This article presents a systematic review of the literature investigating the antitumor effects of the somatostatin analogs octreotide and lanreotide for patients with secretory and nonsecretory neuroendocrine tumors.
Keywords: Octreotide, Lanreotide, Pancreatic neuroendocrine tumors, Gastrointestinal neuroendocrine tumors, Carcinoid
Abstract
Background.
For decades, somatostatin analogs (including octreotide and lanreotide) have been indicated for relief of the symptoms of flushing, diarrhea, and wheezing associated with secretory neuroendocrine tumors (NETs). Recently, it has been suggested that somatostatin analogs may provide direct and indirect antitumor effects in secretory and nonsecretory NETs in addition to symptom control in secretory NETs.
Methods.
A systematic review of MEDLINE was conducted to identify studies that investigated the antitumor effects of octreotide or lanreotide for patients with NETs. Additional studies not published in the peer-reviewed literature were identified by searching online abstracts.
Results.
In all, 17 octreotide trials and 11 lanreotide trials that included antitumor effects were identified. Partial response rates were between 0% and 31%, and stable disease rates were between 15% and 89%. Octreotide was the only somatostatin analog for which results of a phase III, randomized, placebo-controlled clinical trial that investigated antitumor effects were published. After 6 months of treatment in this randomized phase III trial, stable disease was observed in 67% of patients (hazard ratio for time to disease progression: 0.34; 95% confidence interval: 0.20–0.59; p = .000072).
Conclusions.
In addition to symptom control for NETs, the data support an antitumor effect of somatostatin analogs and suggest that they may slow tumor growth. Long-acting repeatable octreotide has been shown to have an antitumor effect in a randomized phase III trial in midgut NETs, whereas results are pending in a corresponding controlled trial with lanreotide for patients with intestinal and pancreatic primary NETs.
Introduction
Advanced neuroendocrine tumors (NETs) are a family of malignancies with diverse origins, including the gastrointestinal (GI) tract, lung, and pancreas [1–3]. Primary treatment guidelines for NETs include surgical resection of the tumor, if possible [1, 4]. The 5-year survival rate is 51%–80%, depending on the disease location and stage [4]. However, tumors may recur after surgery; for example, liver metastases have an 84%–91% probability of recurrence at 5 years [4]. In addition, up to 50% of patients with NETs have metastases at diagnosis [3], and the presence of metastasis portends a significantly worse prognosis [4].
Despite common histopathologic characteristics, GI-NETs (previously known as carcinoid tumors) and pancreatic NETs (pNETs) should be differentiated on the basis of biological behavior and therapeutic strategy. Although NETs have the potential to secrete specific hormones or vasoactive peptides into the systemic circulation, thus resulting in a broad range of symptoms [2], most patients (60%) have nonfunctioning tumors. Although most pNETs are nonsecreting, many GI-NETs are associated with symptoms of flushing, diarrhea, and wheezing [2]; the constellation of these symptoms is called carcinoid syndrome. Secreting pNETs occur, in decreasing frequency, as insulinomas (causing hypoglycemia), gastrinomas (causing Zollinger-Ellison syndrome), glucagonomas (causing weight loss, diabetes, and/or skin lesions), VIPomas (i.e., Verner-Morrison syndrome; causing profuse diarrhea, hypokalemia, and flushing), and others (e.g., somatostatinomas) [2, 5].
Most NETs express G-protein–coupled transmembrane somatostatin receptors (SSTRs) [6]. There are five subtypes of SSTRs, and different NETs have differing proportions of SSTR expression [6]. Somatostatin inhibits the release of neuroendocrine hormones, including those released from NETs. However, somatostatin has a short half-life in vivo [6], making it unsuitable for therapeutic use; thus, synthetic somatostatin analogs were developed for NET symptom control [7–11]. A subcutaneous (SC) formulation of octreotide was approved in New Zealand (1987), followed by octreotide long-acting repeatable (LAR) some years later; both are approved in more than 90 countries worldwide for the control of hormonal symptoms in patients with GI-NETs and pNETs [10]. Lanreotide SC first became available in 1988 in Europe; lanreotide long-acting microparticle formulation (LA) was approved for relief of NET symptoms in 1995 in France, and lanreotide Autogel was approved in 2001 in the European Union [9].
Like somatostatin, octreotide and lanreotide bind to the SSTR and produce a range of effects, including decreased hormonal secretion, decreased growth and proliferation, increased apoptosis, inhibition of cell signaling, and inhibition of protein synthesis; they may also have direct antiproliferative activity (Fig. 1) [12, 13]. There are 25 years of evidence that octreotide controls the symptoms of severe diarrhea and flushing in patients with carcinoid syndrome [14–16]. Analysis of the Surveillance, Epidemiology, and End Results (SEER) database found that patients with metastatic NETs who were diagnosed between 1988 and 2004 had significantly greater median survival times than patients with metastatic NETs who were diagnosed between 1973 and 1987 (39 vs. 18 months, respectively; hazard ratio [HR]: 0.73; 95% confidence interval [CI]: 0.69–0.77; p < .001) [3]. Notably, this improvement coincides with the introduction of octreotide in 1987. Before the introduction of somatostatin analogs, many patients may have died from the effects of associated secretion syndromes (e.g., long-term diarrhea, ultimately resulting in severe dehydration and renal failure [17]); today, the highest mortality rate is for patients with high tumor burdens.
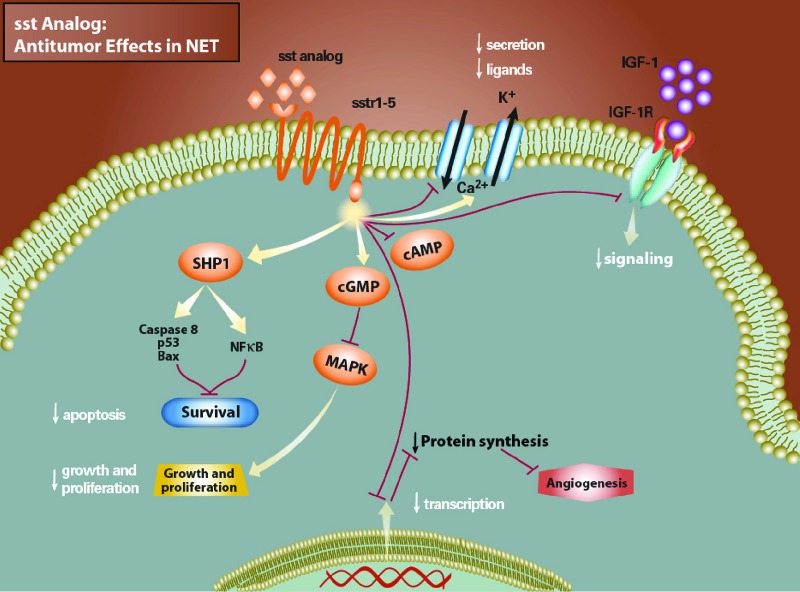
Figure 1.
Effect of somatostatin analog in neuroendocrine tumors. Somatostatin analogs bind to G-protein–linked receptors on the cell surface and cause decreased hormonal secretion by inhibiting cyclic adenosine monophosphate, increased apoptosis by activating the protein tyrosine phosphatase SHP1, decreased growth and proliferation through mitogen-activated protein kinase, inhibition of insulin-like growth factor receptor 1 signaling, and inhibition of protein synthesis caused by decreased transcription [12].
Abbreviations: cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; IGF, insulin-like growth factor; IGF-1R, insulin-like growth factor receptor 1; MAPK, mitogen-activated protein kinase; NET, neuroendocrine tumor; NFκB, nuclear factor-κB; SHP1, Src homology phosphatase-1; sst, somatostatin.
In addition to symptom control, it has long been suggested that somatostatin analogs may exert antitumor effects for NETs. Even before its approval, case reports suggested that octreotide showed antitumor properties for patients with NETs [18, 19]. Reviews of preclinical studies suggest direct receptor-mediated antitumor effects of cell cycle inhibition, growth factor inhibition, and proapoptotic activity [20, 21]. Furthermore, there may be indirect effects, including inhibition of the release of growth factor and trophic hormones, inhibition of angiogenesis, and modulation of the immune system [20, 21]. Based on these data, we reviewed the literature to identify published primary clinical studies and retrospective analyses that supported an antitumor role for the somatostatin analogs octreotide and lanreotide.
Methods
Systematic review of the literature was conducted to identify primary prospective and retrospective studies examining the antitumor effects of octreotide or lanreotide in patients with NETs. Additional studies not published in the peer-reviewed literature were identified by searching online abstracts from the American Society of Clinical Oncology (ASCO), ASCO Gastrointestinal Cancers Symposium (ASCO-GI), European Society for Medical Oncology (ESMO), and the North American Neuroendocrine Tumor Society (NANETS) annual congresses.
Peer-Reviewed Literature
PubMed was searched using the search terms of “endocrine tumor” or “endocrine tumour” or “carcinoid” or “gastroenteropancreatic” (defined as the disease search). The search was limited to English language studies of humans. Results of the disease search were further limited to articles containing the terms “octreotide” or “Sandostatin” or “lanreotide” or “Somatuline Autogel” or “somatostatin analog” in the title or abstract (defined as the drug search). Results of the drug search were critically evaluated to select primary clinical trials or retrospective analyses of octreotide or lanreotide on tumor progression (defined as studies reporting tumor response rates and/or survival rates). In the first instance, titles and abstracts from the drug search were examined to assess whether publications fit the defined criteria for inclusion in our analysis; if insufficient information was available, then the complete published article was obtained and examined.
Review articles and case studies were excluded, as were articles pertaining to symptom control of NETs, radiolabeled octreotide, preclinical studies, octreotide in imaging/diagnosis, and acromegaly. To capture as many relevant articles as possible, citations of selected articles and other recent reviews on octreotide or lanreotide for NETs (published in the past 5 years) were also examined. For each article included in our analysis, the complete publication was obtained and evaluated.
Congress Abstracts
ASCO, ASCO-GI, ESMO, and NANETS online abstracts were searched for those containing “octreotide” or “Sandostatin” or “lanreotide” or “Somatuline Autogel.” The search results were evaluated to select primary clinical trials or retrospective analyses of octreotide or lanreotide on tumor progression. The first author was then searched through PubMed to determine whether the studies had since been published in the peer-reviewed literature.
Results
The disease search yielded 9,590 articles published in the English language that contained the search terms “endocrine tumor” or “endocrine tumour” or “carcinoid” or “gastroenteropancreatic” in humans.
Octreotide
Further limitation to the drug search using the terms “octreotide” or “Sandostatin” or “somatostatin analog” in the title or abstract narrowed the results down to 671 articles. Evaluation of those 671 articles identified 12 primary clinical trials for inclusion in this review [22–33]. Another three articles for inclusion were selected based on the citations of these studies and/or recent reviews [34–36]. Review of ASCO, ASCO-GI, ESMO, and NANETS abstracts online identified one additional study for inclusion [37]. No retrospective analyses were identified, and one placebo-controlled trial was identified. The final search results for octreotide are shown in Table 1.
Table 1.
Prospective clinical trials of octreotide for neuroendocrine tumors
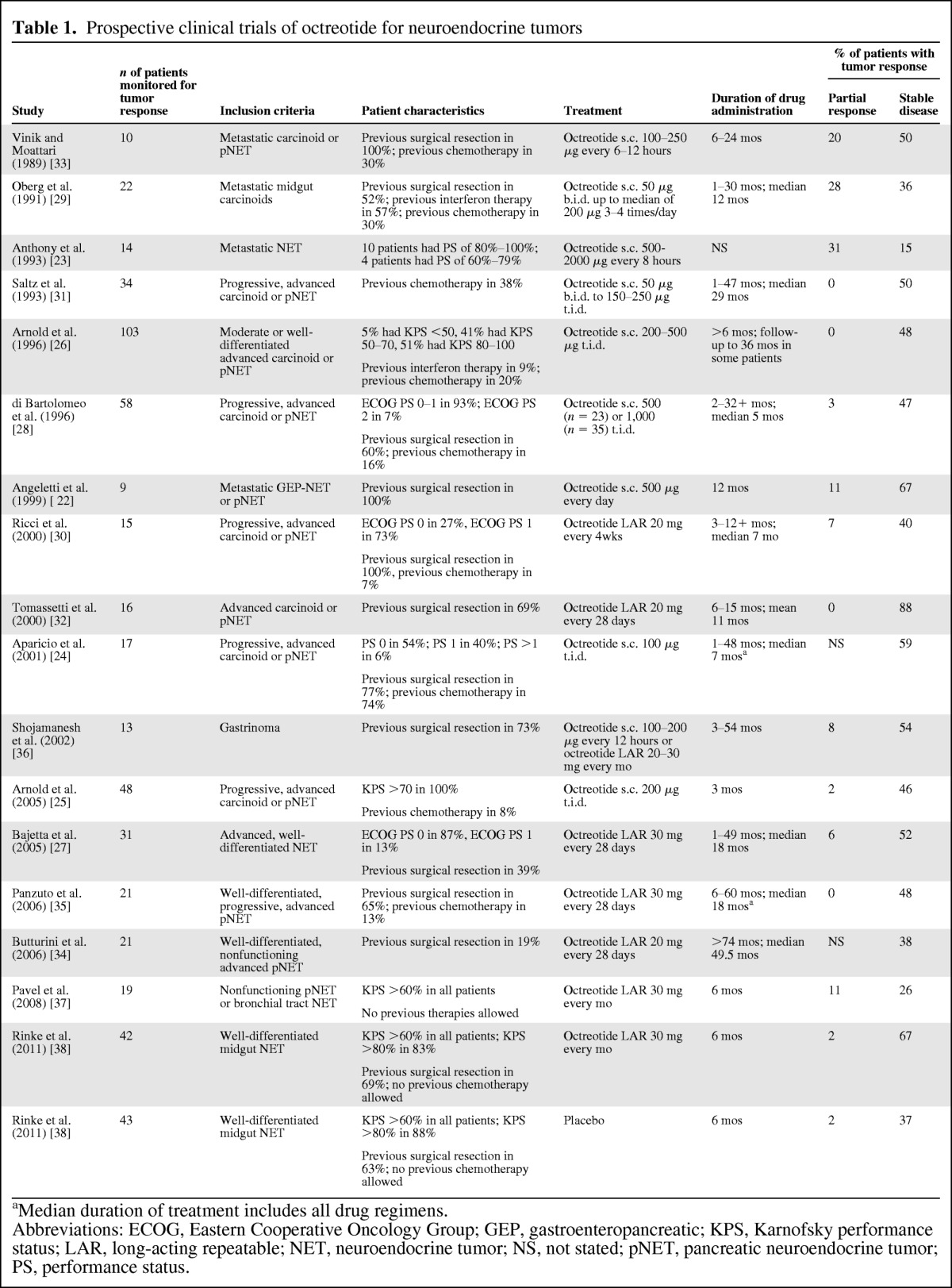
aMedian duration of treatment includes all drug regimens.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; GEP, gastroenteropancreatic; KPS, Karnofsky performance status; LAR, long-acting repeatable; NET, neuroendocrine tumor; NS, not stated; pNET, pancreatic neuroendocrine tumor; PS, performance status.
Shortly after octreotide approval in 1987, Vinik and Moattari [33] reported results from an uncontrolled open-label trial of octreotide SC and demonstrated partial response and stable disease in 20% and 50% of patients, respectively. Six studies in the following decade showed that 15%–86% of patients with advanced NETs achieved stable disease with octreotide SC (Table 1) [22, 23, 26, 28, 29, 31]. After the introduction of octreotide LAR, several more studies reinforced the results observed with the SC formulation [27, 30, 32, 34–37]. Overall, stable disease was observed in 15%–88% of patients with advanced, functioning, or nonfunctioning NETs (Table 1). The studies described in Table 1 revealed that stable disease was observed in up to 86% of patients who received octreotide SC, up to 88% of patients who received octreotide LAR, and approximately 50% of patients with progressive disease before treatment. Partial response (when reported) was observed in up to 31% of patients receiving octreotide SC and up to 11% of patients who received octreotide LAR; however, in most newer trials using a standardized partial response definition such as Response Evaluation Criteria in Solid Tumors (RECIST), the partial response rate did not exceed 10% (Table 1) [38].
Evidence of an antitumor effect of octreotide in patients with NETs was confirmed with the results of the Placebo-Controlled, Double-Blind, Prospective, Randomized Study of the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID) [38]. PROMID was a phase IIIb, prospective, randomized, placebo-controlled, double-blind study of 85 evaluable patients with well-differentiated advanced midgut NETs [38]. Median time to tumor progression was 14.3 months (95% CI: 11.0–28.8 months) with octreotide LAR and 6 months (95% CI: 3.7–9.4 months) with placebo, with 26 and 40 instances of disease progression and tumor-related deaths, respectively (HR: 0.34; 95% CI: 0.20–0.59; p = .000072) [38]. After 6 months of treatment, SD was observed in 67% of patients in the octreotide LAR group compared with 37% of patients in the placebo group [38]. Importantly, there was no difference in response between functionally active and inactive tumors; the most favorable effect was observed in patients with low hepatic tumor load and resected primary tumor [38]. Median overall survival time could not be estimated in the octreotide LAR group because of the crossover design of the study and was not considered statistically robust in the placebo group because of the low number of deaths.
Analyses to identify prognostic factors supporting an antitumor effect were conducted in several studies. A Karnofsky score ≥80 [26] and hepatic tumor burden ≤10% indicated favorable prognosis [35, 38], whereas fast tumor growth rate in the 3–6 months before treatment [24, 36] and pancreatic primary tumor localization [35] indicated poor prognosis; all were significant in their respective studies. Interestingly, the ability to control symptoms or affect laboratory values was not an indicator of antitumor effect in any analysis. Prognostic factors with a trend toward response included tumor location, resection of primary tumor, time from diagnosis to drug initiation, tumor differentiation, and somatostatin analog scintigraphy response [24, 26–28, 35, 38].
Whether the observed benefit in progression-free survival coincides with the prolongation of overall survival is unclear. Median survival or overall survival rates were reported in several studies, but the variability in time periods (1-, 2-, and 3-year survival rates were variously reported) made them difficult to compare [24, 28, 31, 34, 35, 39]. The HR for overall survival in an interim analysis of PROMID was 0.81 (95% CI: 0.30–2.18; p = .77) [38], with further evaluation of overall survival to be made. A trend toward better prognosis is consistent with analysis of the SEER database, which identified increased survival after the introduction of octreotide in 1987 [3].
Lanreotide
Limiting the disease search using the terms “lanreotide” or “Somatuline Autogel” or “somatostatin analog” in the title or abstract identified 174 potential articles. Critical evaluation of these articles yielded 10 final articles for inclusion [23, 24, 35, 40–46]. Review of ASCO, ASCO-GI, ESMO, and NANETS abstracts online identified one other study for inclusion [47]. No retrospective analyses or placebo-controlled results were identified. The final search results for lanreotide are shown in Table 2.
Table 2.
Prospective clinical trials of lanreotide in neuroendocrine tumors
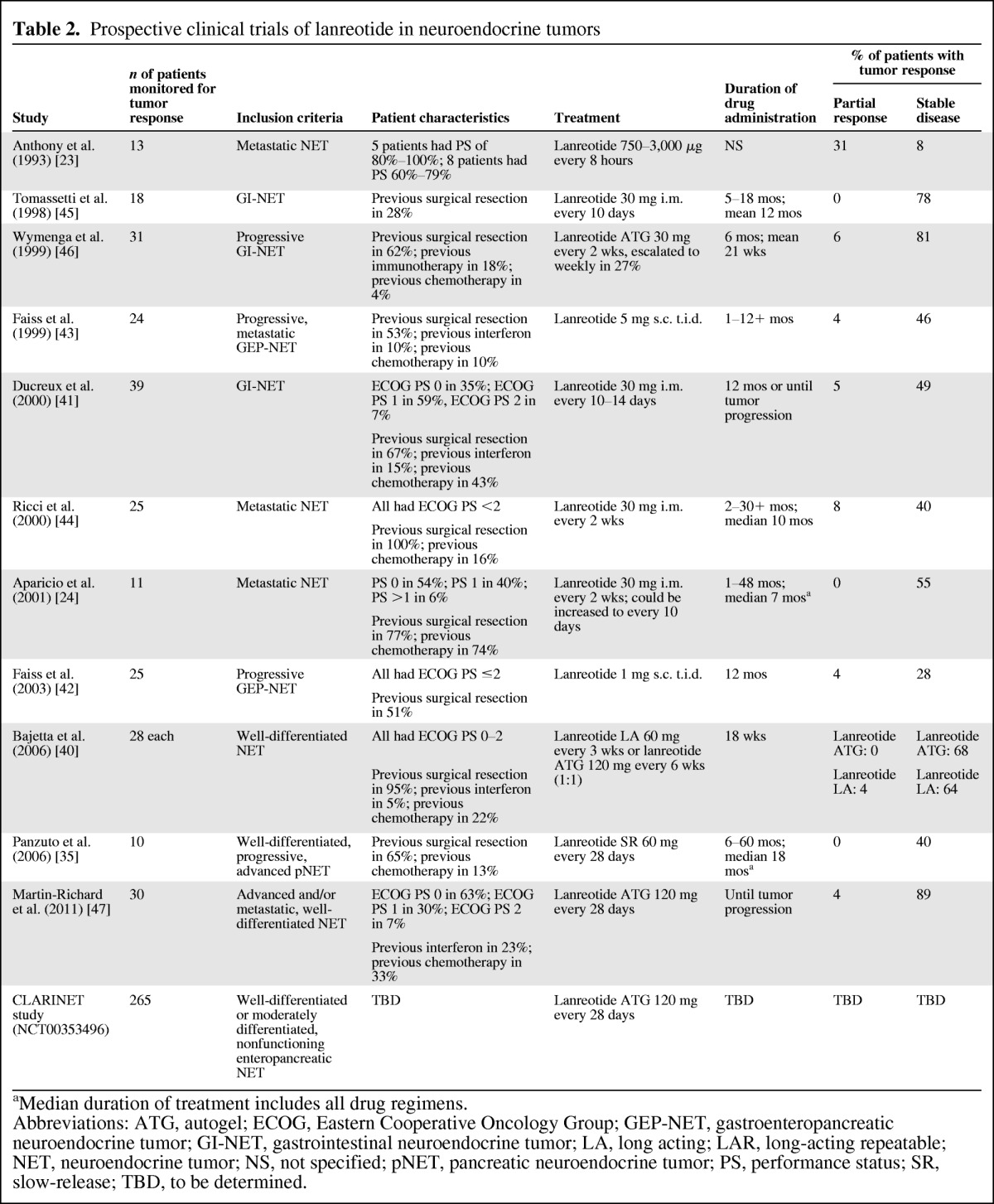
aMedian duration of treatment includes all drug regimens.
Abbreviations: ATG, autogel; ECOG, Eastern Cooperative Oncology Group; GEP-NET, gastroenteropancreatic neuroendocrine tumor; GI-NET, gastrointestinal neuroendocrine tumor; LA, long acting; LAR, long-acting repeatable; NET, neuroendocrine tumor; NS, not specified; pNET, pancreatic neuroendocrine tumor; PS, performance status; SR, slow-release; TBD, to be determined.
In comparison with octreotide, fewer studies could be found evaluating the potential antitumor effect of lanreotide, probably because of its shorter availability period and the more restricted geographic approval for NET treatment. Partial response and stable disease rates in published prospective studies (most phase I or II) are similar to those reported for octreotide (Table 2). Data from the CLARINET trial (NCT00353496; a randomized, placebo-controlled, phase III trial of patients with nonfunctioning intestinal and pancreatic NETs) are not yet available, although enrollment was completed in June 2011 (n = 265).
Analyses to identify prognostic factors for antitumor response identified tumor location [35, 42, 43], tumor progression (fast vs. slow) [24], and distant extrahepatic metastases [35] as significant factors. Trends were also found between functional and nonfunctional tumors, tumor locations, and hepatic tumor burdens [35, 43, 44].
Survival benefits of patients responding to somatostatin analog treatment compared with nonresponders were reported in two studies that investigated both octreotide and lanreotide [24, 35]. However, drug type was not investigated as a predictive factor.
Discussion
The present analysis identified a number of studies supportive of the antitumor effects of the somatostatin analogs octreotide and lanreotide for patients with NETs. Although most of the octreotide studies were uncontrolled trials in heterogeneous populations, results were consistent with those from PROMID, which was the only randomized, placebo-controlled, phase III study [38]. PROMID demonstrated for the first time that octreotide LAR inhibits tumor progression in patients with well-differentiated advanced midgut (or unknown location) NETs with or without secretory symptoms [38]. On the basis of these results, 30 mg of octreotide LAR should be considered for patients with symptomatic and asymptomatic, well-differentiated, advanced midgut NETs.
In response to results from PROMID, several guidelines (including those by the National Comprehensive Cancer Network, the North American Neuroendocrine Tumor Society, and the European Society for Medical Oncology) have been updated to recommend 20–30 mg of octreotide LAR as a management option for patients with recurrent or unresectable metastatic GI-NETs [1, 48–50]. Further studies including patients with pNETs and patients with primary tumor locations outside the midgut should be undertaken to clarify whether the antiproliferative effects of octreotide can be proven in these groups. Additional controlled trials in a larger number of patients are needed to identify prognostic factors to antitumor response and to confirm whether the antitumor effect of octreotide translates to a survival benefit.
No placebo-controlled studies have been published to date on the antitumor effect of lanreotide for patients with NETs. A double-blind, randomized, placebo-controlled, phase III study of lanreotide Autogel compared with placebo investigating progression-free survival in patients with nonsecretory enteropancreatic NETs (CLARINET) is ongoing. As noted here, results from uncontrolled prospective trials identified in the present literature search suggest that the rates of stable disease in patients treated with lanreotide (up to 89%) are comparable with those observed in patients treated with octreotide in uncontrolled studies (up to 88%).
There is also evidence to suggest that pasireotide (SOM230), a novel somatostatin analog, may have potential antitumor properties. Pasireotide targets multiple somatostatin receptors, including subtype 3, and has a higher affinity for subtypes 1 and 5 than octreotide and lanreotide [51]. Whether the ability to target additional somatostatin receptor subtypes translates to higher antitumor efficacy needs further investigation. Pasireotide has shown antiproliferative effects in vitro [52, 53]. A randomized, double-blind, phase III trial of pasireotide LAR compared with octreotide LAR in patients with metastatic GI-NETs is ongoing (NCT00690430), and tumor response as measured by RECIST version 1.0 is a secondary endpoint of this trial.
Somatostatin analogs may also be used in combination with other treatments to enhance antiproliferative effects. The effect of octreotide in combination with the mammalian target of rapamycin (mTOR) inhibitor everolimus was examined for patients with GI-NET in the phase III, randomized, controlled RAD001 in Advanced Neuroendocrine Tumors (RADIANT-2) study [54]. Progression-free survival for octreotide plus placebo (11.3 months) is consistent with that reported in PROMID (14.3 months) [38]. Patients treated with everolimus plus octreotide LAR were more likely to experience tumor shrinkage than patients receiving placebo plus octreotide LAR (75% vs. 45%), although the extent of shrinkage was not sufficient to be classified as an objective response as defined by RECIST [54].
In the RADIANT-1 phase II study of everolimus in patients with advanced pNETs, everolimus was added to octreotide LAR in a subset of patients (n = 45) with progressive pNETs who had been treated with octreotide LAR alone [55]. The median progression-free survival for these patients was 16.7 months (95% CI: 11.1–not reached), and the objective response rate was 4.4% [55]. Thus, there is a rationale for the everolimus plus octreotide LAR therapeutic combination because antitumor effects may be enhanced by simultaneously targeting upstream and downstream components of the mTOR pathway (Fig. 2) [56, 57]. Other treatment options being assessed in combination with somatostatin analogs include agents targeted against vascular endothelial growth factor (NCT00569127, NCT00427349), epidermal growth factor receptor (NCT01121939), insulin-like growth factor-1 receptor (NCT01204476), and techniques such as radioembolization with resin microspheres (NCT00466856). The results of these studies are awaited with interest.
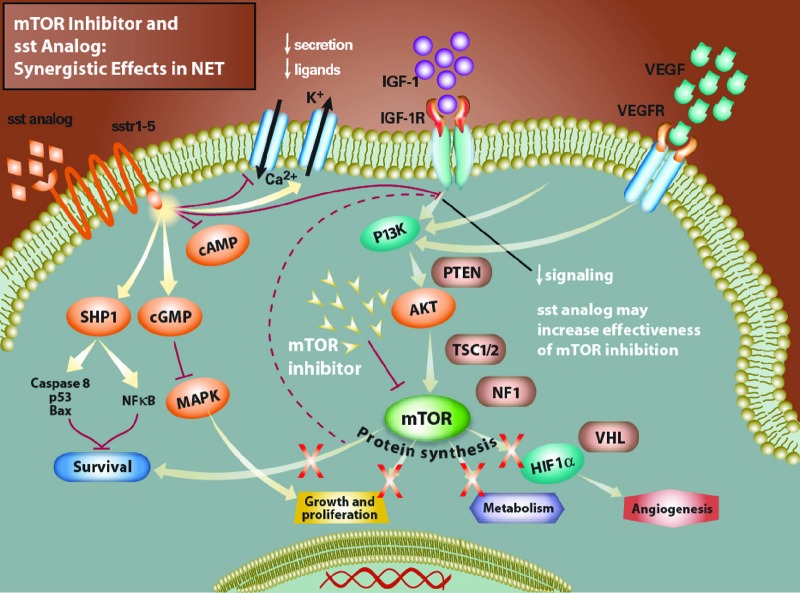
Figure 2.
Effect of combined somatostatin analog and mammalian target of rapamycin (mTOR) inhibitor therapy in neuroendocrine tumors. Combined treatment with an mTOR inhibitor and a somatostatin analog has been shown to cause tumor cell cycle arrest in vitro [57]. The indirect inhibition of mTOR through phosphoinositase-3-kinase/Akt resulting from the somatostatin analog seems to increase sensitivity to mTOR inhibition.
Abbreviations: cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; HIF, hypoxia-inducible factor; IGF, insulin-like growth factor; IGF-1R, insulin-like growth factor receptor 1, MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NET, neuroendocrine tumor; NF1, nuclear factor 1; NFκB, nuclear factor-κB; PI3K, phosphoinositase-3-kinase; PTEN, phosphatase and tensin homolog; SHP1, Src homology phosphatase-1; sst, somatostatin; TSC, tuberous sclerosis complex; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; VHL, Von Hippel-Lindau.
Conclusions
The randomized phase III PROMID trial is the only placebo-controlled clinical trial to date that demonstrates the antitumor effects of somatostatin analogs, specifically octreotide. Published results of uncontrolled clinical trials of the antitumor effect of octreotide in patients with NETs are consistent with these results. In addition to symptom control for patients with NETs, the data support an antitumor effect of octreotide and suggest that it may slow tumor growth. Data for lanreotide in phase II trials show similar results, although results of the placebo-controlled, phase III trial of patients with intestinal NETs and pNETs (CLARINET) are not expected until 2013. Further controlled trials in a larger number of patients are needed to identify prognostic factors to antitumor response and to confirm whether the antitumor effect of octreotide translates to a survival benefit.
Acknowledgments
We thank Sara Duggan, Ph.D., and Jennifer M. Kulak, Ph.D., of ApotheCom for editing assistance in the preparation of this manuscript. Editorial compensation was provided by Novartis Oncology.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Lucas Sidéris, Pierre Dubé, Anja Rinke
Manuscript writing: Lucas Sidéris, Pierre Dubé, Anja Rinke
Final approval of manuscript: Lucas Sidéris, Pierre Dubé, Anja Rinke
References
- 1.Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: Consensus Report of the National Cancer Institute Neuroendocrine Tumor Clinical Trials Planning Meeting. J Clin Oncol. 2011;29:934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinik AI, Woltering EA, Warner RR, et al. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713–734. doi: 10.1097/MPA.0b013e3181ebaffd. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Plockinger U, Rindi G, Arnold R, et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours: A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS) Neuroendocrinology. 2004;80:394–424. doi: 10.1159/000085237. [DOI] [PubMed] [Google Scholar]
- 5.Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: Well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735–752. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appetecchia M, Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J Exp Clin Cancer Res. 2010;29:19. doi: 10.1186/1756-9966-29-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somatuline Autogel: Summary of product characteristics. Uppsala, Sweden: Pharmacia; 2002. [Google Scholar]
- 8.Sandostatin LAR depot (octreotide acetate for injectable suspension) East Hanover, NJ: Novartis Pharmaceutical Corporation; 2010. [Google Scholar]
- 9.Lightman S. Somatuline autogel: An extended release lanreotide formulation. Hosp Med. 2002;63:162–165. doi: 10.12968/hosp.2002.63.3.2062. [DOI] [PubMed] [Google Scholar]
- 10.Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966–973. doi: 10.1093/annonc/mdh216. [DOI] [PubMed] [Google Scholar]
- 11.Somatuline LA: Summary of product characteristics. Brisbane, CA: Ipsen; 2002. [Google Scholar]
- 12.De Herder WW, Hofland LJ, Van Der Lely AJ, Lamberts SW. Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr Relat Cancer. 2003;10:451–458. doi: 10.1677/erc.0.0100451. [DOI] [PubMed] [Google Scholar]
- 13.Villaume K, Blanc M, Gouysse G, et al. VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/AKT/mTOR pathway. Neuroendocrinology. 2010;91:268–278. doi: 10.1159/000289569. [DOI] [PubMed] [Google Scholar]
- 14.Sandostatin LAR: Summary of product characteristics. Uppsala, Sweden: Pharmacia; 2002. [Google Scholar]
- 15.Kvols LK, Moertel CG, O'Connell MJ, et al. Treatment of the malignant carcinoid syndrome: Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315:663–666. doi: 10.1056/NEJM198609113151102. [DOI] [PubMed] [Google Scholar]
- 16.Rubin J, Ajani J, Schirmer W, et al. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol. 1999;17:600–606. doi: 10.1200/JCO.1999.17.2.600. [DOI] [PubMed] [Google Scholar]
- 17.Smith CS, Houston M, Jensen B, et al. A 32-year-old man with copious, watery diarrhea. N C Med J. 2001;62:134–139. [PubMed] [Google Scholar]
- 18.Clements D, Elias E. Regression of metastatic vipoma with somatostatin analogue SMS 201-995. Lancet. 1985;1:874–875. doi: 10.1016/s0140-6736(85)92235-4. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd JJ, Senator GB. Regression of liver metastases in patient with gastrin-secreting tumour treated with SMS 201-995. Lancet. 1986;2:574. doi: 10.1016/s0140-6736(86)90139-x. [DOI] [PubMed] [Google Scholar]
- 20.Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17:1733–1742. doi: 10.1093/annonc/mdl105. [DOI] [PubMed] [Google Scholar]
- 21.Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16:2963–2970. doi: 10.3748/wjg.v16.i24.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angeletti S, Corleto VD, Schillaci O, et al. Single dose of octreotide stabilize metastatic gastro-entero-pancreatic endocrine tumours. Italian J Gastroenterol Hepatol. 1999;31:23–27. [PubMed] [Google Scholar]
- 23.Anthony L, Johnson D, Hande K, et al. Somatostatin analogue phase I trials in neuroendocrine neoplasms. Acta Oncol. 1993;32:217–223. doi: 10.3109/02841869309083915. [DOI] [PubMed] [Google Scholar]
- 24.Aparicio T, Ducreux M, Baudin E, et al. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer. 2001;37:1014–1019. doi: 10.1016/s0959-8049(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 25.Arnold R, Rinke A, Klose KJ, et al. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: A randomized trial. Clin Gastroenterol Hepatol. 2005;3:761–771. doi: 10.1016/s1542-3565(05)00481-7. [DOI] [PubMed] [Google Scholar]
- 26.Arnold R, Trautmann ME, Creutzfeldt W, et al. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut. 1996;38:430–438. doi: 10.1136/gut.38.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajetta E, Catena L, Procopio G, et al. Is the new WHO classification of neuroendocrine tumours useful for selecting an appropriate treatment? Ann Oncol. 2005;16:1374–1380. doi: 10.1093/annonc/mdi258. [DOI] [PubMed] [Google Scholar]
- 28.di Bartolomeo M, Bajetta E, Buzzoni R, et al. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors: A study by the Italian Trials in Medical Oncology Group. Cancer. 1996;77:402–408. doi: 10.1002/(SICI)1097-0142(19960115)77:2<402::AID-CNCR25>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Oberg K, Norheim I, Theodorsson E. Treatment of malignant midgut carcinoid tumours with a long-acting somatostatin analogue octreotide. Acta Oncol. 1991;30:503–507. doi: 10.3109/02841869109092409. [DOI] [PubMed] [Google Scholar]
- 30.Ricci S, Antonuzzo A, Galli L, et al. Octreotide acetate long-acting release in patients with metastatic neuroendocrine tumors pretreated with lanreotide. Ann Oncol. 2000;11:1127–1130. doi: 10.1023/a:1008383132024. [DOI] [PubMed] [Google Scholar]
- 31.Saltz L, Trochanowski B, Buckley M, et al. Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer. 1993;72:244–248. doi: 10.1002/1097-0142(19930701)72:1<244::aid-cncr2820720143>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Tomassetti P, Migliori M, Corinaldesi R, Gullo L. Treatment of gastroenteropancreatic neuroendocrine tumours with octreotide LAR. Aliment Pharmacol Ther. 2000;14:557–560. doi: 10.1046/j.1365-2036.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- 33.Vinik A, Moattari AR. Use of somatostatin analog in management of carcinoid syndrome. Dig Dis Sci. 1989;34:14S–27S. doi: 10.1007/BF01536042. [DOI] [PubMed] [Google Scholar]
- 34.Butturini G, Bettini R, Missiaglia E, et al. Predictive factors of efficacy of the somatostatin analogue octreotide as first line therapy for advanced pancreatic endocrine carcinoma. Endocr Relat Cancer. 2006;13:1213–1221. doi: 10.1677/erc.1.01200. [DOI] [PubMed] [Google Scholar]
- 35.Panzuto F, Di Fonzo M, Iannicelli E, et al. Long-term clinical outcome of somatostatin analogues for treatment of progressive, metastatic, well-differentiated entero-pancreatic endocrine carcinoma. Ann Oncol. 2006;17:461–466. doi: 10.1093/annonc/mdj113. [DOI] [PubMed] [Google Scholar]
- 36.Shojamanesh H, Gibril F, Louie A, et al. Prospective study of the antitumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinoma. Cancer. 2001;94:331–343. doi: 10.1002/cncr.10195. [DOI] [PubMed] [Google Scholar]
- 37.Pavel ME, Heuck F, Plockinger U, et al. Prospective randomized trial: Biotherapy versus chemotherapy in malignant nonfunctional neuroendocrine tumors of the pancreas and bronchial tract (ENET-1). Paper presented at the Gastrointestinal Cancers Symposium; January 25–27, 2008; Orlando, FL. [Google Scholar]
- 38.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 39.Chadha MK, Lombardo J, Mashtare T, et al. High-dose octreotide acetate for management of gastroenteropancreatic neuroendocrine tumors. Anticancer Res. 2009;29:4127–4130. [PubMed] [Google Scholar]
- 40.Bajetta E, Procopio G, Catena L, et al. Lanreotide autogel every 6 weeks compared with lanreotide microparticles every 3 weeks in patients with well differentiated neuroendocrine tumors: A phase III study. Cancer. 2006;107:2474–2481. doi: 10.1002/cncr.22272. [DOI] [PubMed] [Google Scholar]
- 41.Ducreux M, Ruszniewski P, Chayvialle JA, et al. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am J Gastroenterol. 2000;95:3276–3281. doi: 10.1111/j.1572-0241.2000.03210.x. [DOI] [PubMed] [Google Scholar]
- 42.Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors: The International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 43.Faiss S, Rath U, Mansmann U, et al. Ultra-high-dose lanreotide treatment in patients with metastatic neuroendocrine gastroenteropancreatic tumors. Digestion. 1999;60:469–476. doi: 10.1159/000007693. [DOI] [PubMed] [Google Scholar]
- 44.Ricci S, Antonuzzo A, Galli L, et al. Long-acting depot lanreotide in the treatment of patients with advanced neuroendocrine tumors. Am J Clin Oncol. 2000;23:412–415. doi: 10.1097/00000421-200008000-00020. [DOI] [PubMed] [Google Scholar]
- 45.Tomassetti P, Migliori M, Gullo L. Slow-release lanreotide treatment in endocrine gastrointestinal tumors. Am J Gastroenterol. 1998;93:1468–1471. doi: 10.1111/j.1572-0241.1998.465_q.x. [DOI] [PubMed] [Google Scholar]
- 46.Wymenga AN, Eriksson B, Salmela PI, et al. Efficacy and safety of prolonged-release lanreotide in patients with gastrointestinal neuroendocrine tumors and hormone-related symptoms. J Clin Oncol. 1999;17:1111. doi: 10.1200/JCO.1999.17.4.1111. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Richard M, Alonzo V, Marmol M, et al. Evaluation of the efficacy and the safety of lanreotide autogel 120 mg on tumor growth stabilization in patients with progressive neuroendocrine tumors (NET) who are not eligible to surgery or chemotherapy. J Clin Oncol. 2011;29:e14660. [Google Scholar]
- 48.Anthony LB, Strosberg JR, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (NETS): Well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767–774. doi: 10.1097/MPA.0b013e3181ec1261. [DOI] [PubMed] [Google Scholar]
- 49.Boudreaux JP, Klimstra DS, Hassan MM, et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: Well-differentiated neuroendocrine tumors of the jejunum, ileum, appendix, and cecum. Pancreas. 2010;39:753–766. doi: 10.1097/MPA.0b013e3181ebb2a5. [DOI] [PubMed] [Google Scholar]
- 50.Oberg K, Akerstrom G, Rindi G, Jelic S. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v223–v227. doi: 10.1093/annonc/mdq192. [DOI] [PubMed] [Google Scholar]
- 51.Bruns C, Lewis I, Briner U, et al. SOM230: A novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146:707–716. doi: 10.1530/eje.0.1460707. [DOI] [PubMed] [Google Scholar]
- 52.Pavel M, Hassler G, Schmid HA, et al. Pasireotide strongly inhibits migration and proliferation of human micro- and macrovascular endothelial cells mainly via somatostatin receptor subtype 5 and FAK, ERK1/2 and p38 pathways. Endocrinology. 2006;147:352. [Google Scholar]
- 53.Pasquali D, Rossi V, Conzo G, et al. Effects of somatostatin analog SOM230 on cell proliferation, apop-tosis, and catecholamine levels in cultured pheochromocytoma cells. J Mol Endocrinol. 2008;40:263–271. doi: 10.1677/JME-08-0012. [DOI] [PubMed] [Google Scholar]
- 54.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT 2): A randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 55.Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: A phase II trial. J Clin Oncol. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strosberg JR, Kvols LK. A review of the current clinical trials for gastroenteropancreatic neuroendocrine tumours. Expert Opin Invest Drugs. 2007;16:219–224. doi: 10.1517/13543784.16.2.219. [DOI] [PubMed] [Google Scholar]
- 57.Cerovac V, Monteserin-Garcia J, Rubinfeld HB, et al. The somatostatin analogue octreotide confers sensitivity to rapamycin treatment on pituitary tumor cells. Cancer Res. 2010;70:666–674. doi: 10.1158/0008-5472.CAN-09-2951. [DOI] [PubMed] [Google Scholar]