Abstract.
Near-infrared confocal microendoscopy is a promising technique for deep in vivo imaging of tissues and can generate high-resolution cross-sectional images at the micron-scale. We demonstrate the use of a dual-axis confocal (DAC) near-infrared fluorescence microendoscope with a 5.5-mm outer diameter for obtaining clinical images of human colorectal mucosa. High-speed two-dimensional en face scanning was achieved through a microelectromechanical systems (MEMS) scanner while a micromotor was used for adjusting the axial focus. In vivo images of human patients are collected at with a field of view of and a maximum imaging depth of 140 μm. During routine endoscopy, indocyanine green (ICG) was topically applied a nonspecific optical contrasting agent to regions of the human colon. The DAC microendoscope was then used to obtain microanatomic images of the mucosa by detecting near-infrared fluorescence from ICG. These results suggest that DAC microendoscopy may have utility for visualizing the anatomical and, perhaps, functional changes associated with colorectal pathology for the early detection of colorectal cancer.
Keywords: confocal, dual-axis, Gastrointestinal Tract, MEMS, microendoscope, near-infrared fluorescence
1. Introduction
Colorectal cancer is one of the most common cancers, ranking third worldwide in frequency of incidence after cancers of the lung and breast.1 There are over 1.2 million new cases each year and over 600,000 individuals will eventually die from this malignancy. Survival rates depend largely on the stage of diagnosis. Statistics show that about 90% of patients diagnosed with localized cancer survive beyond 5 years, compared with 68% of those diagnosed with regional disease.2 Therefore, there is a pressing need to detect colorectal cancer at early stages, with the aim to improve prognosis and reduce mortality.
Confocal microscopy is an optical imaging technique that has been widely used in various biological and biomedical applications.3 This imaging modality can generate high-resolution (micron-scale) cross-sectional images of biological tissues. The high axial resolution provided by confocal microscopy allows imaging of thin “optical sections” within live intact tissue. Typically, either reflectance or fluorescence images can be collected to identify morphological or molecular features, respectively, of cells and tissues.
The development of confocal microendoscopy has been accelerated by advances and cost reductions in its photonic components, such as optical fibers and diode lasers, as well as microfabrication technologies, including laser beam-scanning micromirrors. These microendoscopes are being integrated in to a wide range of medical devices, including catheters, endoscopes, and surgical instruments, with the goal of enabling in vivo imaging inside the human body for early disease detection, the staging of lesions, and guiding therapies.4–6
2. Dual-Axis Confocal Microendoscope Architecture
The aforementioned efforts present great technical challenges for satisfy the demanding specifications for high axial resolution, deep penetration depth, high contrast, and fast frame rates in miniature devices. A variety of miniature configurations,7,8 including single9 and multiple fiber4 strategies, have been devised to meet some of these requirements. Thus far, miniature confocal microscopes have all employed a conventional single-axis architecture with the illumination and collection paths aligned along the same optical axis. Excellent lateral resolution can be achieved using a sufficiently high numerical aperture (NA) lens at the expense of a limited working distance (WD) and field-of-view (FOV), which decreases as the lens is scaled down in size. Therefore, due to the limited WD of a high-NA lens, the beam-scanning mechanisms of confocal scanning microscopes are typically located proximal to the objective lens (i.e., preobjective position). Unfortunately, preobjective beam scanning leads to off-axis aberrations, such as coma and astigmatism, that require mitigation through complex optical designs.
The dual-axis confocal (DAC) configuration was developed to overcome these limitations for endoscope compatibility and in vivo imaging by utilizing two optical fibers oriented along intersecting optical axes of two low-NA objectives in order to spatially separate the light paths for illumination and collection.10,11 The overlapping region between the foci of the two beams defines the focal volume, and, hence, the resolution, which is sub-cellular in all three dimensions. Furthermore, low-NA objectives enable an increased WD, such that a scanning mirror can be located on the distal side of the lens (i.e., postobjective position), thus eliminating off-axis aberrations. In other words, the beams always pass through the low-NA objectives in a direction that is collinear to the optical axis of each lens, resulting in a diffraction-limited focus that can then be scanned over a large FOV by a nonaberrating mirror surface. This configuration allows the instrument to be scaled down in size to millimeter dimensions for compatibility with medical endoscopes. Furthermore, the DAC configuration has been shown through Monte-Carlo simulations, tissue-phantom experiments, and imaging studies to enable superior rejection of out-of-focus and multiply scattered light in thick tissues.12
3. DAC Microendoscope Components and Performance
The design and integration of our microscope requires a package that allows for precise alignment of the following optical elements: (1) two fiber-coupled collimators, (2) a two-dimensional (2D) microelectromechanical (MEMS) scanner,13 (3) a parabolic focusing mirror, and (4) a hemispherical index-matching solid immersion lens (SIL). The basic design of the microendoscope scanhead is shown in Fig. 1(a). The 5-mm-diameter parabolic mirror () is fabricated using a replicated molding process that provides the high-quality surface profile that is needed for diffraction-limited focusing of two collimated beams. Once the beams are aligned parallel to each other, the parabolic mirror then causes the focused beams to intersect at a common focal point below the tissue surface. Since each of the two collimators are only 1 mm in diameter, the collimated beams underfill the parabolic mirror where they are focused with an effective NA of 0.1.
Fig. 1.
A 5.5-mm diameter DAC microendoscope scanhead. (a) Two collimated beams are focused by a parabolic mirror. Real-time en face scanning is performed by a 2-D MEMS scanner. (b) Photograph of the microendoscope without its cap showing a 2-D MEMS scanner mounted on the axial translation stage; .
The components used in earlier handheld microscopes14–16 were used in the 5.5-mm diameter microendoscope package, which is based on the design shown in Fig. 1. This smaller prototype uses the same replicated parabolic focusing mirror but with a smaller diameter (5 mm). The MEMS scanner being used in this microendoscope is also similar in design to the one employed in previous 10-mm diameter handheld versions of the DAC microscope. A pair of 1-mm diameter fiber-coupled gradient-index (GRIN) collimating lenses, which were reduced in diameter from 1.8 mm, are employed in this DAC microendoscope. In order for the illumination and collection beams to intersect and focus at a single point in space, the collimated beams must be made exactly parallel to each other prior to impinging upon the parabolic focusing mirror. Beam alignment is achieved by manually rotating a pair of 1-mm diameter Risley prisms that are inserted into the path of one of the collimated beams. The collimators and Risley prisms are both held by precision wire-electrical discharge machined (EDM) v-grooves and fixed in place with a UV-curing epoxy. As with the larger prototypes, the combined precision of the v-grooves and the pointing accuracy of the preassembled fiber collimators allow for the collimated beams to be made parallel to each other to within 0.05 deg. The Risley prisms have a small wedge angle (0.1 deg), allowing the collimated beams to be steered over a range of 0.05 deg such that the beams are perfectly parallel with each other. Fig. 2(a) shows a fully packaged DAC microendoscope that is distally loaded through the instrument channel of an Olympus XT-160 therapeutic upper endoscope (6-mm diameter instrument channel). Fig. 2(b) shows the distal end of an endoscope with the DAC microendoscope protruding out of the instrument channel.
Fig. 2.
(a) A DAC microendoscope is passed through the instrument channel of an Olympus XT-160 endoscope that has a 6-mm diameter instrument channel. (b) Distal end of an endoscope showing the protruding DAC microendoscope; . (c) Cropped reflectance image of a 1951 United States Air Force resolution test chart collected using the DAC microendoscope. Shows a transverse resolution of 5 μm; .
The MEMS mirror is electrostatically actuated to raster scan. Both the illumination and collection beams with one of its axis oscillate at resonance (1.1 kHz with an actuation voltage of 90 V) and the orthogonal axis oscillates near DC (1 to 5 Hz with actuation voltage of 170 V). The differential drive is performed on the MEMS driving bias in order to maximize linearization of the beam trajectory.17 Each 2D en face image is continuously displayed on a computer monitor with a custom frame grabber, using National Instruments (NI) data acquisitions boards (NI models: PXI-6711 and PXI-6251) that are controlled using LabVIEW. A computer-controlled micromotor within the microscope package is used to actuate an axial sliding mechanism that serves as the MEMS mirror mount, thus providing real-time imaging-depth adjustments. The micromotor (Faulhaber GmbH & Co. KG) consists of a brushless DC motor (model 0308), a micro-planetary gearhead (model 03A), and a linear actuator (model 03A-S3). Each 3D volumetric image is created using postprocessing to render a series of 2D en face images. A near-infrared laser light source with a center wavelength of 785 nm was used in this experiment. Imaging was performed in either the reflectance or fluorescence mode; in the latter case, a 790-nm long-pass optical filter is inserted in to the collection path. The maximum laser power directed at the sample is 3.6 mW with a FOV of (). The axial resolution is 6.5 μm, as measured by axially translating a reflecting surface through the focal plane. The transverse resolution is 5 μm, as derived from the reflectance images shown in Fig. 2(c).
4. Experimental Results and Conclusion
4.1. Ex vivo Fluorescence Images
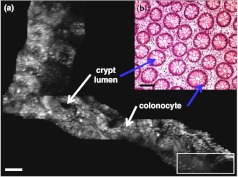
The 3D fluorescence imaging capability of the handheld dual-axis confocal instrument is shown in Fig. 3. Excised tissue specimens from normal and dysplastic colonic mucosa were soaked in 0.5 mg IRDye® 800 continuous-wave N-hydroxysuccinimide (CW NHS) Ester (LI-COR Biosciences, Inc) diluted in 10 ml of phosphate-buffered saline (PBS) at neutral pH for 5 min and then rinsed with PBS to remove excess dye. After imaging, the specimens were fixed in 10% buffered formalin, embedded in paraffin, cut into 5-μm sections, and processed for histology with hematoxylin and eosin (H&E) staining. All ex vivo images were obtained from freshly excised human tissues (after informed consent and institutional review board approval was obtained from Stanford University and the Veterans Affairs Palo Alto Health Care System). Fig. 3(a)–(c) show the en face image, H&E, and 3D volumetric images of normal colonic mucosa, respectively. The features of the colonic crypts, including colonocytes and crypt lumens, are clearly resolved. Fig. 3(c) shows three extracted en face planes at 30, 90, and 140 μm below the tissue surface. The photomultiplier tube (PMT) gain was increased with depth in order to compensate for signal attenuation.
Fig. 3.
Ex vivo fluorescence images obtained using a DAC microendoscope. En face image of (a) normal colonic mucosa, (b) corresponding histology (H&E) of (a), and (c) 3D volumetric image of (a). .
4.2. In vivo Fluorescence Images
Real-time in vivo fluorescence imaging was performed in the colons of human patients with our DAC microendoscope. While performing standard video colonoscopy, the confocal images were simultaneously collected from the MEMS-based microendoscope, which occupied the instrument channel of the endoscope. Before each imaging session, lyophilized indocyanine green (ICG) (Akorn, Inc.) was dissolved in an aqueous solvent () and was topically applied to the gastrointestinal (GI) mucosal surface for 2–3 min before washing out the excess dye with water. Fig. 4(a) shows a sequence of approximately 180 individual en face mosaiced images (post processed) of normal colonic mucosa acquired at a depth of 60 μm beneath the mucosal surface. The white rectangle represents an individual en face image (; ) obtained with the DAC microendoscope. Normal colonic microarchitecture is seen as circular and regularly spaced crypts that line the epithelial layer [Fig. 4(a)]. The images were mosaiced by first correcting the image borders for scanning distortions. Then, each new image was registered and merged before proceeding to the subsequent image.18 Fig. 4(b) shows a representative histologic image derived from H&E staining of normal colonic mucosa. After each use, the whole microendoscope is soaked in Cidex Solution (Civco Medical Solutions, Inc.) for 45 min at room temperature in order to disinfect and sterilize the imaging probe.
Fig. 4.
In summary, we have engineered the first MEMS-based DAC microendoscope (5.5-mm diameter) to be used for obtaining near-infrared fluorescence images in vivo from the colon of human patients. This MEMS-based microendoscope is a step towards enabling point-of-care pathology for the in vivo diagnosis, staging of colorectal lesions, and guiding biopsies and therapies.
Acknowledgments
WP was partially supported by grants from the National Research Council, the Higher Education Research Promotion & National Research University Project, and the Office of the Higher Education Commission of Thailand (HR1162A and HR1166I). This work was supported in part by grants from the National Institutes of Health, including U54 CA105296, R33 CA109988, K08 DK67618, P50 CA93990, and U54 CA136429.
References
- 1.Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D., “Global cancer statistics,” CA. Cancer J. Clin. 61(2), 69–90 (2011). 10.3322/caac.v61:2 [DOI] [PubMed] [Google Scholar]
- 2.Howlader N., Noone A. M., Krapcho M., Neyman N., Aminou R., Waldron W., Altekruse S. F., Kosary C. L., Ruhl J., Tatalovich Z., Cho H., Mariotto A., Eisner M. P., Lewis D. R., Chen H. S., Feuer E. J., Cronin K. A., Edwards B. K., Seer cancer statistics review, 1975-2008,National Cancer Institute,Bethesda, MD: (2011). [Google Scholar]
- 3.Corle T. R., Kino G. S., Confocal scanning optical microscopy and related imaging systems, Academic Press,San Diego: (1996). [Google Scholar]
- 4.Laemmel E., Genet M., Le Goualher G., Perchant A., Le Gargasson J. F., Vicaut E., “Fibered confocal fluorescence microscopy (cell-vizio) facilitates extended imaging in the field of microcirculation. A comparison with intravital microscopy,” J. Vasc. Res. 41(5), 400–411 (2004). 10.1159/000081209 [DOI] [PubMed] [Google Scholar]
- 5.Thekkek N., Richards-Kortum R., “Optical imaging for cervical cancer detection: solutions for a continuing global problem,” Nat. Rev. Cancer 8(9), 725–731 (2008). 10.1038/nrc2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurlstone D. P., Tiffin N., Brown S. R., Baraza W., Thomson M., Cross S. S., “In vivo confocal laser scanning chromo-endomicroscopy of colorectal neoplasia: Changing the technological paradigm,” Histopathology 52(4), 417–426 (2008). 10.1111/his.2008.52.issue-4 [DOI] [PubMed] [Google Scholar]
- 7.Dickensheets D. L., Kino G. S., “Silicon-micromachined scanning confocal optical microscope,” IEEE J. Microelectromech. Syst. 7(1), 38–47 (1998). 10.1109/84.661382 [DOI] [PubMed] [Google Scholar]
- 8.Aguirre A. D., Hertz P. R., Chen Y., Fujimoto J. G., Piyawattanametha W., Fan L., Wu M. C., “Two-axis mems scanning catheter for ultrahigh resolution three-dimensional and en face imaging,” Opt. Express 15(5), 2445–2453 (2007). 10.1364/OE.15.002445 [DOI] [PubMed] [Google Scholar]
- 9.Delaney P. M., Harris M. R., King R. G., “Fiber-optic laser scanning confocal microscope suitable for fluorescence imaging,” Appl. Opt. 33(4), 573–577 (1994). 10.1364/AO.33.000573 [DOI] [PubMed] [Google Scholar]
- 10.Wang T. D., Mandella M. J., Contag C. H., Kino G. S., “Dual-axis confocal microscope for high-resolution in vivo imaging,” Opt. Lett. 28(6), 414–416 (2003). 10.1364/OL.28.000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piyawattanametha W., Wang T. D., In vivo microendoscopy, McGraw Hill Professional, New York, NY, 45–76 (2011). [Google Scholar]
- 12.Liu J. T. C., Mandella M. J., Crawford J. M., Contag C. H., Wang T. D., Kino G. S., “Efficient rejection of scattered light enables deep optical sectioning in turbid media with low-numerical-aperture optics in a dual-axis confocal architecture,” J Biomed Opt 13(3), 034020 (2008). 10.1117/1.2939428 [DOI] [PubMed] [Google Scholar]
- 13.Ra H., Piyawattanametha W., Taguchi Y., Lee D., Mandella M. J., Solgaard O., “Two-dimensional mems scanner for dual-axes confocal microscopy,” IEEE J. Microelectromech. Syst. 16(4), 969–976 (2007). 10.1109/JMEMS.2007.892900 [DOI] [Google Scholar]
- 14.Liu J. T. C., Mandella M. J., Ra H., Wong L. K., Solgaard O., Kino G. S., Piyawattanametha W., Contag C. H., Wang T. D., “Miniature near-infrared dual-axes confocal microscope utilizing a two-dimensional microelectromechanical systems scanner,” Opt. Lett. 32(3), 256–258 (2007). 10.1364/OL.32.000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ra H., Piyawattanametha W., Mandella M. J., Hsiung P.-L., Hardy J., Wang T. D., Contag C. H., Kino G. S., Solgaard O., “Three-dimensional in vivo imaging by a handheld dual-axes confocal microscope,” Opt. Express 16(10), 7224–7232 (2008). 10.1364/OE.16.007224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piyawattanametha W., Ra H., Mandella M. J., Loewke K., Wang T. D., Kino G. S., Solgaard O., Contag C. H., “3-d near-infrared fluorescence imaging using an mems-based miniature dual-axis confocal microscope,” IEEE J. Sel. Top. Quantum Electron. 15(5), 1344–1350 (2009). 10.1109/JSTQE.2009.2021533 [DOI] [Google Scholar]
- 17.Piyawattanametha W., Barretto R. P. J., Ko T. H., Flusberg B. A., Cocker E. D., Ra H. J., Lee D. S., Solgaard O., Schnitzer M. J., “Fast-scanning two-photon fluorescence imaging based on a microelectromechanical systems two-dimensional scanning mirror,” Opt. Lett. 31(13), 2018–2020 (2006). 10.1364/OL.31.002018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loewke K. E., Camarillo D. B., Piyawattanametha W., Mandella M. J., Contag C. H., Thrun S., Salisbury J. K., “In vivo micro-image mosaicing,” IEEE Trans. Biomed. Eng. 58(1), 159–171 (2011). 10.1109/TBME.2010.2085082 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.