synaptic weight is strengthened when an input neuron and output neuron are active together. Some recent explications of procedural learning liken it almost to a type of high-level priming, in which existing structures are made efficient through recent use (e.g., ref. 4).
The second idea emphasizes that skill acquisition goes through “stages” (1, 7), or that different processes are associated with the different levels of skill, perhaps allowing the performance of a task to be programmed at different levels of complexity (8–10). The first time someone tries to drive seems so different from skilled driving that they seem to be qualitatively different tasks. The different levels of performance may entail changes in storage of and/or access to different associations and information across time, processes that were not necessarily part of the earliest performance. These “different tasks” then might be expected to utilize different neural substrates. A common view is that early task performance includes recognition of a patterned behavioral demand. This recognition may be explicit (the subject consciously recognizes what needs to be learned, and some basic level of conscious problem-solving might be applied); in other cases, this recognition might be implicit. Following this phase, correction of gross errors in performance takes place. Then, a final phase of fine honing of behavior and overlearning occurs, during which less effort or attention is needed for task performance.
Although these two types of mechanisms sound very different, we would like to emphasize that we do not think that they are mutually exclusive. We believe that both circuit-efficiency changes and processing differences are part of skill acquisition in almost all situations. What we would like to emphasize in this paper, however, is the way that imaging can highlight the latter: changes that take place in the brain putatively related to processing differences between unskilled and skilled performance of a task.
The first section of this paper focuses on a series of experiments on simple processing of single words. It begins with a comparison between several single-word processing tasks (11, 12). A reanalysis of the data, which went beyond that performed in the original study, led to the idea that there were separate pathways between visual word inputs and verbal outputs in a verb generation task related to unskilled and skilled associations (13, 14). Finally, results of a study that explicitly tested this idea in a practice paradigm (15) will be discussed.
To see if a similar switch of activation for unskilled and skilled performance could be observed in another task domain, practice-related results on a maze tracing task are reported. This study was developed to be different from the verb-generation experiments in the domain of information used (language vs. spatial/motor), output modality (verbal vs. manual), and sensory information used (visual vs. kinesthetic/somatosensory).
One final introductory point worth making: The examples that will be presented here are of the effects of practice on a set of items, or on a particular learning instance. As such, they do not explicitly represent the development of a general skill, like typing or playing tennis. We will argue, however, that the nature of results themselves suggests relevance to skill acquisition in a more general way than might be thought given the item-based nature of the practice effects.
SINGLE WORD STUDY
Background/Experimental Design.
In an early set of studies (11, 12), subjects performed a hierarchy of four single word processing tasks. These tasks were done with both visual and auditory word stimuli. During separate scans, subjects did the following: 1, fixated on a centrally displayed fixation point; 2, fixated and passively viewed or heard nouns; 3, fixated and repeated the seen or heard nouns; and 4, fixated and said aloud a verb appropriate for heard or seen nouns.
In that original study, a hierarchical subtraction design was used to identify regions at different “levels” of the processing of single words. In other words, in each modality, task 1 was subtracted from task 2, task 2 from task 3, etc. and only positive changes were reported (see Table 1). This produced some interesting results, but had some design problems, which will be discussed below.
Table 1.
| Active state | Control state | Activated areas |
|---|---|---|
| Passive words | Fixation point | Visual: bilateral primary and extrastriate regions |
| Auditory: bilateral primary auditory and association areas | ||
| Repeat/read words | Passive words | Visual + auditory: bilateral primary motor cortex, insula, premotor, SMA, medial cerebellum |
| Generate verbs | Repeat/read words | Visual + auditory: left prefrontal cortex, anterior cingulate, right cerebellum |
All subtractions are made within modality of presentation. SMA, supplementary motor area.
Each task (except fixation point) was performed in a set of scans with visual presentation as well as in a set of scans with auditory presentation.
Results.
In the first subtraction (passive presentation minus fixation), activation was seen in modality-specific primary and nonprimary sensory processing regions. For visual input, bilateral primary and extrastriate regions were clearly activated. For auditory input, bilateral primary auditory and auditory association areas were active, as well as a region at the left temporoparietal junction. By virtue of the localizations and the tasks involved these activations were attributed to modality-specific processing of the word stimuli. These experiments could go no further in assessing the type or specificity of that processing [although further work on this issue has been done (16, 17)].
In the second subtraction (word reading or repetition minus passive presentation), areas commonly related to motor processing were seen. Bilateral primary motor and insular cortex, premotor, SMA, and medial cerebellum were active irrespective of the modality of stimulus input, and these activations were attributed to the common output demands of the reading and repetition tasks.
In the final subtraction (verb generation minus word reading or repetition), activation was seen in the left prefrontal cortex, the anterior cingulate, and the right cerebellar hemisphere. Again, these activations were seen irrespective of modality of input, and they were attributed to the additional processing demands of the more complex verb-generation tasks.
“Problems” with “Cognitive Subtraction.”
In several instances in the literature, hierarchical subtraction designs such as described above have been criticized as having inherent assumptions that may well not be met. The most problematic of these assumptions is that of “pure insertion.” This difficulty is not new with the use of subtraction in functional imaging, and indeed it has been discussed for several decades in psychology, particularly in dealing with certain types of reaction time studies (e.g., ref. 18).
The problem with pure insertion can be conceptualized like this. Take two tasks—for example, seeing a word and saying it out loud (word reading) and seeing a word and saying an appropriate verb related to that word (verb generation). Both tasks entail visual processing and motor output, and processes to translate the visual input into a motor output. When the reaction times for these two tasks are studied, it turns out that word reading on the average takes about 550–600 ms, and verb generation about 1000 ms (15). One way of interpreting these results is that all of the processing that occurs during word reading also occurs during verb generation, plus some more processing that takes about 400 ms. This interpretation assumes “pure insertion” in that the extra processing that is related to the verb generation task is just added to that of the reading task.
The problem with such a subtractive design and interpretation in a reaction time experiment is that it is quite possible that certain processes that occur during the word reading task may not occur during the verb generation task. In other words, some of the processes used in word reading are replaced when verb generation is performed. It is the sum of the time saved by dropping some processes used in simple reading and the time for additional processes for verb generation that in total contribute to the longer reaction time. By just looking at reaction times for these two tasks, the two alternative explanations cannot be deconfounded. Different approaches to this issue in cognitive psychology have led to more complex experimental methods, such as additive factors and factorial designs, that allow the investigation of process interactions in two tasks. Some of these approaches have also been used in imaging research (e.g., ref. 19).
The hierarchical subtraction analysis used in the imaging study presented above “buys into” this problem, because the design of the analysis and the interpretation drawn from it uses pure insertion logic (and assumptions).
However, this is where the problem ends and confusion in the imaging literature begins. The confusion results from the lack of appreciation for the distinction between “cognitive subtraction” as an experimental design and interpretive strategy, and image subtraction as an analysis methodology. As seen above, cognitive subtraction can be used to design and interpret an imaging study, and imaging subtraction can be used to mirror the cognitive strategy. However, image subtraction does not make the assumption of pure insertion: experimental designs, analysis choices, and interpretive strategies do. Image subtraction is performed in part to mirror experimental design strategy, but more importantly it is done to reveal the differences in the hemodynamic signal between two conditions by subtracting the large amplitude complex anatomical background present in hemodynamic images.
Reanalysis of Original Results
In recognition of the interpretive problems with the early analysis presented above, Fiez et al. (13, 14) reanalyzed the same data set and, using only image subtraction, tested the assumption of pure insertion.
Reanalysis/Results.
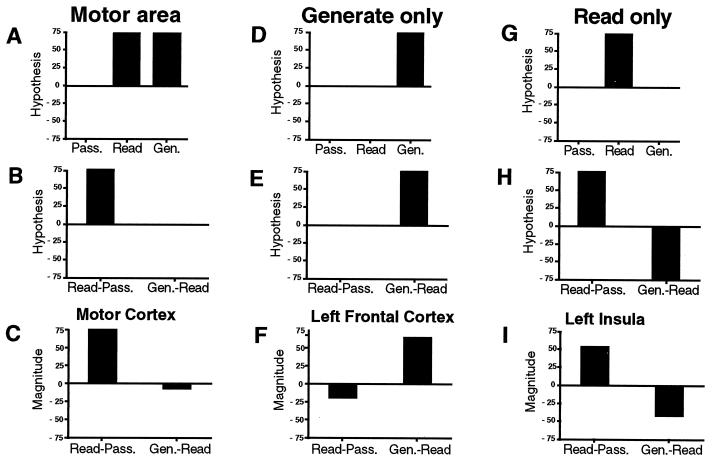
The reanalysis took the form of a tracking analysis, that is, both positive and negative changes at each region identified in any task were analyzed across all subtraction pairings. Fig. 1 shows how such a tracking analysis might work. Take for instance a region that is most likely to be related to motor output. Such an area might be about equally activated in both the reading and generation tasks because there is a common nearly equivalent demand for motor output in the two tasks. It would, however, not be activated by the simple presentation of the visual stimuli. When compared with the low-level fixation control, an activation pattern similar to that shown in Fig. 1A would be expected. When the reading minus passive presentation and verb generating minus reading subtractions are made, the theoretical results would look like the graph in Fig. 1B. The actual results from primary motor cortex are shown in Fig. 1C and follow very closely what would be expected from regions that are commonly activated by the two conditions. In this case, the areas are adhering to the ideas of pure insertion, in that something present in reading is carried through with similar activation to the verb generation task.
Figure 1.
Absolute magnitudes in hypothetical brain areas (Top three graphs) during passively viewing words, reading words, and generating verbs; difference magnitudes in hypothetical brain areas (Middle three graphs) for reading minus passively viewing words and verb generation minus reading words subtractions; and difference magnitudes in brain areas of interest (Bottom three graphs) for reading minus passively viewing words and verb generating minus reading words subtractions in areas with activations related to motor output (A–C), to generating a verb (D–F), and to simple reading of words (G–I).
What would happen in the tracking analysis for a region that is specifically related to verb generation? Such a region would not be active during either passive presentation or reading, and it would be activated only during the verb generation task (see Fig. 1D). The theoretical subtractions of read minus passive and verb generation minus read would appear as seen in Fig. 1E. Actual activations in left frontal cortex that follow this theoretical construct are shown in Fig. 1F. Again, these areas follow the assumption of pure insertion.
In fact, most of the regions passed through the tracking analysis did follow the assumption of pure insertion across conditions. However, the reanalysis revealed an interesting violation of pure insertion that is exploited in the learning paradigm (14).
What might such a violation of pure insertion look like? One example would be a region that was used for reading but not for the verb generation task. When compared against the low-level baseline, it would show up as an isolated activation in the reading task (Fig. 1G) and in the higher level subtraction as a positive in the read minus passive subtraction and an essentially equivalent negative in the verb generation minus read subtraction (Fig. 1H). Such a pattern was seen bilaterally for the insular activation described in the original paper and shown for the left insula in Fig. 1I. By using image subtraction, a violation of the assumption of pure insertion underlying “cognitive subtraction” can be easily described if the analysis is complete, and appropriate control conditions are included at imaging time.
This presentation is not meant to show that subtraction is the only viable method for exploring images. What it is meant to show instead is that even when simple subtraction is used, complex issues such as pure insertion can be examined in imaging data if the question is clearly conceptualized. Rather than focusing on particular experimental forms (such as subtractive, factorial, or additive factors designs) in a prescriptive way, emphasis should be placed on whether a particular instance of a design and analysis strategy appropriately addresses a specific question or set of questions.
Unskilled and Skilled Use of Words.
Beyond the observation that tracking analyses can illuminate violations of pure insertion, this analysis led to a second interesting idea. The observation that similar visual and motor regions, but different intermediate regions, are used in the reading and verb generation tasks suggests that two distinct pathways are used to convert visual information into a verbal response. One potential distinction that might explain the difference between these two pathways is the level of skilled association between the visual input and the verbal output. When the stimulus–response pairing is overlearned, as in the simple reading task, a Sylvian-insular pathway may be used to convert a sensory input into an appropriate motor response; when this condition is not met, further processing may be necessary, using an alternate frontal/cingulate/cerebellar pathway. This idea led to the question: “Would practicing the verb generation task (thus increasing the strength of association between the input and output) change the areas of the brain used to those activated during the more skilled task of simple reading?”
PRACTICE EFFECTS ON VERB GENERATION
Experimental Design.
As a test of this question, positron-emission tomography (PET) and performance studies examined the functional and behavioral effects of practice on the verb generation task. These studies are described more completely in Raichle et al. (15).
Subjects performed the verb generation task (say aloud an appropriate verb for each presented noun) in three different states of practice: 1, naive—initial (unpracticed) performance of the verb generation task; 2, practiced—following nine blocks of practice with the same set of nouns; and 3, novel—following practice with the task, but with a novel set of nouns as stimuli. As a control state, subjects were asked to read aloud the nouns. This paradigm and the hypothesized effects are shown in Table 2.
Table 2.
Hypothesized brain activations during Naive, Practiced, and Novel verb generation as well as during reading and hypothesized increased (+) and decreased (−) activations when reading is subtracted from verb generation
| Practice state | Generation activation | Read activation | Generation–read activation |
|---|---|---|---|
| Naive, no prior practice | L frontal Ant. cingulate R cerebellum | Insula | (−) Insula (+) L frontal (+) Ant. cingulate (+) R cerebellum |
| Practiced, after 9 blocks with same nouns | Insula | Insula | No activation |
| Novel, after 10 blocks but with new nouns | L frontal Ant. cingulate R cerebellum | Insula | (−) Insula (+) L frontal (+) Ant. cingulate (+) R cerebellum |
L, left; R, right; Ant., anterior.
Results.
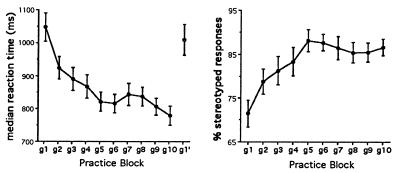
Behavioral results. When subjects practiced nine times with the same list of 40 nouns, several observations could be made about their behavior, which are shown in Fig. 2. First, as subjects practiced, the time between the onset of the visual word and the onset of their vocalization became shorter (decrease in voice reaction time). Second, the words that were used as a response to each of the 40 nouns became increasingly stereotyped (e.g., a subject responds repeatedly to the stimulus word “DOG” with the verb “pet”). As a measure of this, we plotted, by practice trial number, the percentage of words completed with the most frequently used answer. By the end of nine practice trials, about 95% of the words are being responded to with the most frequently used verb.
Figure 2.
Median reaction times (Left) and percentage of stereotyped responses (Right) across verb generation practice blocks. Means and standard error are presented. g1–g10 represent the 10 verb generate blocks, all on the same list of 40 nouns, g1′ is verb generate on a novel list of nouns. Subjects were scanned during g1 (naive verb generate), g10 (practiced verb generate), and g1′ (novel verb generate).
Imaging results.
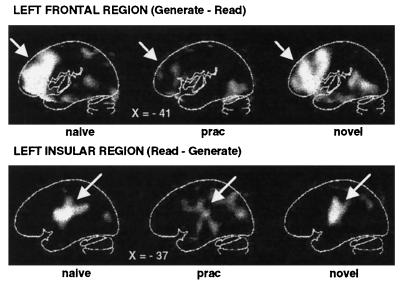
What happens in the brain as a result of practice? For naive performance, the regions found in the original comparison between verb generation and reading were found again. Left prefrontal cortex (see Fig. 3 Upper images), anterior cingulate cortex, and right cerebellum were all significantly more active during verb generation than during reading, whereas bilateral insular areas (see Fig. 3 Lower images) were more active in the reading task. If our hypothesis is correct, and the frontal, cingulate, and cerebellar activations are replaced by insular activation after practice, then all of these differences should disappear. As can be seen in Fig. 3, this indeed did happen. Most of these functional and behavioral effects of practice appear to be item-specific, because introduction of a novel list of nouns produces results similar to those observed during the first performance of the task (naive condition) (see Figs. 3 and 4).
Figure 3.
PET difference (subtraction) images showing areas of increased (Upper images) and decreased (Lower images) blood flow when verb generation (under Naive, Practiced, and Novel conditions) is compared with reading. During naive (Left images) and novel (Right images) verb generation, increased blood flow in left frontal cortex was found compared with simple reading, whereas decreased blood flow was observed in left insular cortex. The Center images show that blood flow in these areas changed to a level almost identical to that found during simple reading after the verb generation was practiced. A linear gray scale is used with white representing maximal activation and black, minimal activation. The brain outlines were traced from the stereotaxic atlas of Talairach and Tournoux (20) and represent sagittal sections with their x-axis (left–right axis, left being negative) positions in millimeters noted.
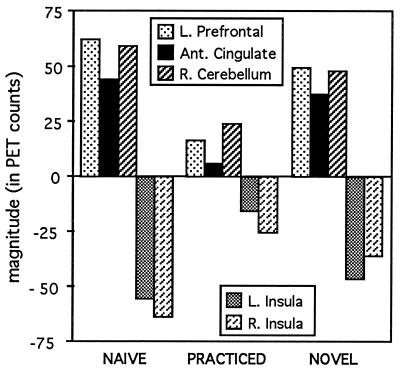
Figure 4.
Magnitudes in left prefrontal cortex, anterior cingulate, right cerebellum, and bilateral insular cortex for the verb generation conditions (naive, practiced, and novel) minus word reading.
One might argue that these results just represent habituation or a decrease in the activation in the left frontal, cingulate, and cerebellar areas related to more efficient processing. If all of the effect of practice were simply made through a decrease in activity in frontal, cingulate, and cerebellar regions, then there would be no change in the insular activation across practice level. What was observed, however, was that the level of activation in the insula increased after practice so that the difference between the level of activation in the reading and generation tasks equalized, as if the frontal, cingulate, and cerebellar activation were replaced with activation in the insula. In addition to the areas presented here, there were also practice effects in two other areas. A region of the temporal cortex paralleled the activations of the frontal and cingulate cortex, and therefore may be related to unskilled performance of the task. A region of extrastriate cortex paralleled the insula, indicating that it may be relied upon more heavily for skilled performance of the task [see Raichle et al. (15)].
A Switch of Areas Used in Task Performance.
Thus, following a brief period of practice (less than 15 min), the cortical circuitry used to perform the verb generation task became almost indistinguishable from that used for reading single words. These results provide converging evidence for the existence of two pathways available for the selection of a verbal response, and they support the hypothesis that the use of these pathways is strongly affected by the degree to which a particular response is learned or automatic.
PRACTICE EFFECTS ON MAZE TRACING
Background/Experimental Design.
One question that could be raised about the practice effects on the verb generation task is the generality of the finding. The verb generation task is quite artificial and in a specific domain (language) that might not generalize well to other domains. The maze-tracing task was developed to be different from the verb generation experiments in domain information, output modality, and sensory input. The general paradigm of the experiment, however, was designed to parallel, in part, the verb generation studies.
This study was performed on two groups of normal right-handed subjects. One group of 16 subjects performed the maze tracing tasks with their (dominant) right hand, whereas the other 16 subjects performed the tasks with their (nondominant) left hand. For the motor learning tasks, subjects traced through cut-out cardboard designs fastened to a bit pad, which was connected to a computer. A special pen (21) was used for the tracing that allowed accurate recording of the behavior. When traced correctly, all of the designs had a path length of 24 cm. In all conditions, subjects had no visual feedback. The cutout paths allowed the subjects to “find” their way by moving the pen along the path until they bumped into the end of the path, after which they had to change direction to move further through the design. The designs were continuous so that when the subjects successfully traversed the 24 cm path, they could begin the next trip through the design.
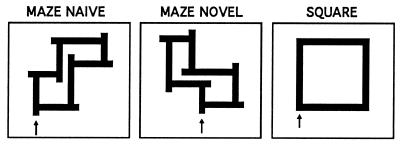
Two different types of designs were used, mazes and simple squares (see Fig. 5). For the square tasks, the designs were squares 6 cm on a side. The mazes consisted of eight segments. At the end of each maze segment, subjects had two choices, either a correct path which led to the next choice point, or a dead end 1 cm from the choice point. When subjects reached a dead end, they had to reverse direction to take the correct path to the next choice point.
Figure 5.
Maze and square designs used in the study. Arrows indicate the starting position for the tracing of each design. Shown are the mazes presented during right-hand performance. During left-hand performance, mirror images of the mazes were presented and tracing had to be done in a counterclockwise direction. Starting position for left-hand square tracing was at the lower right corner, with counterclockwise tracing.
Subjects were imaged during initial unpracticed performance on a specific maze, after practice on the same maze, and during the performance of a different maze. These three conditions paralleled the Naive, Practiced, and Novel conditions of the verb generation task. A control condition (Square Fast), comparable to the simple reading task, was used as the basic control for this study [for more complete analysis, see van Mier et al. (22, 23)]. In the Square Fast condition, subjects traced the simple square design as quickly as possible. Subjects show highly skilled performance in this Square Fast condition almost immediately (9).
Several considerations have to be made about the behavior in this task. Only when an increase in velocity is accompanied by a decrease in errors can an increase in skill be assumed (1). Furthermore, a decrease in the number and duration of stops at corners is an indication that the movements are performed smoothly (9). Fast, accurate, and smooth movement execution characterizes overlearned performance. Because subjects get faster with practice, and bump less into sides and endpoints, an extra control condition was included by instructing subjects to trace the square slowly, and bump into the side at each turn (Square Slow). The subjects obtained a high level of skill quickly. The hope was that the skilled Square Fast and Square Slow conditions would bracket the complex maze conditions in the performance variables of stops and velocity, so that performance variables such as velocity and number of stops could be dissociated from level of skill. Finally, a simple “Rest” condition, with subjects just holding the pen, was used as a low-level control for comparison of all five active conditions.
In terms of the imaging results, the main comparisons that are shown in Table 3 are between the Naive, Practiced, and Novel maze conditions and the fast tracing of the simple square (Square Fast), to make the comparisons as similar in form of analysis to the preceding section.
Table 3.
Hypothesized brain activations during Naive, Practiced, and Novel maze tracing as well as during Fast Square tracing and hypothesized increased (+) and decreased (−) activations when Square Fast is subtracted from maze tracing
| Practice state | Maze activation | Square Fast activation | Maze–Square Fast activation |
|---|---|---|---|
| Naive, no prior practice | R premotor R par. (7 + 40) L cerebellum | SMA | (−) SMA (+) R premotor (+) R par. (7 + 40) (+) L cerebellum |
| Practiced, after 10 min on same maze | SMA | SMA | No activation |
| Novel, after practice but with new maze | R premotor R par. (7 + 40) L cerebellum | SMA | (−) SMA (+) R premotor (+) R par. (7 + 40) (+) L cerebellum |
L, left; R, right; par., parietal cortex.
Results.
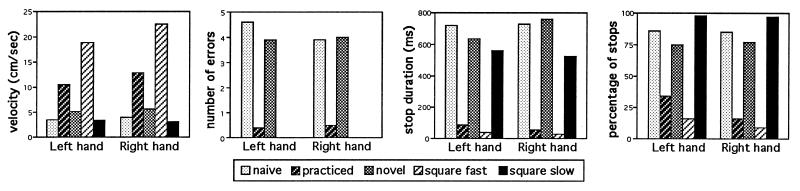
Behavioral results. Performance results for velocity, errors, and stops for the five conditions are presented in Fig. 6. For both right- and left-hand performance, it was found that after practice, movements were performed more quickly with hardly any errors or stops. With presentation of a new maze, performance nearly returns to that found in the unlearned, naive state. As hoped, the Square Fast and Slow conditions essentially bracket the complex maze conditions in velocity and stops. There are no errors in the square conditions, because there are no dead ends. Repeated measurement ANOVAs, using the Greenhouse–Geisser epsilon adjustment, showed that the mean effect of condition was highly significant (P < 0.001) for each dependent variable. Post-hoc tests verified that naive and novel performance was significantly different from practiced performance.
Figure 6.
Mean velocity, number of errors, and duration and percentage of stops for right- and left-hand performance as a function of condition.
Imaging results.
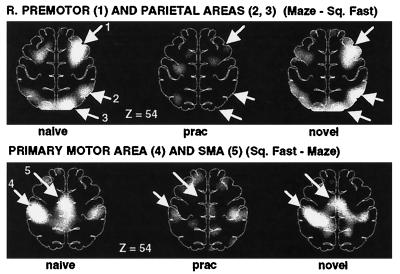
Some regions were more active during unpracticed than practiced performance of the mazes. These included right premotor and right parietal regions (Fig. 7, Upper images), as well as unpictured activation in the left cerebellum. Other regions were more active during practiced than unpracticed performance, including SMA and primary motor cortex (Fig. 7, Lower images). The levels of activity observed during right-hand performance in these areas across the three maze conditions (against Square Fast) are shown in Fig. 8. Note that left-hand performance showed activations in the same areas, with the exception of right-lateralized activation in primary motor cortex. Because practice changed the level of activity in these regions, they were candidate regions relating to unskilled and skilled performance, respectively. There is still the potential confound that performance change (e.g., moving faster) accounts for the change in activity.
Figure 7.
PET difference (subtraction) images showing areas of increased (Upper images) and decreased (Lower images) blood flow when maze tracing (under Naive, Practiced, and Novel conditions) is compared with fast square tracing. During naive (Left images) and novel (Right images) maze tracing, increased blood flow in right premotor and parietal areas was found compared with square tracing, whereas decreased blood flow was observed in primary and supplementary motor cortex. The Center images show that blood flow in these areas changed to a level almost identical to that found during simple square tracing after the maze was practiced. A linear gray scale is used, with white representing maximal activation and black, minimal activation. The brain outlines were traced from the stereotaxic atlas of Talairach and Tournoux (20) and represent a transverse section 54 mm above the AC–PC line.
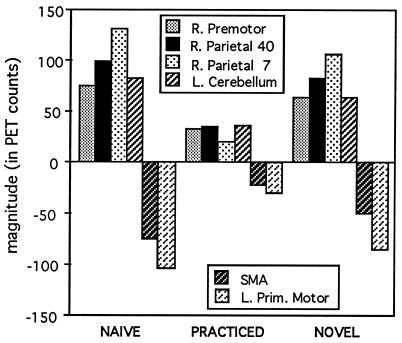
Figure 8.
Magnitudes in right premotor and parietal areas, left cerebellum, SMA, and left primary motor cortex for the maze-tracing conditions (Naive, Practiced, and Novel) minus fast square tracing.
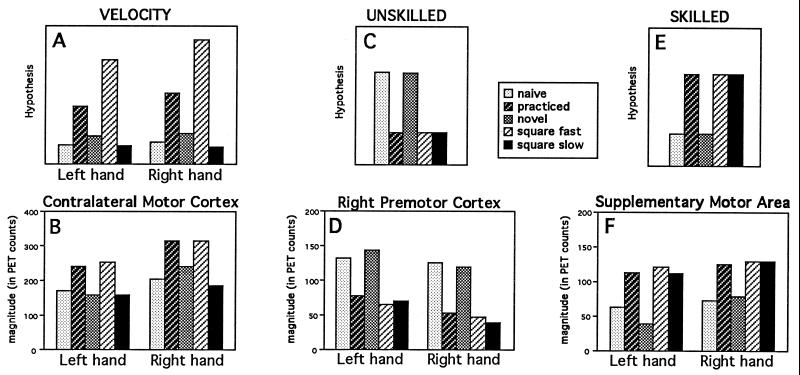
One way to explore this possibility is to track the level of activation across all five conditions compared with a low-level control. If an area were related specifically to a performance variable such as velocity, then its activation should track the velocity profile. The example of a hypothesized velocity profile is shown in Fig. 9A. Because velocity is highest in the Practiced and Square Fast conditions, a region related to velocity would be expected to be highest in those two conditions. Primary motor cortex followed such a pattern (Fig. 9B).
Figure 9.
Absolute magnitudes in hypothetical brain areas (Upper three graphs) during the five tracing conditions minus the control rest condition and in brain areas of interest (Lower three graphs) in areas with activations related to velocity (A and B), to unskilled performance (C and D), and to skilled performance (E and F).
This pattern does not follow skill level, however, since tracing the simple square, which was performed skillfully, was done quickly as well as slowly. For areas to be related specifically to level of skill, they would have to follow the hypothesized patterns shown in Fig. 9 C and E. For areas related to unskilled performance, activity would be expected to be highest in the Naive and Novel maze conditions, with lower levels in the other three conditions (in spite of their differences in velocity and stops). Areas related to skilled performance should show the opposite pattern: the three skilled conditions—Practiced, Square Fast, and Square Slow—should show higher activations than the other two conditions.
For unskilled behavior, all of the candidate regions identified earlier [right premotor (Fig. 9D), right parietal regions, and the left cerebellum] showed the activity profile for unskilled performance. For skilled behavior, only the SMA activation showed the appropriate activity profile (Fig. 9F). A more complete description of these results will be published elsewhere (H.v.M., L. W. Tempel, J. S. Perlmutter, M.E.R., and S.E.P., unpublished work).
As was observed in the verb generation task, there was a shift in activity between regions as subjects acquired skillful performance at the maze task. The switch was from initial high activation of right premotor and parietal cortex and left cerebellum, to high activation of SMA during skilled performance of the same task.
GENERAL DISCUSSION
From the similarities between the two experiments, as well as consistent results from several other learning studies in the imaging literature, it seems clear that changes in activation across areas may well be a common theme in learning paradigms (24–27). At first glance, these results were very surprising. The fact that the areas used to perform nominally the same task changed over the course of 15 min sounds a note of caution in interpreting the results, and it encourages more careful collection and assessment of performance measurements. Such changes have several implications, both methodological for the performance of imaging experiments and more theoretical for our understanding of the cognitive neuroscience of procedural learning and skill acquisition.
Methodologically, the idea that the use of different systems is skill dependent has some simple practical consequences for imaging experiments. First, this would often lead to the use of parallel pathways for different skill levels and violations of pure insertion (as described at length in the verbal learning study). As shown, this is not a problematic issue when data are completely analyzed, and appropriate control conditions are collected during imaging. More interestingly, there is the implication that practice may produce activation changes over the course of a single scan session, so that care must be taken either that subjects are imaged at a single level of skill or that such changes are accounted for in the analysis.
More conceptually, the body of results presented here, and those that exist in the imaging literature on learning as a whole, emphasize the importance of multiple processes in procedural learning or skill acquisition. Multiple-level, or process, models of skill learning, as has been advanced by many people over the years, are supported by these imaging results. These results do not map well onto the idea that skill acquisition takes place only through changes in synaptic efficiency within circuits used for naive performance of a task.
A major issue with these studies is whether these practice-related changes are related to skill acquisition at all. The tasks presented are item-specific and thus not nearly as “practiced” or as generalized as higher level skills such as language fluency or locomotion through the environment. We would like to make arguments that in both experiments, the results have relevance.
In the verbal learning experiment, even though the effect is item specific, the practice produces a switch in activation to the insula. The insula is the region that is used in the overlearned simple reading task. Given this observation, it seems unlikely that the activation of the insular region in practiced verb generation is simply related to item-based practice effects.
In the maze-learning task, we would argue that the task itself is item specific, so that a general skilled processor is much less likely to preexist for the performance of the task. Even so, the switch in activity is to a region that is highly active in the almost immediately learned Square Fast condition. Also, there is evidence that the information used is abstracted from the specific motor demands of the task. The observation that hand of performance does not affect the learning-related areas used for either skilled or unskilled performance argues that there is abstraction of the information used to perform the task. More recent behavioral studies showing that transfer of learning between hands is specific to the maze itself, and not to muscle agonist–antagonist relationships, furthers this idea (28).
One way of thinking about these results is in a “scaffolding-storage” framework. For unskilled effortful performance, a scaffolding set of regions is used to cope with the novel task demands (e.g., novel input–output relations, novel sequencing of behavior, etc.). After practice, processes or associations are more efficiently stored, and they can be accessed as rote programs or sets of programs in a way different from naive performance (8, 29). In this light, the idea that unskilled and skilled performance of a task in some sense represents performance of “different tasks” in a neurobiological sense maps on to our intuitions. The idea that different brain regions might then be involved does not seem so far-fetched.
Other conceptual distinctions can be made within this framework. In one scenario, both “scaffolding” and “storage” areas are active in parallel at all stages of learning, and what switches with practice is the balance of activity between the pathways. In the other scenario, one set of areas is essentially or exclusively active early, and when the task is overlearned passes the activity necessary to the performance of the task to other areas. In neither of our experiments does the activity in the “scaffolding regions” ever completely disappear, seeming more consistent with the parallel activity idea and the notions that control gradually shifts from scaffolding to storage areas and that both areas may contribute to the task, especially during intermediate skill levels. However, in neither experiment is the task truly overlearned (task performance does not reach asymptote for an extended period of time with only 10 min of practice). On the other hand, some evidence does exist in the verbal learning case for the complete transfer idea. In that case, simple reading (the overlearned control) actually appears to inhibit some of the verb generation “scaffolding” areas. At this point, either type of explanation seems plausible, and it may be that each is most relevant for different learning tasks.
Another interesting issue is the identification of specific processes represented in the scaffolding and storage regions. For this issue as well, many alternative explanations exist. For example, left frontal opercular activation at or near that seen in the verb generation task has been variously attributed to episodic encoding (30), (lexical) retrieval (31), semantic processing (12, 32, 33), willed generation (34), working memory for verbal material (35, 36), high-level phonological processing (17, 37–39), etc. On the storage side, the insular activation in the verbal learning task, and the SMA activation in the maze-learning task, may represent regions where specific information for the performance of the task is stored. SMA might be a likely candidate for the storage of the sequential (40, 41) and/or temporal aspects of a motor sequence (22, 23). Alternatively, SMA and insula may represent regions controlling access to that information, which might be stored elsewhere.
We believe that the observations of change in functional anatomy through practice provide an interesting foundation for understanding processing distinctions in learning. As can be seen from the incomplete outline of remaining issues, there is still much to be learned about the functional anatomy of skill learning.
ABBREVIATIONS
- SMA
supplementary motor area
- PET
positron-emission tomography
References
- 1.Fitts P M. In: Categories of Human Learning. Melton A W, editor. New York: Academic; 1964. pp. 243–285. [Google Scholar]
- 2.Rosenbaum D A. Human Motor Control. San Diego: Academic; 1991. [Google Scholar]
- 3.Cohen A, Ivry R I, Keele S W. J Exp Psychol Learn Mem Cognit. 1990;16:17–30. [Google Scholar]
- 4.Mishkin M, Petri H L. In: Neuropsychology of Memory. Squire L R, Butters N, editors. New York: Guilford; 1984. pp. 287–296. [Google Scholar]
- 5.Thompson R F. Science. 1987;238:1728–1730. [Google Scholar]
- 6.Robinson D A. J Neurophysiol. 1976;39:954–969. doi: 10.1152/jn.1976.39.5.954. [DOI] [PubMed] [Google Scholar]
- 7.Posner M I, Keele S W. J Exp Psychol. 1968;77:353–363. doi: 10.1037/h0025953. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum D A. In: Higher Brain Functions: Recent Explorations of the Brain’s Emergent Properties. Wise S P, editor. New York: Wiley; 1987. pp. 45–66. [Google Scholar]
- 9.van Mier H, Hulstijn W, Petersen S E. Acta Psychol. 1993;82:291–312. doi: 10.1016/0001-6918(93)90017-l. [DOI] [PubMed] [Google Scholar]
- 10.van Mier H, Hulstijn W. Acta Psychol. 1993;84:231–251. doi: 10.1016/0001-6918(93)90062-v. [DOI] [PubMed] [Google Scholar]
- 11.Petersen S E, Fox P T, Posner M I, Mintun M, Raichle M E. Nature (London) 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 12.Petersen S E, Fox P T, Posner M I, Mintun M, Raichle M E. J Cognit Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- 13.Fiez J A, Petersen S E. Psychol Sci. 1993;4:287–293. [Google Scholar]
- 14.Fiez J A, Raichle M E, Petersen S E. In: Developmental Dyslexia: Neural, Cognitive, and Genetic Mechanisms. Chase C, Rosen G, Sherman G F, editors. Baltimore: York; 1996. pp. 227–258. [Google Scholar]
- 15.Raichle M E, Fiez J A, Videen T O, MacLeod A K, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 16.Petersen S E, Fox P T, Snyder A Z, Raichle M E. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- 17.Fiez J A, Tallal P, Raichle M E, Miezin F M, Katz W F, Petersen S E. J Cognit Neurosci. 1995;7:357–375. doi: 10.1162/jocn.1995.7.3.357. [DOI] [PubMed] [Google Scholar]
- 18.Sternberg S. Acta Psychol. 1969;30:276–315. [Google Scholar]
- 19.Jonides J, Schumacher E H, Smith E E, Lauber E J, Awh E, Minoshima S, Koeppe R A. J Cognit Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- 20.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 21.Maarse F J, Janssen H J J, Dexel F. In: Computers in Psychology; Methods, Instrumentation, and Psychodiagnostics. Maarse F J, Mulder L J M, Sjouw W P B, Akkerman A E, editors. Amsterdam: Swets & Zeitlinger; 1988. pp. 133–139. [Google Scholar]
- 22.Tanji J, Mushiake H. Cognit Brain Res. 1996;3:143–150. doi: 10.1016/0926-6410(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 23.Rao S M, Harrington D L, Haaland K Y, Bobholz J A, Cox R W, Binder J R. J Neurosci. 1997;17:5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazziotta J C, Grafton S T, Woods R C. In: Brain Work and Mental Activity. Lassen N A, Ingvar D H, Raichle M E, Friberg L, editors. Vol. 31. Copenhagen: Munksgaard; 1991. pp. 280–290. [Google Scholar]
- 25.Grafton S T, Mazziotta J C, Presty S, Friston K J, Frackowiak R S J, Phelps M E. J Neurosci. 1992;12:2542–2548. doi: 10.1523/JNEUROSCI.12-07-02542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkins I H, Brooks D J, Nixon P D, Frackowiak R S J, Passingham R E. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jueptner M, Stephan K M, Frith C D, Brooks D J, Frackowiak R S J, Passingham R E. J Neurophysiol. 1997;77:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- 28.van Mier, H., Hunton, D. L. & Petersen, S. E. (1996) Cognit. Neurosci. Abstr. 144.
- 29.Rosenbaum D A, Kenny S B, Derr M A. J Exp Psychol. 1983;9:86–102. doi: 10.1037//0096-1523.9.1.86. [DOI] [PubMed] [Google Scholar]
- 30.Tulving E, Kapur S, Craik F I M, Habib R, Houle S. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckner R L, Petersen S E, Ojemann J G, Miezin F M, Squire L R, Raichle M E. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demb J B, Desmond J E, Wagner A D, Vaidya C J, Glover G H, Gabrieli J D E. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roskies A L, Fiez J A, Balota D A, Ojemann J G, Raichle M E, Petersen S E. Soc Neurosci Abstr. 1996;22:1110. [Google Scholar]
- 34.Frith C D, Friston K, Liddle P F, Frackowiak R S J. Proc R Soc London Ser B. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- 35.Fiez J A, Raife E A, Balota D A, Schwarz J P, Raichle M E, Petersen S E. J Neurosci. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J D, Forman S D, Braver T S, Casey B J, Servan-Schreiber D, Noll D C. Hum Brain Mapp. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 37.Zatorre R J, Evans A C, Meyer E, Gjedde A. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
- 38.Demonet J-F, Chollet R, Ramsay S, Cardebat D, Nespoulous J-L, Wise R, Rascol A, Frackowiak R. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 39.Paulesu E, Frith C D, Frackowiak R S J. Nature (London) 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 40.Tanji J, Mushiake H, Inase M. In: Brain Mechanisms of Perception and Memory. Ono T, Squire L R, Raichle M E, Perrett D I, Fukuda M, editors. New York: Oxford Univ. Press; 1993. pp. 464–472. [Google Scholar]
- 41.Tanji J, Shima K. Nature (London) 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]