Abstract
Tumor necrosis factor alpha (TNFα) and its two receptors (TNFR1 and 2) are known to stimulate dendritic cell (DC) maturation and T cell response. However, the specific receptor and mechanisms involved in vivo are still controversial. Here we show that in response to an attenuated mouse hepatitis virus (MHV) infection, DCs fail to mobilize and up-regulate CD40, CD80, CD86, and MHC class I in TNFR1−/− mice as compared to the wild-type and TNFR2−/− mice. Correspondingly, virus-specific CD8 T cell response was dramatically diminished in TNFR1−/− mice. Adoptive transfer of TNFR1-expressing DCs into TNFR1−/− mice rescues CD8 T cell response. Interestingly, adoptive transfer of TNFR1-expressing naïve T cells also restores DC mobilization and maturation and endogenous CD8 T cell response. These results show that TNFR1, not TNFR2, mediates TNFα stimulation of DC maturation and T cell response to MHV in vivo. They also suggest two mechanisms by which TNFR1 mediates TNFα-driven DC maturation: a direct effect through TNFR1 expressed on immature DCs and an indirect effect through TNFR1 expressed on naïve T cells.
Introduction
Initiation of a T cell response requires T cell-dendritic cell (DC) interactions through two sets of receptors: T cell receptor (TCR) recognition of antigen in the form of peptides presented by the major histocompatibility complex (MHC) molecules and binding of the co-stimulatory molecule CD28 on naïve T cells to its ligands CD80 (B7.1) and CD86 (B7.2) expressed on mature DCs (1, 2, 3). DCs are sentinels and normally reside in the tissues in an immature state without expressing CD80 and CD86 (5). Following microbial infection, immature DCs take up and process antigens to present them as peptides with MHC (5, 11). Immature DCs are also activated to express the co-stimulatory molecules through pathogen recognition receptors, such as toll-like receptors (TLR) (6), that recognize evolutionarily conserved microbial components or pathogen-associated molecular patterns (PAMPs) (7). By the time DCs migrate from the site of infection to the draining lymph nodes, they have matured, present antigenic peptides and express co-stimulatory molecules (4, 8). In the draining lymph node, the mature DCs activate cognate naïve T cells by providing two stimulating signals (8).
In addition to PAMPs, cytokines can also stimulate DC maturation and expression of CD80 and CD86 (9). Tumor necrosis factor α (TNFα) and its family member lymphotoxin α (LTα) are potent cytokines secreted by activated macrophages and T cells (10, 11, 12) and exerts pleiotropic effects on inflammation and immunity through two receptors: TNFR1 (p55) and TNFR2 (p75), which are ubiquitously expressed by most nucleated cells (13, 14). Treatment of DCs with TNFα, but not LTα, in vitro leads to up-regulation of surface expression of MHC class I, class II, CD80 and CD86 and an enhanced T cell stimulatory activity (12, 38). Transgenic expression of TNFα in DCs results in elevated levels of CD40, CD80 and ICAM-13 expression and induction of a stronger mixed lymphocyte reaction (15). In TNFα-deficient (TNFα−/−) mice, DC maturation is impaired and T cell response is diminished following infection with a replication deficient recombinant adenovirus (rAd) (16). Adoptive transfer of antigen-primed wild-type DCs into TNFα−/− mice rescues T cell response. Although in LTα−/− mice the number of DCs in the spleen was reduced, the observed deficiency is likely due to a lack of the membrane-bound LTα/β but not soluble LTα, that signals through LTβR (39, 40, 41, 42). Furthermore, the defective DC maturation and CD8 T cell responses in TNFα−/− mice suggest that LTα does not replace TNFα function in these processes. Together, these results suggest a critical role of TNFα, but not LTα, in DC maturation in vivo.
The role of TNFα/TNFR in DC maturation and T cell response, however, is more complex. In contrast to the diminished T cell response to rAd in TNFα−/− mice (16), following Mycobacterium infection, antigen-specific CD8 T cells, as identified by peptide-MHC tetramer staining, are significantly elevated at 14 and 27 days post infection (dpi) (17). Similarly, following acute or chronic infections with lymphocytic choriomeningitis virus (LCMV), while the magnitude of virus-specific CD8 T cell response is similar between wild-type and TNFR1−/− or TNFR2−/− mice 8 days post infection (dpi), the response is much greater in TNFR1−/− and TNFR2−/− double knockout mice (18, 19). Seven days post influenza virus infection, virus-specific CD8 T cell response is enhanced in TNFR2−/− mice (20). These apparently contradictory results of CD8 T cell responses to different infections in TNFα−/−, TNFR1−/−, TNFR2−/−, and TNFR1−/− and TNFR2−/− double knockout mice raise at least two questions: What is the likely cause contributing to the different effects of TNFα and TNFR on CD8 T cell responses to different infections in knockout mice? Through which receptor does TNFα mediate DC maturation in vivo? Consistent with a much lesser important role of LTα in DC maturation, LTα−/− mice make delayed but effective CD8 T cell response to influenza virus infection (43) and the defective CD8 T cell responses to LCMV likely result from abnormal lymphoid architecture (44).
Further complicating the delineation of the role of TNFα/TNFR in DC maturation is the observation that T cells are required for efficient DC maturation, including CD80 and CD86 expression. In RAG1−/− mice, which are deficient in T cells, the number of DCs is significantly reduced, and the residual DCs are defective in expressing CD80 and CD86 and activating naïve T cells (21). Both the deficiency in DC number and function is restored by adoptive transfer of naïve T cells into the RAG1−/− mice (22). Recently, B7-H1 (PD-L1) expressed by naïve T cells is shown to condition immature DCs to undergo efficient maturation following stimulation by influenza virus (23). Given the complex effects of TNFα/TNFR on CD8 T cell responses, it is possible that TNFα or TNFRs may affect DC maturation and T cell response through multiple pathways.
In this study, we have constructed an attenuated mouse hepatitis virus A59 (MHV-A59) that infects mice but does not cause apparent liver damage. By comparing DC maturation and CD8 T cell response in wild-type, TNFR1−/− and TNFR2−/− mice following infection with the attenuated MHV-A59, we show that CD8 T cell response is impaired in the absence of TNFR1, but not TNFR2. The impaired CD8 T cell response is associated with an impaired DC maturation and mobilization and can be corrected by adoptive transfer of wild-type DCs into TNFR1−/− mice. Furthermore, DC mobilization and maturation and CD8 T cell response in TNFR1−/− mice are also restored by adoptive transfer of TNFR1-expressing naïve T cells. These findings suggest that TNFR1 mediates DC maturation through two mechanisms: a direct effect through TNFR1 expressed on immature DCs and an indirect effect through TNFR1 expressed on naïve T cells. Our results also suggest a possible explanation for the divergent effects of TNFα and TNFR deficiencies on CD8 T cell responses to different infections.
Materials and Methods
Viruses, mice and infection
Wild-type MHV-A59, referred to as A59/WT, was engineered to express eGFP, ISQAVHAAHAEINEAGR (OVA323–339) and SIYRYYGL (SIY), referred to as RA59/GOS. The recombinant RA59/GOS was constructed by replacing ORF4 in A59/WT with a sequence coding for eGFP-OVA-SIY fusion protein through targeted RNA recombination (24, 33). Briefly, a 90 bp DNA fragment containing OVA-SIY sequences was cloned in frame into the 3′ end of eGFP. The DNA fragment encoding eGFP-OVA-SIY fusion protein was then cut out and cloned into pMH54 plasmid (25, 33) via Sal I and Not I sites, referred to as pMH54-eGFP-OVA-SIY. In vitro transcription using pMH54-eGFP-OVA-SIY plasmid as a template led to production of corresponding RNA that was flanked by A59/WT sequences. To construct RA59/GOS virus, AK-D cells were infected for 4 hrs with feline MHV (fMHV) (25, 33), which is a recombinant MHV-A59 containing the ectodomain of the feline infectious peritonitis virus spike glycoprotein in place of the A59/WT spike protein. The infected AK-D cells were electroporated with in vitro transcribed RNA for eGFP-OVA-SIY and plated on a layer of murine 17Cl-1 cells. Targeted recombination between fMHV and in vitro transcribed RNA led to generation of recombinant MHV where ORF4 of A59/WT was replaced by eGFP-OVA-SIY. The correct recombinant RA59/GOS was identified by eGFP expression and sequencing, and plaque purified twice on 17Cl-1 cells. Both A59/WT and RA59/GOS viruses were amplified and titrated by plaque assay in 17Cl-1 cells (26). MHV-A59, fMHV, pMH54 plasmid, 17Cl-1 and AK-D cell lines are all kindly provided by Dr. Ralph Baric (Chapel Hill, USA).
C57BL/6 (B6) mice were purchased from Vitalriver (Beijing, China). TNFR1−/− mice on a mixed genetic background of 129/Sv/Ev and B6 were kindly provided by Dr. Horst Bluethmann (27). TNFR2−/− mice were obtained from the Jackson Laboratory (Bar Harbor, USA). Both strains of mice were backcrossed for more than 18 generations onto the B6 background. Thy1.1+ B6 mice were a gift from Dr. Shengdian Wang. Mice were housed under specific-pathogen-free conditions in the animal facilities at the Institute of Biophysics, Chinese Academy of Sciences. All mouse work was performed according to the guidelines of the Animal Ethics and Experimentation Committee of the Institute of Biophysics. Every batch of mice used in this study was sampled to ensure no prior MHV infection (i.e. negative for anti-MHV antibody in the serum). Mice at 6–8 weeks of age were infected with A59/WT or RA59/GOS virus intraperitoneally (i.p.) in 500 μl phosphate-buffered saline (PBS).
Antibodies, tissue preparation and flow cytometry
Mice were sacrificed and perfused with 5 ml of PBS. Spleens were removed and gently homogenized through a nylon filter with a syringe plunger in RPMI 1640 medium supplemented with 2% fetal calf serum (FCS). Red blood cells in splenocyte suspension were lysed with 144 mM ammonium chloride and 17 mM Tris-HCl, pH7.4 solution. Single-cell suspension from liver was obtained by cutting liver tissue into pieces followed by 1mg/ml collagenase IV digestion at 37°C for 30 minutes. The lysate was centrifuged at 50g for 1 minute to remove debris, re-suspended in 5 ml of 40% Percoll solution and then layered over 5 ml of 70% Percoll solution and centrifuged at 1500 g for 20 minutes without break at room temperature. Cells were removed from the interface, washed, and counted by trypan blue exclusion.
All antibodies were purchased as direct conjugates. Anti-CD3-FITC, CD8-APC, CD40-PE, CD80-PE, CD86-PE, and H-2Kb:Ig fusion protein-PE were from BD Biosciences. B220-FITC, Gr-1-FITC, CD11c-FITC and Thy1.1 (CD90.1)-FITC were from eBiosciences. To load SIY peptide, SIY and H-2Kb:Ig fusion protein at a molar ratio of 40:1 were incubated in 37°C for 6 hours. The SIY-Kb was used within one week. For staining, 0.5–2 × 106 cells from spleen and liver were incubated with anti-CD16/32 (Fc blocker) at 4°C for 5 minutes and then incubated with a cocktail of appropriate antibodies at 4°C for 30 minutes in PBS supplemented with 2% FCS and 0.1% azide. Dead cells were excluded by 7AAD staining. Stained cells were analyzed on a FACSCalibur™ flow cytometer (BD Biosciences). Data analysis was carried out using FlowJo™ software (Tree Star, Inc., Ashland, OR).
For intracellular IFNγ and TNFα staining, splenocytes and intrahepatic lymphocytes (1 × 106) were stimulated with or without SIY peptide (1 μg/ml) for 5h at 37°C in the presence of 10U of human recombinant IL-2 and brefeldin A (Golgiplug; PharMingen) in a total volume of 200 μl of RPMI 1640 medium supplemented with 10% FCS. As positive controls, cells were stimulated by PMA and ionomycin in the presence of brefeldin A. As negative control, cells were cultured in medium only with brefeldin A. Cells were stained for surface expression of CD8 and SIY-Kb dimer, then were fixed and permeabilized with the Cytofix/Cytoperm kit (PharMingen) and stained with APC-conjugated monoclonal rat anti-mouse IFNγ antibody or FITC-conjugated monoclonal rat anti-mouse TNFα antibody. Cells were analyzed with a FACSCalibur cytometer.
Differentiation and transfer of dendritic cells
Bone marrow cells from B6 mice were cultured at 4 × 105 cells per ml in RPMI 1640 supplemented with 10% FCS, β-mercaptoethanol, L-glutamine, streptomycin, penicillin and 20ng/ml GM-CSF in 6-well plates (28). At day 2 and 4, half of the culture medium was replaced with fresh medium. At day 7, non-adherent and loosely adherent cells were collected by gentle pipetting, and centrifuged at 400g for 5 min. Cells were counted by trypan blue exclusion and analyzed for CD11c expression (~90% CD11c+) by flow cytometry. For adoptive transfer, 8 × 105 cells in 200μl of PBS were administrated per mouse by tail vein injection.
Purification and transfer of T cells
Total T cells were purified from lymph nodes of B6 mice (Thy1.1+) by negative depletion using FITC-conjugated anti-CD11c, B220, or Gr-1 antibodies followed with anti-FITC magnetic beads (Miltenyi Biotech). CD4+ and CD8+ T cells were purified from lymph nodes of B6 mice by positive selection using anti-CD4 or anti-CD8 conjugated magnetic beads. Purified cells were counted and analyzed for CD3 by flow cytometry. Purified T cells (~95% CD3+) were injected intravenously into Thy1.2+ TNFR1−/− mice (5–8 × 106 per recipient in 200μl of PBS).
Statistical analyses
Statistical significance between groups was assessed by Student t test using Prism 5 software (GraphPad Software). A P-value of <0.05 was considered significant.
Results
Construction and characterization of recombinant MHV
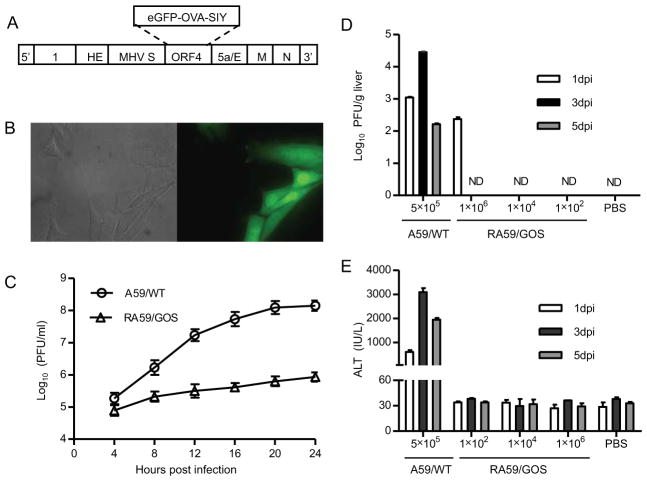
Although a CD8 epitope, RCQIFANI (29), has been identified in wild-type MHV-A59 (A59/WT), its binding to MHC class I Kb is weak. Kb tetramer loaded with RCQIFANI failed to stain CD8 T cells in the spleen of MHV infected C57BL/6 (B6) mice (data not shown). To facilitate studying T cell responses to A59/WT, we constructed a recombinant MHV-A59 expressing eGFP fused to a CD4 epitope, ISQAVHAAHAEINEAGR (OVA323–339), and a CD8 epitope, SIYRYYGL (SIY). The recombinant MHV, referred to as RA59/GOS, was constructed by targeted RNA recombination replacing a non-essential gene, open reading frame 4 (ORF4), with sequence encoding eGFP-OVA-SIY fusion protein (Fig. 1A, see Materials and Methods). Recombinant virus was selected via eGFP-positive cells (Fig. 1B) and further plaque purified.
FIGURE 1. Construction and characterization of recombinant MHV-A59 expressing an eGFP-OVA-SIY fusion protein.
(A) Schematic diagram of recombinant MHV virus. Targeted RNA recombination was used to replace ORF4 of MHV-A59 with a sequence encoding eGFP-OVA-SIY fusion protein. The eGFP-OVA-SIY fusion gene was first cloned into pMH54 plasmid via Sal I and Not I sites, and then transcribed into RNA for recombination with feline MHV in AK-D cells. See Materials and Methods for details.
(B) eGFP fluorescence of RA59/GOS infected cells. 17Cl-1 cells were seeded on cover glass in 6-well plate followed by RA59/GOS infection at MOI of 1. Eight hours post infection, cells were fixed and visualized by fluorescence microscopy. Bright field and fluorescent images of the same area are shown.
(C) Comparison of replication of wild-type and recombinant MHV-A59 in cultured cells. 17Cl-1 cells were infected in triplicates with A59/WT or RA59/GOS at MOI of 1. Culture supernatants were harvested every 4 hrs and assayed for virus titer by plaque assay. The mean virus titer ± standard deviation (SD) of triplicate samples is shown. Representative results from one of three experiments are shown.
(D, E) Comparison of virus titers and ALT levels in B6 mice infected with A59/WT or RA59/GOS. B6 mice were inoculated i.p. with A59/WT (5×105 pfu/mouse) or the indicated doses of RA59/GOS. PBS injected mice were used as control. At 1, 3 and 5 dpi, sera and livers were harvested for assaying ALT levels and virus titers, respectively. Virus titers (D) and ALT levels (E) are shown as mean ± SD of 5–6 mice per group. ND, not detectable. Representative results from one of three experiments are shown.
The recombinant RA59/GOS virus was further characterized for its replication in vitro and infection in mice. Murine fibroblasts 17Cl-1 were inoculated with 5 × 105 pfu of either A59/WT or RA59/GOS (MOI=1) and virus titer in the culture supernatants was measured every 4 hrs for the next 24 hrs. The titer of A59/WT increased steadily, reaching a peak level of 108 pfu/ml around 20 hrs post infection (Fig. 1C). In contrast, the titer of RA59/GOS only reached 106 pfu/ml 24 hrs post infection, indicating a reduction of replication capacity by approximately 100-fold. Furthermore, B6 mice were inoculated intraperitoneally (i.p.) with A59/WT or RA59/GOS and virus titers were measured in the liver 1, 3, and 5 days post infection (dpi). Following inoculation with 5 × 105 pfu of A59/WT, virus was readily detected in the liver at all three time points, peaking at 3 dpi (Fig. 1D). In contrast, RA59/GOS virus was detected in the liver of inoculated mice only at 1 dpi and only when 1 × 106 pfu was used for the inoculation. No virus was detected at 3 or 5 dpi or when lower doses of virus were inoculated. Correlating with virus replication, elevated levels of serum alanine aminotransferase (ALT) were detected in B6 mice infected with A59/WT virus at 1, 3, and 5 dpi (Fig. 1E), whereas no elevated ALT level was detected in RA59/GOS inoculated mice at any day or virus doses. Taken together, these results suggest that RA59/GOS is an attenuated virus with dramatically reduced capacity to replicate in cultured cells and mice.
RA59/GOS induces CD8 T cell responses in a dose dependent manner
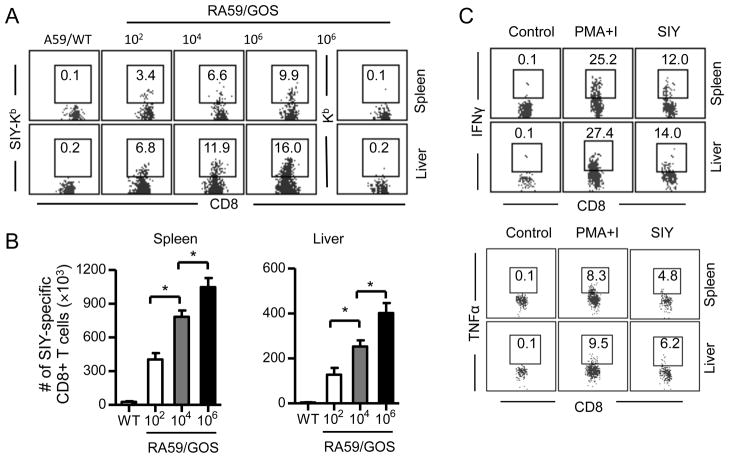
To study CD8 T cell response to MHV infection, we inoculated B6 mice with 102, 104 or 106 pfu of RA59/GOS. Seven dpi, SIY-specific CD8 T cells were identified in the spleen and liver by staining with anti-CD8 antibody and a H-2Kb:Ig fusion protein, the Kb component of which was loaded with SIY peptide (SIY-Kb), followed by flow cytometry. When mice were infected with A59/WT virus, a background level (0.1–0.2%) of SIY-positive CD8 T cells was detected in the spleen and liver (Fig. 2A). Similar background level of SIY-positive CD8 T cells was detected in the spleen and liver of RA59/GOS infected mice when H-2Kb:Ig fusion protein used for staining was not loaded with SIY peptide (Fig. 2A far right panel). In contrast, the frequency of SIY-positive CD8 T cells was increased at least 10-fold over the background level in the spleen and liver of RA59/GOS infected mice that were stained with both anti-CD8 and SIY-Kb. Notably, the frequency of SIY-Kb-positive CD8 T cells increased with the increasing inoculation virus doses. Similarly, the total numbers of SIY-positive CD8 T cells in the spleen and liver increased with the increasing inoculation virus doses (Fig. 2B). In addition, a significant fraction of SIY-Kb-positive CD8 T cells from both the spleen and liver was induced to express IFNγ or TNFα by SIY peptide in vitro (Fig. 2C). These results suggest that attenuated RA59/GOS virus can cause infection in mice but probably does not replicate significantly, resulting in functional CD8 T cell responses proportional to the doses of inoculating virus.
FIGURE 2. RA59/GOS induces CD8 T cell responses in a dose dependent manner.
Groups of B6 mice were infected i.p. with different doses of RA59/GOS virus or with 5 × 105 pfu of A59/WT virus. Seven dpi, cells from spleen and liver were enumerated and analyzed for CD3, CD8, SIY-Kb and 7AAD (A and B). Alternatively, cells were stimulated with SIY peptide or PMA plus ionomycin (PMA+I) or without any stimulation (Control) for 5 hrs and stained for CD8, SIY-Kb and intracellular IFNγ or TNFα (C). Kb indicates the same stains except that H-2Kb:Ig fusion protein was not loaded with SIY peptide. (A) Representative SIY-Kb (or Kb) versus CD8 staining profiles of CD3+ CD8+ live cells (7AAD−) from spleen and liver are shown. The number indicates percentage of SIY-Kb-positive cells among CD8+ cells. (B) Comparison of mean ± SD of SIY-Kb+ CD8+ cells in the spleen (left panel) and liver (right panel) of 4 mice per group. Data from one of two similar experiments are shown. * indicates p value of <0.05. (C) Intracellular staining of IFNγ and TNFα gating on SIY-Kb+ and CD8+ T cells in the spleen and liver. Representative data from two independent experiments are shown.
Defective CD8 T cell response to RA59/GOS in TNFR1-deficient mice
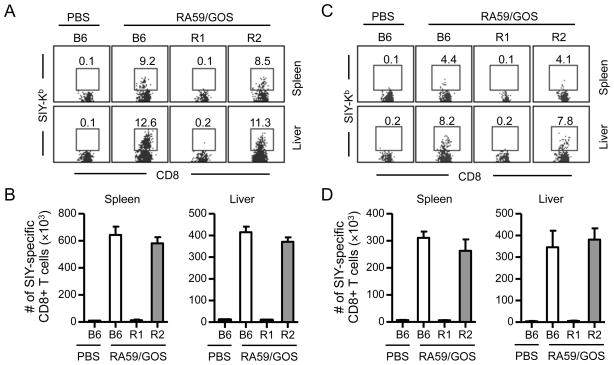
To investigate the effect of TNFR in CD8 T cell response to MHV, we compared SIY-specific CD8 T cell responses to RA59/GOS among B6 mice, B6 mice deficient in TNFR1 (TNFR1−/−), and B6 mice deficient in TNFR2 (TNFR2−/−). Seven dpi similar frequency and number of SIY-specific CD8 T cells were detected in the spleen and liver of B6 and TNFR2−/− mice (Fig. 3A and B). However, only background level of SIY-specific CD8 T cells was detected in the spleen and liver of TNFR1−/− mice.
FIGURE 3. Defective CD8 T cell response to RA59/GOS in TNFR1-deficient mice.
(A, B) B6, TNFR1−/− (R1) and TNFR2−/− (R2) mice were inoculated i.p. with 1 × 106 pfu of RA59/GOS or the same volume of PBS. Seven dpi, cells from spleen and liver were enumerated and analyzed for CD3, CD8, SIY-Kb and 7AAD as in Fig. 2. (A) Representative SIY-Kb versus CD8 staining profiles of CD3+ CD8+ live cells from spleen and liver. (B) Comparison of mean ± SD of SIY-Kb+ CD8+ cells in the spleen and liver of 4 mice per group. Combined data from two experiments are shown.
(C, D) B6, TNFR1−/− and TNFR2−/− mice were infected and analyzed as in A and B, except analysis was done 11 dpi. Data shown are from 3–4 mice per group.
To exclude the possibility that CD8 T cell response in TNFR1−/− mice was delayed, the frequency and total number of SIY-specific CD8 T cells were measured in the spleen and liver 11 dpi. Again, while similarly robust SIY-specific CD8 T cell responses were detected in the spleen and liver of B6 and TNFR2−/− mice, no significant SIY-specific CD8 T cell response was detected in TNFR1−/− mice (Fig. 3C and D). These results show that TNFR1, but not TNFR2, is required for efficient CD8 T cell response to MHV infection.
Impaired DC mobilization and maturation in TNFR1-deficient mice following RA59/GOS infection
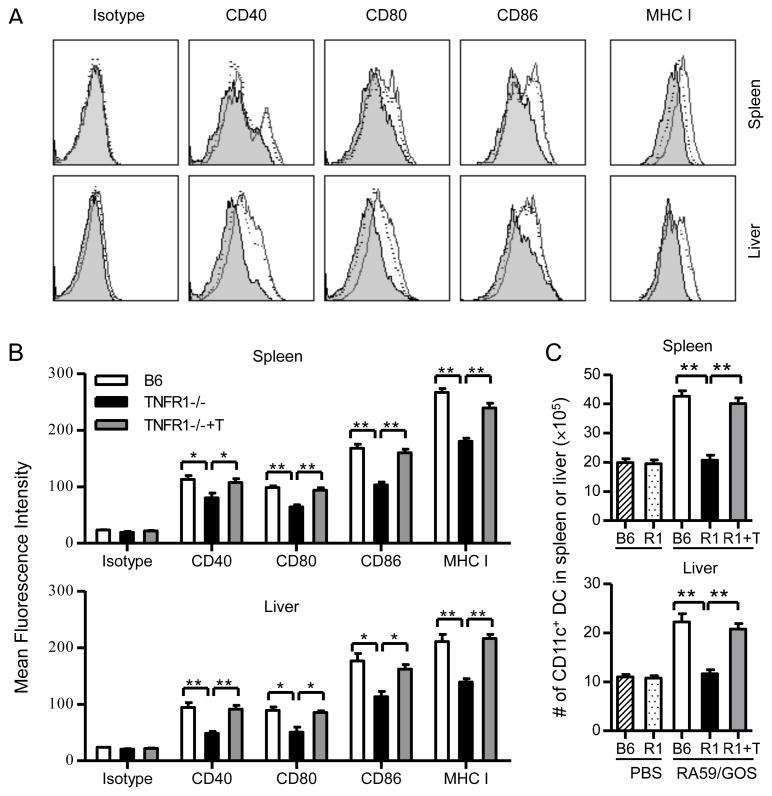
Because of the critical role of DCs in mediating CD8 T cell responses, we determined DC numbers and maturation status in TNFR1−/− mice following RA59/GOS infection. Three dpi, cells from spleen and liver of B6 and TNFR1−/− mice were enumerated and analyzed for CD11c plus CD40, CD80, CD86 or MHC class I. Compared to CD11c+ cells from spleen or liver of B6 mice, a significantly lower percentage of CD11c+ cells from spleen and liver of TNFR1−/− mice expressed CD40, CD80, CD86 or class I (Fig. 4A and B, p<0.05). More dramatically, while the number of CD11c+ DCs was similar in the spleen or liver between PBS-injected B6 and TNFR1−/− mice (Fig. 4C), the number of CD11c+ DCs was increased approximately 2 fold in both spleen and liver of B6 mice following RA59/GOS infection. However, the number of CD11c+ DCs was not increased at all in the spleen and liver of TNFR1−/− mice following RA59/GOS infection. Thus, in the absence of TNFR1, DC mobilization and maturation are impaired following RA59/GOS infection.
FIGURE 4. DC maturation and mobilization is impaired in TNFR1-deficient mice and the impaired DC response is restored by adoptive transfer of TNFR1-positive T cells.
B6 mice, TNFR1−/− mice and TNFR1−/− mice that were transferred with purified CD3+ T cells (TNFR1−/−+T or R1+T) one day earlier were inoculated with 1 × 106 pfu of RA59/GOS virus or the same volume of PBS. Three days later, cells from spleen and liver were enumerated and analyzed for CD11c plus CD40, CD80, CD86 or MHC class I. (A) Comparison of CD40, CD80, CD86 and MHC I expression by CD11c+ cells from B6 mice (histograms with solid lines), TNFR1−/− mice (shaded histograms), and TNFR1−/− mice injected with T cells (histograms with dotted lines). (B) Comparison of mean fluorescence intensity (MFI) of CD40, CD80, CD86, and MHC I by CD11c+ cells from the spleen (upper panel) or liver (lower panel) from B6 mice, TNFR1−/− mice, and TNFR1−/− mice injected with T cells. (C) Comparison of the total numbers of CD11c+ cells in the spleen (upper panel) and liver (lower panel) of B6 mice, TNFR1−/− mice (R1), and TNFR1−/− mice injected with T cells (R1+T). * p< 0.05; ** p< 0.01.
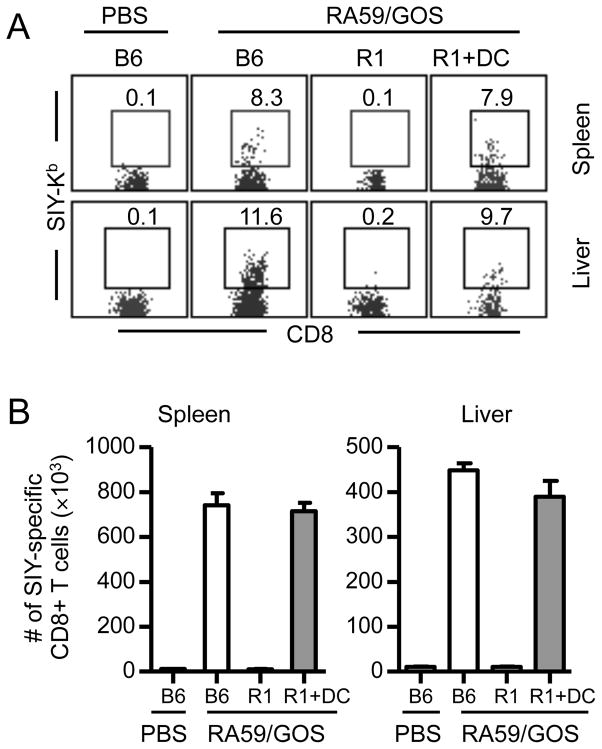
Next, we determined whether SIY-specific CD8 T cell response to RA59/GOS infection can be rescued by transfer of TNFR1-expressing DCs into TNFR1−/− mice. GM-CSF induced bone marrow DCs from B6 mice were adoptively transferred into TNFR1−/− mice, followed by infection with RA59/GOS and analysis at 7 dpi. Without DC transfer, virtually no SIY-specific CD8 T cells were detected in the spleen or liver of TNFR1−/− mice. With DC transfer, SIY-specific CD8 T cells were detected in the spleen and liver of TNFR1−/− mice (Fig. 5). Statistically, there was no difference in both the frequency and the number of SIY-specific CD8 T cells in the spleen or liver between B6 mice and TNFR1−/− mice that were transferred with DCs. These results show that the impaired DC maturation and mobilization is a major factor contributing to the defective CD8 T cell response to RA59/GOS infection in TNFR1−/− mice.
FIGURE 5. Rescue of CD8 T cell response in TNFR1-deficient mice by adoptive transfer of TNFR1-expressing DCs.
Bone marrow cells from B6 mice were cultured in the presence of GM-CSF for 7 days. DCs (75% CD11c+) were injected intravenously into TNFR1−/− mice (R1+DC, 8 × 105 per recipient). One day later, mice were infected with 1 × 106 pfu of RA59/GOS virus and the frequency and the number of SIY-specific CD8 T cells were analyzed in the spleen and liver at 7 dpi as in Fig. 2. (A) Representative SIY-Kb versus CD8 staining profiles of CD3+ CD8+ cells from spleen and liver. (B) Comparison of mean ± SD of SIY-Kb+ CD8+ cells in the spleen and liver of 4 mice per group. Data shown are from one of two independent experiments.
Transfer of TNFR1-expressing T cells also rescues endogenous CD8 T cell response to RA59/GOS in TNFR1-deficient mice
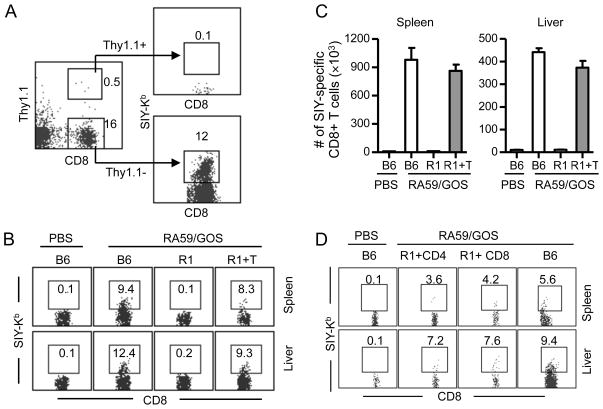
We also tested whether TNFR1-expressing T cells can rescue CD8 T cell response to RA59/GOS infection in TNFR1−/− mice. Total T cells were purified from lymph nodes of Thy1.1+ B6 mice. Purified Thy1.1+ T cells (>95% CD3+) were transferred into TNFR1−/− mice on Thy1.2+ background (5 × 106 cells per recipient), followed by RA59/GOS infection and analysis for Thy1.1, CD8 and SIY-Kb at 7 dpi. In both spleen and liver, less than 0.5% of live cells were transferred Thy1.1+ T cells (Fig. 6A). As expected, without T cell transfer, the frequency and number of SIY-specific endogenous (Thy1.1−) CD8 T cells were minimal in the spleen or liver of TNFR1−/− mice. With T cell transfer, significant levels of SIY-specific Thy1.1−CD8 T cells were detected in the spleen and liver of TNFR1−/− mice (Fig. 6B, C). Statistically, there was no difference in both the frequency and the number of SIY-specific Thy1.1− CD8 T cells in the spleen or liver between B6 mice and TNFR1−/− mice transferred with T cells. Furthermore, when purified CD4 and CD8 T cells were transferred separately into TNFR1−/− mice, endogenous CD8 T cell responses to MHV were also elevated to similar levels as in B6 mice (Fig. 6D). These results show that transfer of TNFR-expressing T cells into TNFR1−/− mice also restores the endogenous CD8 T cell response to RA59/GOS infection.
FIGURE 6. Rescue of CD8 T cell response in TNFR1-deficient mice by adoptive transfer of TNFR1-expressing T cells.
Total, CD4+ and CD8+ T cells (>95% CD3+) were purified from lymph nodes of B6 mice (Thy1.1+) and injected intravenously into TNFR1−/− mice (R1+T, 5–8 × 106 per recipient). One day later, mice were infected with 1 × 106 pfu of RA59/GOS virus and 7 dpi cells from spleen and liver were enumerated and analyzed for Thy1.1, CD8, and SIY-Kb. (A) Representative Thy1.1 versus CD8 staining profiles of live cells from liver. Note, very few transferred T cells (Thy1.1+) were positive for SIY-Kb. (B) Representative SIY-Kb versus CD8 staining profiles of endogenous (Thy1.1−) CD8+ cells from spleen and liver as gated in A. The number indicates percentage of cells in the gated regions. R1, TNFR1−/− mice; R1+T, TNFR1−/− mice transferred with purified total T cells. (C) Comparison of mean ± SD of SIY-Kb+ CD8+ Thy1.1− endogenous T cells in the spleen and liver of B6 mice, TNFR1−/− mice and TNFR1−/− mice transferred with total T cells. (D) Representative SIY-Kb versus CD8 staining profiles of endogenous (Thy1.1−) CD8+ cells from spleen and liver as gated in A. The number indicates percentage of cells in the gated regions. R1+CD4, TNFR1−/− mice transferred with purified CD4 T cells; R1+CD8, TNFR1−/− mice transferred with purified CD8 T cells. Data shown are from one of two independent experiments with 4 mice per group per experiment.
TNFR1-expressing T cells restore DC mobilization and maturation in TNFR1-deficient mice
We further determined whether DC mobilization and maturation in TNFR1−/− mice were restored by adoptive transfer of TNFR1-expressing T cells. Purified Thy1.1+ T cells were transferred into TNFR1−/− mice (5 × 106 cells per recipient), followed by RA59/GOS infection and analysis for CD11c, CD40, CD80, CD86 and MHC I at 3 dpi. As shown in Fig. 4A and B, DCs from T cell-transferred TNFR1−/− mice up-regulated CD40, CD80, CD86 and MHC I to the similar levels as those in B6 mice. In addition, the numbers of DCs in the spleen and liver were restored to the similar levels as those in B6 mice (Fig. 4C). These results show that transferred T cells rescue the virus-specific CD8 T cell response in TNFR1−/− mice through DC activation and mobilization.
Discussion
Studies have shown a critical role of TNFα in DC maturation both in vitro and in vivo (12, 15, 16, 38). Although LTα also binds to TNFR1 and TNFR2, its effect on DC accumulation and/or homeostasis in the spleen is primarily through signaling via LTβR by membrane LTα/β but not soluble LTα (39, 40, 41, 42). Consistently, LTα−/− mice produce delayed but effective CD8 T cell responses to influenza virus infection (43) and the defective CD8 T cells responses to LCMV likely result from abnormal lymphoid architecture (44). Furthermore, the defective DC maturation and CD8 T cell responses in TNFα−/− mice suggest that LTα does not replace TNFα function in these processes. These findings suggest that signaling through TNFR by soluble LTα is unlikely critical for DC maturation or CD8 T cell responses. Nevertheless, because LTα−/− mice lack both soluble LTα and membrane LTα/β and because there is no reagent for selectively blocking soluble LTα, current studies have not conclusively ruled out an essential involvement of soluble LTα in DC maturation and CD8 T cell responses.
Results presented here reveal two distinct mechanisms by which TNFR stimulates DC maturation and initiates T cell response following MHV infection. First, our findings suggest that TNFα can stimulate DCs maturation in vivo directly through TNFR1 expressed on immature DCs. Although maturation of DCs is impaired in TNFα−/− mice following infection with a replication defective rAd (16), which receptor mediates TNFα’s effect on DC maturation in vivo was not identified. Our observation that DC maturation and CD8 T cell response are impaired in TNFR1−/−, but not TNFR2−/−, mice suggests that it is TNFR1 that mediates DC maturation in vivo. Our findings are consistent with and further extend the studies showing that TNFα stimulates DC maturation in vitro via TNFR1 (30). Furthermore, because of complex cell-cell interactions in vivo, previous studies did not address whether TNFα can directly stimulate DC maturation through TNFRs expressed on immature DCs. For example, T cell response to rAd in TNFα−/− mice is restored by adoptive transfer of antigen primed mature wild-type DCs. Because TNFα is secreted primarily by activated macrophages and T cells (10, 11, 12), the transferred DCs is unlikely to secrete TNFα to activate maturation of endogenous DCs to restore T cell response. It is more likely that the transferred DCs bypass endogenous DCs and directly activate T cell response. Like TNFα−/− mice, TNFR1−/− mice have normal numbers of DCs in the spleen and liver and normal T cell and macrophage development. Unlike TNFα−/− mice, TNFα is still present in TNFR1−/− mice. Because only the transferred DCs can respond to TNFα in TNFR1−/− mice, restoration of CD8 T cell response in TNFR1−/− mice by adoptive transfer of bone marrow derived wild-type DCs (not antigen-primed) suggests that the transferred DCs can respond directly to TNFα in TNFR1−/− mice. Together, these results suggest that in vivo TNFα also directly stimulates DC maturation through TNFR1 expressed on immature DCs.
Second, our results reveal an alternative T cell-dependent pathway by which TNFα may stimulate DC maturation and CD8 T cell response in vivo. We found that in TNFR1−/− mice DC mobilization and maturation and CD8 T cell response to the attenuated MHV are also restored by adoptive transfer of TNFR1-expressing naïve T cells. Because in these mice, only the transferred T cells can respond to TNFα, restoration of DC maturation and mobilization and response by endogenous CD8 T cells must go through the transferred T cells. Macrophages play a critical role in the clearance of MHV (31). It can be envisioned that TNFα secreted by activated macrophages could stimulate the transferred naïve T cells, resulting in expression of molecules that can in turn stimulate DC mobilization and maturation. For example, CD40L is expressed by activated T cells and can engage CD40 expressed on immature DCs to stimulate DC maturation (32). Nevertheless, the molecular mechanism mediating the T cell-dependent pathway of DC maturation and mobilization has yet to be elucidated.
Our results also provide a possible explanation for the apparently contradictory results of CD8 T cell responses to different infections in TNFα−/−, TNFR1−/−, TNFR2−/−, and TNFR1−/− and TNFR2−/− double knockout mice (16, 18, 22). The recombinant RA59/GOS was constructed by replacing a 283 bp fragment of ORF4 with eGFP-OVA-SIY. The resulting virus is attenuated based on the observation that the virus titer was reduced over 100 fold in both 17C1-1 cells and mice when compared to the wild-type MHV-A59 virus (Figure 1B, C). In addition, the duration of virimia was reduced to the first day post infection with RA59/GOS whereas virimia was detected for three days post infection with RA59/WT (Figure 1C). Consistent with the minimal viral replication, no serum ALT was detected following inoculation of RA59/GOS. However, virus infection and limited translation occur because CD8 T cell response to SIY can be detected in a dose-dependent manner (Figure 2). Das Sarma et al have reported a recombinant MHV-A59 in which ORF4 was replaced with GFP. The resulting virus replicated similarly as wild-type A59 in vitro (33). However, Sperry et al reported that single-amino-acid substitutions in ORF1b-nsp14 and ORF2a of the mouse hepatitis virus are attenuating in vivo (34). The difference between the two recombinant viruses is the addition of OVA-SIY sequences to the GFP sequences in our virus, resulting in an insert of 860 bp fragment in our virus versus an insert of 720 bp in Das Sarma’s virus. As the replaced fragment of ORF is only 283 bp, the increased insert size could have interfered with virus replication, resulting in an attenuated RA59/GOS.
It is notable that T cell response is impaired in TNFα−/− mice following infection with a replication defective rAd (16) and in TNFR1−/− mice following infection with an attenuated MHV. In contrast, T cell responses were all enhanced in TNFα−/− or TNFR−/− mice following acute or chronic infection with replication competent pathogens, such as LCMV, influenza virus and Mycobacterium (16, 17, 18, 19, 20). Studies have shown that DCs can be stimulated to mature independent of TNFα (35), probably through direct interaction between microbial components and TLRs (6) or other pathogen recognition receptors (36, 37). The TNFα-independent pathway of DC maturation is more likely to occur when pathogen can replicate, whereas the replication defective or highly attenuated pathogens may not produce sufficient amount of microbial components to directly engage TLRs and other pathogen recognition receptors to active DCs directly. Thus, T cell response is selectively impaired in TNFα−/− or TNFR1−/− mice only in response to infection with replication defective rAd or highly attenuated MHV. It is also possible that different pathogens, such as MHV, rAd, LCMV, influenza virus and Mycobacterium express different microbial components, infect different cell types in mice and have different replication cycles; these differences may also contribute to the observed different outcomes of CD8 T cell responses in TNFα- or TNFR-deficient mice.
In summary, our results show that in vivo TNFR1 mediates TNFα-dependent DC maturation either through a direct binding of TNFα to TNFR1 on immature DCs or through an indirect T cell-dependent pathway. The divergent CD8 T cell responses to different infections in TNFα-and TNFR-deficient mice may also relate to the pathways by which DCs are activated.
Acknowledgments
We thank Dr. Ralph Baric for kindly providing MHV-A59, fMHV, pMH54 plasmid, 17Cl-1 and AK-D cell lines and for the advice with generating the recombinant MHV, Dr. Shengdian Wang for kindly providing Thy1.1 mice, and members of the Deng laboratory for helpful discussion.
Work in the authors’ laboratories was supported by grants from the Ministry of Science and Technology of China (2009CB522505, 2006CB910901 and 2006CB504304) and NIH AI69208 (to JC).
Abbreviations used in this paper
- DCs
dendritic cells
- TNFR1
tumor necrosis factor receptor 1
- TNFR2
tumor necrosis factor receptor 2
- TLRs
toll-like receptors
- PAMPs
pathogen-associated molecular patterns
- A59/WT
wild-type mouse hepatitis virus A59
- RA59/GOS
recombinant mouse hepatitis virus A59 expressing eGFP-OVA-SIY fusion protein
- fMHV
feline mouse hepatitis virus
- rAd
recombinant adenovirus
- LCMV
lymphocytic choriomeningitis virus
- dpi
days post infection
- MOI
multiplicity of infection
- ALT
alanine aminotransferase
References
- 1.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 3.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 4.Matsuno K, Ezaki T, Kudo S, Uehara Y. A life stage of particle-laden rat dendritic cellsin vivo: their terminal division, active phagocytosis, and translocation from the liver to the draining lymph. J Exp Med. 1996;183:1865–1878. doi: 10.1084/jem.183.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores-Romo L. In vivo maturation and migration of dendritic cells. Immunology. 2001;102:255–262. doi: 10.1046/j.1365-2567.2001.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Brunner C, Seiderer J, Schlamp A, Bidlingmaier M, Eigler A, Haimerl W, Lehr HA, Krieg AM, Hartmann G, Endres S. Enhanced dendritic cell maturation by TNF-alpha or cytidine-phosphate-guanosine DNA drives T cell activation. in vitro and therapeutic anti-tumor immune responses in vivo. J Immunol. 2000;165:6278–6286. doi: 10.4049/jimmunol.165.11.6278. [DOI] [PubMed] [Google Scholar]
- 10.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabot S, Williams G, Yong VW. Microglial production of TNF-alpha is induced by activated T lymphocytes. Involvement of VLA-4 and inhibition by interferonbeta-1b. J Clin Invest. 1997;100:604–612. doi: 10.1172/JCI119571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai EY, Jain J, Pesavento PA, Rao A, Goldfeld AE. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol Cell Biol. 1996;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 14.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 15.Miller G, Lahrs S, Shah AB, DeMatteo RP. Optimization of dendritic cell maturation and gene transfer by recombinant adenovirus. Cancer Immunol Immunother. 2003;52:347–358. doi: 10.1007/s00262-003-0379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trevejo JM, Marino MW, Philpott N, Josien R, Richards EC, Elkon KB, Falck-Pedersen E. TNF-alpha -dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc Natl Acad Sci USA. 2001;98:12162–12167. doi: 10.1073/pnas.211423598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zganiacz A, Santosuosso M, Wang J, Yang T, Chen L, Anzulovic M, Alexander S, Gicquel B, Wan Y, Bramson J, Inman M, Xing Z. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest. 2004;113:401–413. doi: 10.1172/JCI18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suresh M, Singh A, Fischer C. Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection. J Virol. 2005;79:202–213. doi: 10.1128/JVI.79.1.202-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A, Svaren J, Grayson J, Suresh M. CD8 T cell responses to lymphocytic choriomeningitis virus in early growth response gene 1-deficient mice. J Immunol. 2004;173:3855–3862. doi: 10.4049/jimmunol.173.6.3855. [DOI] [PubMed] [Google Scholar]
- 20.Turner SJ, La Gruta NL, Stambas J, Diaz G, Doherty PC. Differential tumor necrosis factor receptor 2-mediated editing of virus-specific CD8+ effector T cells. Proc Natl Acad Sci USA. 2004;101:3545–3550. doi: 10.1073/pnas.0307347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muraille E, De Trez C, Pajak B, Brait M, Urbain J, Leo O. T cell-dependent maturation of dendritic cells in response to bacterial superantigens. J Immunol. 2002;168:4352–4360. doi: 10.4049/jimmunol.168.9.4352. [DOI] [PubMed] [Google Scholar]
- 22.Shreedhar V, Moodycliffe AM, Ullrich SE, Bucana C, Kripke ML, Flores-Romo L. Dendritic cells require T cells for functional maturationin vivo. Immunity. 1999;11:625–636. doi: 10.1016/s1074-7613(00)80137-5. [DOI] [PubMed] [Google Scholar]
- 23.Talay O, Shen CH, Chen L, Chen J. B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proc Natl Acad Sci USA. 2009;106:2741–2746. doi: 10.1073/pnas.0813367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leparc-Goffart I, Hingley ST, Chua MM, Phillips J, Lavi E, Weiss SR. Targeted recombination within the spike gene of murine coronavirus mouse hepatitis virus-A59: Q159 is a determinant of hepatotropism. J Virol. 1998;72:9628–9636. doi: 10.1128/jvi.72.12.9628-9636.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo L, Godeke GJ, Raamsman MJ, Masters PS, Rottier PJ. Retargeting of coronavirus by substitution of the spike glycoprotein ectodomain: crossing the host cell species barrier. J Virol. 2000;74:1393–1406. doi: 10.1128/jvi.74.3.1393-1406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gombold JL, Hingley ST, Weiss SR. Fusion-defective mutants of mouse hepatitis virus A59 contain a mutation in the spike protein cleavage signal. J Virol. 1993;67:4504–4512. doi: 10.1128/jvi.67.8.4504-4512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 28.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pewe L, Wu GF, Barnett EM, Castro RF, Perlman S. Cytotoxic T cell-resistant variants are selected in a virus-induced demyelinating disease. Immunity. 1996;5:253–262. doi: 10.1016/s1074-7613(00)80320-9. [DOI] [PubMed] [Google Scholar]
- 30.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wijburg OL, Heemskerk MH, Boog CJ, Van Rooijen N. Role of spleen macrophages in innate and acquired immune responses against mouse hepatitis virus strain A59. Immunology. 1997;92:252–258. doi: 10.1046/j.1365-2567.1997.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 33.Das Sarma J, Scheen E, Seo SH, Koval M, Weiss SR. Enhanced green fluorescent protein expression may be used to monitor murine coronavirus spreadin vitro and in the mouse central nervous system. J Neurovirol. 2002;8:381–391. doi: 10.1080/13550280260422686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperry SM, Kazi L, Graham RL, Baric RS, Weiss SR, Denison MR. Single-amino-acid substitutions in open reading frame (ORF) 1b-nsp14 and ORF 2a proteins of the coronavirus mouse hepatitis virus are attenuating in mice. J Virol. 2005;79:3391–3400. doi: 10.1128/JVI.79.6.3391-3400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundquist M, Wick MJ. TNF-alpha-dependent and -independent maturation of dendritic cells and recruited CD11c(int)CD11b+ Cells during oral Salmonella infection. J Immunol. 2005;175:3287–3298. doi: 10.4049/jimmunol.175.5.3287. [DOI] [PubMed] [Google Scholar]
- 36.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 37.Sundquist M, Johansson C, Wick MJ. Dendritic cells as inducers of antimicrobial immunityin vivo. APMIS. 2003;111:715–724. doi: 10.1034/j.1600-0463.2003.11107804.x. [DOI] [PubMed] [Google Scholar]
- 38.Ritter U, Meissner A, Ott J, Korner H. Analysis of the maturation process of dendritic cells deficient for TNF and lymphotoxin-alpha reveals an essential role for TNF. J Leukoc Biol. 2003;74:216–22. doi: 10.1189/jlb.1202587. [DOI] [PubMed] [Google Scholar]
- 39.Wu Q, Wang Y, Wang J, Hedgeman EO, Browning JL, Fu YX. The requirement of membrane lymphotoxin for the presence of dendritic cells in lymphoid tissues. J Exp Med. 1999;190:629–38. doi: 10.1084/jem.190.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abe K, Yarovinsky FO, Murakami T, Shakhov AN, Tumanov AV, Ito D, Drutskaya LN, Pfeffer K, Kuprash DV, Komschlies KL, Nedospasov SA. Distinct contributions of TNF and LT cytokines to the development of dendritic cells in vitro and their recruitment in vivo. Blood. 2003;101:1477–1483. doi: 10.1182/blood.V101.4.1477. [DOI] [PubMed] [Google Scholar]
- 41.Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–50. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Wang YG, Kim KD, Wang J, Yu P, Fu YX. Stimulating lymphotoxin beta receptor on the dendritic cells is critical for their homeostasis and expansion. J Immunol. 2005;175:6997–7002. doi: 10.4049/jimmunol.175.10.6997. [DOI] [PubMed] [Google Scholar]
- 43.Lund FE, Partida-Sanchez S, Lee BO, Kusser KL, Hartson L, Hogan RJ, Woodland DL, Randall TD. Lymphotoxin-alpha-deficient mice make delayed, but effective, T and B cell responses to influenza. J Immunol. 2002;169:5236–5243. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
- 44.Suresh M, Lanier G, Large MK, Whitmire JK, Altman JD, Ruddle NH, Ahmed R. Role of lymphotoxin alpha in T-cell responses during an acute viral infection. J Virol. 2002;76:3943–51. doi: 10.1128/JVI.76.8.3943-3951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]