The rationale for the diagnosis and management of hyponatremia in cancer patients is identified, the main causes are reviewed, and treatment options are discussed with a focus on the practical use of the arginine vasopressin antagonist tolvaptan.
Keywords: Cancer, Hyponatremia, Syndrome of inappropriate antidiuretic hormone, Arginine vasopressin, Tolvaptan
Abstract
Hyponatremia, a common electrolyte abnormality in oncology practice, may be a negative prognostic factor in cancer patients based on a systematic analysis of published studies. The largest body of evidence comes from small-cell lung cancer (SCLC), for which hyponatremia was identified as an independent risk factor for poor outcome in six of 13 studies. Hyponatremia in the cancer patient is usually caused by the syndrome of inappropriate antidiuretic hormone (SIADH), which develops more frequently with SCLC than with other malignancies. SIADH may be driven by ectopic production of arginine vasopressin (AVP) by tumors or by effects of anticancer and palliative medications on AVP production or action. Other factors may cause hypovolemic hyponatremia, including diarrhea and vomiting caused by cancer therapy. Hyponatremia may be detected on routine laboratory testing before or during cancer treatment or may be suggested by the presence of mostly neurological symptoms. Treatment depends on several factors, including symptom severity, onset timing, and extracellular volume status. Appropriate diagnosis is important because treatment differs by etiology, and choosing the wrong approach can worsen the electrolyte abnormality. When hyponatremia is caused by SIADH, hypertonic saline is indicated for acute, symptomatic cases, whereas fluid restriction is recommended to achieve a slower rate of correction for chronic asymptomatic hyponatremia. Pharmacological therapy may be necessary when fluid restriction is insufficient. The orally active, selective AVP receptor 2 (V2)-receptor antagonist tolvaptan provides a mechanism-based option for correcting hyponatremia caused by SIADH or other conditions with inappropriate AVP elevations. By blocking AVP effects in the renal collecting duct, tolvaptan promotes aquaresis, leading to a controlled increase in serum sodium levels.
Introduction
Hyponatremia is an electrolyte abnormality commonly encountered in oncology practice and is usually defined by a serum sodium level <135 mEq/L [1, 2]. Although many cases are asymptomatic, hyponatremia may cause neurological symptoms, particularly when serum sodium declines rapidly or by a substantial extent [3]. The incidence and prevalence of hyponatremia vary greatly, depending on the cancer type, clinical setting, and serum sodium cutoff point. Among cancer patients, hyponatremia occurs most frequently with small cell lung cancer (SCLC). In an analysis of nine consecutive clinical trials conducted jointly at four hospitals in Denmark and Sweden, a serum sodium level <136 mEq/L was identified in 415 of 1,684 SCLC patients (24.6%) [4]. Rates of 25%–44% were reported in smaller SCLC cohorts when a similar serum sodium cutoff was used [5–7], whereas rates of ∼15% were found when a serum sodium level <130 mEq/L was used as the cutoff [8, 9].
Most cases of hyponatremia are caused by the syndrome of inappropriate antidiuretic hormone (SIADH), with higher rates of SIADH found with SCLC than with other malignancies [2, 10]. However, hyponatremia—whether precipitated by SIADH, cancer treatment, or other underlying causes—may occur with other solid tumor types besides SCLC, as well as with hematological malignancies. This article identifies the rationale for diagnosis and management of hyponatremia in cancer patients, reviews its main causes, and then discusses treatment options, with a focus on the practical use of the arginine vasopressin (AVP) antagonist tolvaptan.
Rationale for Diagnosis and Management of Hyponatremia in Cancer Patients
A systematic search using PubMed and MEDLINE from inception through December 2011 was conducted to identify English-language studies that investigated the impact of hyponatremia on outcome in cancer patients. Thirteen studies were identified in SCLC, including six prospective and seven retrospective studies (Table 1) [4–9, 11–17]. Study sizes were in the range of 76–1,960 patients, with different serum sodium cutoff points used to define hyponatremia. Hyponatremia was significantly associated with a shorter survival duration on univariate analysis of the study cohorts in seven of the 13 studies (54%) and on multivariate analysis in six studies (46%). The prognostic value of hyponatremia varied among studies depending on the patient population; it was prognostic for a shorter survival time in patients with extensive disease but not in patients with limited disease in a Japanese cohort [14], whereas the opposite was suggested in a Danish cohort [5]. In the largest study, pretreatment hyponatremia was independently associated with a shorter survival time, particularly in the 6- to 24-month period after starting treatment [13]. Failure to normalize serum sodium was a negative prognostic factor in one study, in which the baseline serum sodium level was also prognostic for outcome [7]. A subset of 61 patients had baseline serum sodium levels <130 mEq/L and received at least two cycles of chemotherapy; patients whose serum sodium levels failed to normalize by the second cycle had poorer survival outcomes in both univariate and multivariate analyses than those who had serum sodium levels ≥136 mEq/L.
Table 1.
Prognostic value of hyponatremia for survival of patients with small-cell lung cancer
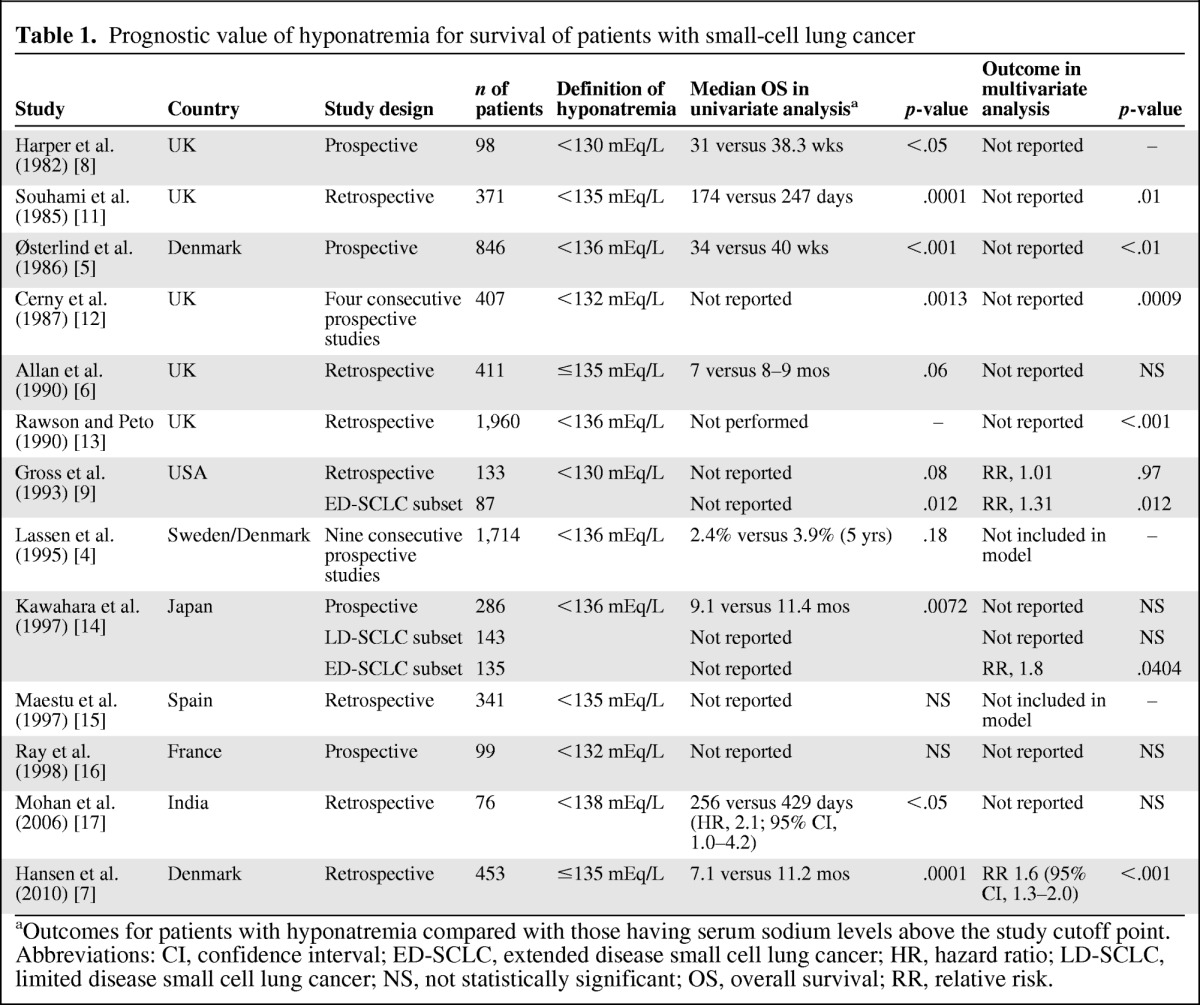
aOutcomes for patients with hyponatremia compared with those having serum sodium levels above the study cutoff point.
Abbreviations: CI, confidence interval; ED-SCLC, extended disease small cell lung cancer; HR, hazard ratio; LD-SCLC, limited disease small cell lung cancer; NS, not statistically significant; OS, overall survival; RR, relative risk.
Several studies were identified that explored the impact of a low serum sodium level on survival outcomes in patients with malignancies other than SCLC (Table 2). Hyponatremia was identified as a negative prognostic factor in both univariate and multivariate analyses in two of three studies of non-small cell lung cancer [16, 18, 19], two studies of renal cell carcinoma [20, 21], and in separate studies of gastric cancer [22, 23] and non-Hodgkin's lymphoma [24].
Table 2.
Prognostic value of hyponatremia for survival of patients with cancer, other than small cell lung cancer
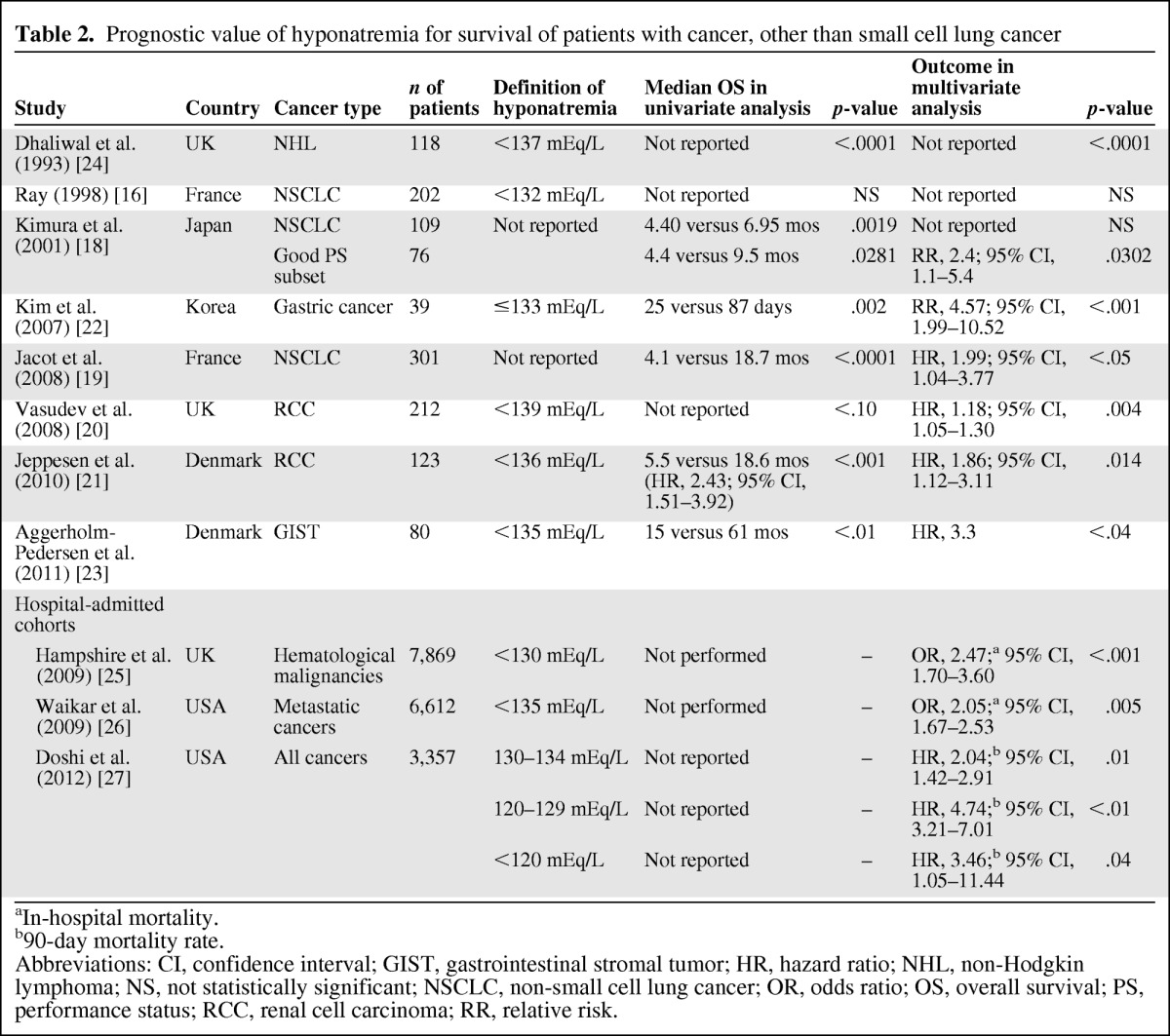
aIn-hospital mortality.
b90-day mortality rate.
Abbreviations: CI, confidence interval; GIST, gastrointestinal stromal tumor; HR, hazard ratio; NHL, non-Hodgkin lymphoma; NS, not statistically significant; NSCLC, non-small cell lung cancer; OR, odds ratio; OS, overall survival; PS, performance status; RCC, renal cell carcinoma; RR, relative risk.
Three large studies explored the prognostic role of hyponatremia at hospital admission (Table 2). The first study assessed 7,689 patients with hematological malignancies who were admitted to intensive care units in the U.K. in 1995–2007 [25]. A serum sodium level <130 mEq/L was identified in 4.2% of 6,766 patients who had assessments at admission, and this was independently associated with a significantly greater risk for in-hospital mortality (odds ratio [OR], 2.47; 95% confidence interval [CI], 1.70–3.60). The second study included 6,612 patients with metastatic cancer admitted to two Boston teaching hospitals in 2000–2002 [26]. A serum sodium level <135 mEq/L was identified in 10.8% of these patients, and this was also significantly associated with a greater risk for in-hospital mortality (OR, 2.05; 95% CI, 1.67–2.53). Of note, the risk for in-hospital mortality increased as serum sodium levels declined, with an OR of 4.8 (95% CI, 1.3–18.2) for patients with a serum sodium level ≤120 mEq/L. The third study evaluated the impact of hyponatremia among 3,357 cancer patients admitted to the M.D. Anderson Cancer Center during a 3-month period in 2006 [27]. Hyponatremia (defined as a serum sodium level <135 mEq/L) was identified in 47% of patients (23% at admission and 24% acquired during hospitalization), and this was independently associated with a poorer 90-day survival probability. Of note, patients whose serum sodium levels did not improve following admission had a higher 90-day mortality risk than those whose serum sodium improved (hazard ratio [HR], 2.09; 95% CI, 1.40–3.15).
Unfortunately, the data from the SCLC studies cannot be combined and subjected to a meta-analysis, given that most did not report HRs for survival outcomes. Moreover, the impact of hyponatremia on outcome in patients with other cancer types has been evaluated in only a limited number of studies. Nevertheless, when these data are considered together, they support the hypothesis that hyponatremia is a negative prognostic factor in cancer patients.
Causes of Hyponatremia in Cancer Patients
Hyponatremia typically develops in the presence of excessive water relative to existing sodium stores in the body [3]. Most cases reflect impaired water excretion, in which the capacity of the kidneys to eliminate water does not keep up with water intake, but in a minority of cases hyponatremia is driven by excessive water intake. Hyponatremia is often caused by SIADH in cancer patients [2, 10]. In this setting, ectopic secretion of AVP (also known an antidiuretic hormone [ADH]) by tumor cells appears to be important in driving the hyponatremic state. Under normal conditions, AVP is secreted by the posterior pituitary in response to increases in plasma osmolality detected by osmoreceptors located in the anterior hypothalamus, or by reductions in blood volume or pressure detected by baroreceptors located in the carotid sinus, aortic arch, atria, and pulmonary venous system [28]. AVP then activates AVP receptor 2 (V2) receptors located on the basolateral membrane of renal collecting duct cells to promote movement of aquaporin-2 containing vesicles from the cytoplasm to the apical membrane and, in turn, enhance the water permeability of the apical membrane [29]. Through this mechanism, AVP promotes water reabsorption and consequently reduces plasma osmolality. Further AVP secretion is normally suppressed once plasma osmolality falls below a genetically defined threshold [28]. In SIADH, however, AVP secretion is not fully suppressed despite the low plasma osmolality, and instead persistently elevated AVP levels are maintained by nonosmotic factors including ectopic production of AVP. In some cases, other neurohormones, such as atrial natriuretic peptide, may also contribute to the persistence of low plasma osmolality [30, 31].
SIADH is most commonly found in patients with SCLC, occurring at a frequency of 11%–15% [32, 33]. SIADH has been reported in ∼3% of patients with head and neck cancer (HNC) [34], most often in patients with lesions in the oral cavity and less frequently in those with lesions in the larynx, nasopharynx, hypopharynx, or other sites [35]. SIADH has also been identified in patients with a wide variety of other solid tumors and hematological malignancies, but at lower rates than those found with SCLC or HNC [2, 10]. Besides cancer, SIADH may be caused by a variety of other conditions, including central nervous system (CNS) disorders (e.g., inflammatory or demyelinating diseases, subarachnoid hemorrhage, head trauma), pulmonary disorders (e.g., tuberculosis, pneumonia, acute respiratory failure, positive-pressure ventilation), HIV infection, and prolonged strenuous exercise [3, 28].
Drugs used in the treatment and palliation of cancer may also cause hyponatremia by inducing SIADH (Table 3). Vincristine and, to a lesser extent, vinblastine induce SIADH by altering normal osmotic control of ADH secretion through a neurotoxic effect on the hypothalamic–pituitary axis [36–38]. Cyclophosphamide induces SIADH by potentiating the actions of AVP in the kidneys, and possibly by stimulating AVP secretion [38]. This can lead to water intoxication, even when moderate doses of cyclophosphamide are used, because patients are encouraged to drink large amounts of fluids in an effort to prevent chemical cystitis [39, 40]. Similarly, opioids and antidepressants, including tricyclics and selective serotonin reuptake inhibitors, stimulate AVP secretion, whereas nonsteroidal anti-inflammatory drugs potentiate the effects of AVP in the renal tubules [38].
Table 3.
Drugs known to cause hyponatremia by affecting arginine vasopressin (AVP) production or action
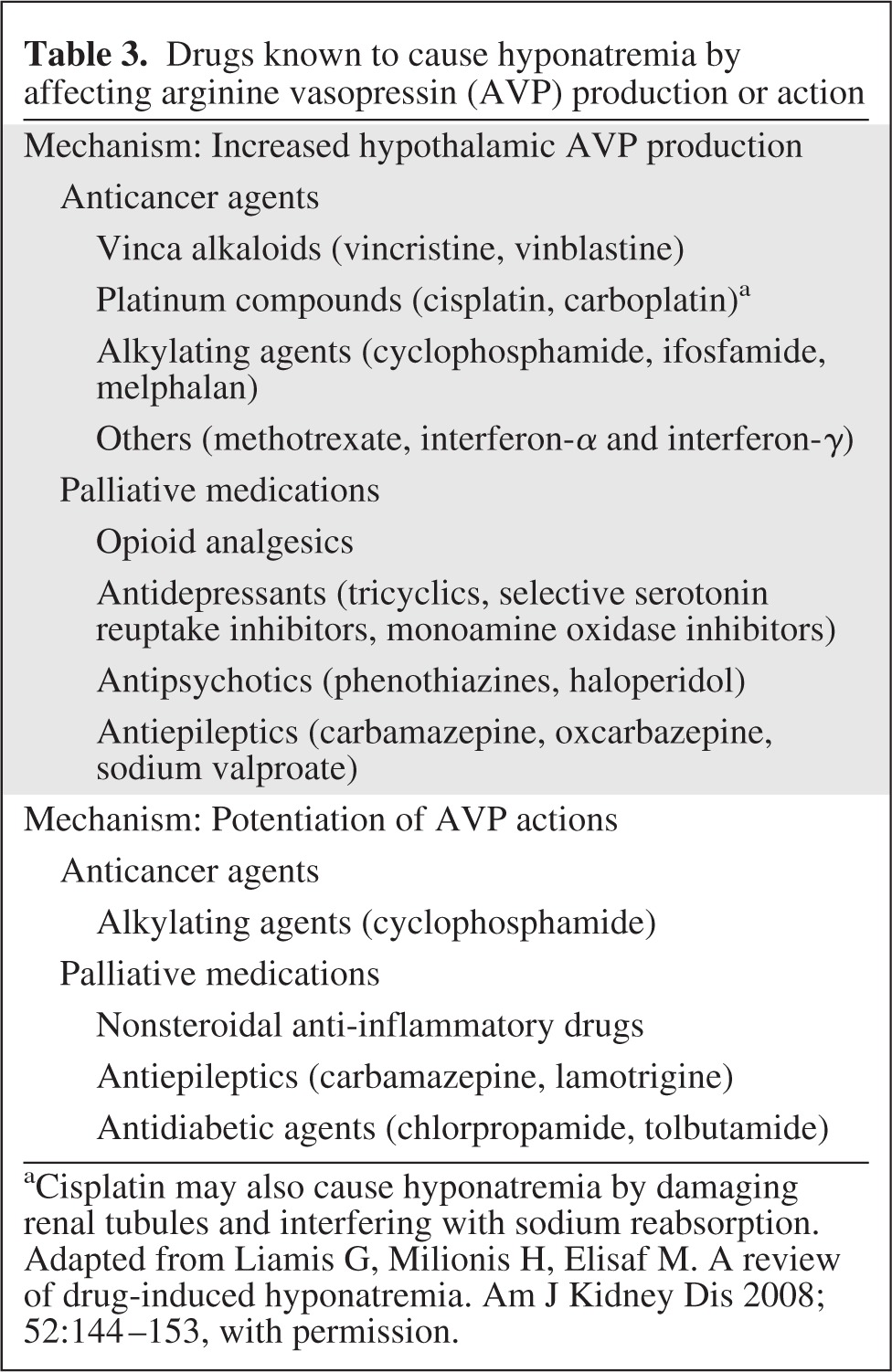
aCisplatin may also cause hyponatremia by damaging renal tubules and interfering with sodium reabsorption.
Adapted from Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis 2008;52:144–153, with permission.
The development of hyponatremia during cisplatin therapy bears special mention. Cisplatin stimulates AVP secretion to cause SIADH, but it can also directly damage renal tubules to interfere with sodium reabsorption, which in rare cases may lead to hyponatremia via salt wasting nephropathy [41]. Similarly, excessive sodium loss resulting from cerebral salt wasting may develop in patients with brain metastases, head trauma, or meningitis, or after CNS surgery [42]. These salt wasting syndromes are often difficult to distinguish from SIADH because each is characterized by low plasma and sodium osmolality, high urine sodium concentration, and higher urine than plasma osmolality. However, the distinction is important because their management differs, and choosing the wrong management approach can lead to worsening of the hyponatremia, with potential adverse consequences [2].
Diagnosis of Hyponatremia
Hyponatremia may be detected incidentally on routine laboratory testing before or during cancer treatment, or it may be suggested by the presence of mostly neurological symptoms (e.g., headache, nausea, vomiting, muscle cramps, lethargy, disorientation, depressed reflexes) [3]. Serious neurological complications may be evident following large or rapid declines in serum sodium, including seizures, coma, respiratory arrest, or brain-stem herniation. The symptoms associated with hyponatremia are attributable to water movement in the brain caused by the low plasma osmolality. Water moves across an osmotic gradient from intravascular spaces into brain cells, thereby raising intracranial pressure. However, adaptive mechanisms are activated to initially extrude inorganic solutes (e.g., sodium and potassium salts) and then organic solutes (e.g., glutamate, myoinositol) from the brain cells in order to limit cerebral edema [43]. The brain's adaptive mechanisms are generally completed within ∼48 hours, which explains why slow serum sodium declines may not cause symptoms. However, these adaptive mechanisms may be overwhelmed by large or rapid serum sodium decreases, thereby accounting for the appearance of symptoms in such cases. On the basis of the brain adaptation time frame, hyponatremia is classified as acute if it develops within 48 hours or chronic if it develops over >48 hours [44].
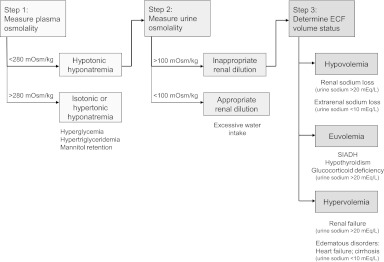
The differential diagnosis of hyponatremia is important for selecting appropriate treatment to correct the abnormality and is based on clinical and laboratory assessments (Fig. 1) [1, 45]. The first step is to determine plasma osmolality; it will be low (<280 mOsm/kg) in most cases. Normal or high plasma osmolality is suggestive of the presence of an osmotically active substance, such as glucose (i.e., hyperglycemia). For patients with low plasma osmolality, the next step is to assess urine osmolality in order to determine if renal diluting mechanisms are intact. Normal kidneys elaborate a maximally dilute urine (<100 mOsm/L) in the face of hyponatremia, and this scenario suggests excessive water intake as a cause. Impaired water excretion is suggested by inappropriately concentrated urine (>100 mOsm/L) in the presence of hyponatremia. For patients with inappropriately concentrated urine, it is critical to assess the extracellular fluid volume status. Volume depletion (hypovolemia) is suggested by orthostatic hypotension or tachycardia, dry mucus membranes, and poor skin turgor, whereas volume expansion (hypervolemia) is suggested by the presence of s.c. edema or ascites [28]. Patients without clinical evidence of hypovolemia or hypervolemia are considered to be euvolemic.
Figure 1.
Algorithm for the differential diagnosis of hyponatremia.
Abbreviations: ECF, extracellular fluid; SIADH, syndrome of inappropriate antidiuretic hormone.
Modified from Palmer BF, Gates JR, Lader M. Causes and management of hyponatremia. Ann Pharmacother 2003;37:1694–1702 and Douglas I. Hyponatremia: Why it matters, how it presents, how we can manage it. Clev Clin J Med 2006;73(suppl 3):S4–S12.
Additional clues about volume status may be obtained from measurements of urine sodium, blood urea nitrogen (BUN), serum uric acid, and serum potassium [28, 45, 46]. Urine sodium >30 mEq/L in the absence of diuretic use or renal disease is supportive of a euvolemic state, whereas a low urine sodium level (<30 mEq/L) is supportive of hypovolemia because the kidneys reabsorb sodium to conserve volume. However, the urine sodium level may be high if the kidneys are responsible for the sodium loss, as in salt wasting syndromes, or in elderly patients who adapt slowly to rapid volume depletion. Low BUN and serum uric acid levels are also supportive of euvolemia, whereas high values are supportive of hypovolemia; variations in the levels of these substances reflect changes in their proximal reabsorption based on volume status.
Potassium depletion may be suggestive of diuretic-induced hyponatremia [45]. In addition, assessment of the acid–base and potassium balance may be useful in patients in whom the diagnosis is not apparent; for example, metabolic alkalosis and hypokalemia suggest diuretic use or vomiting, metabolic acidosis and hypokalemia suggest diarrhea or laxative abuse, and metabolic acidosis and hyperkalemia suggest adrenal insufficiency [47]. On the other hand, plasma bicarbonate and potassium concentrations are typically normal in patients with SIADH [48]. Volume depletion with a high urine sodium level and accompanying hyperkalemia should raise suspicion for mineralocorticoid deficiency; a low urine potassium level or transtubular potassium gradient can provide confirmation [28].
As noted previously, hyponatremia in the oncology setting is usually caused by SIADH—which is characterized by an essentially normal extracellular volume (euvolemia). Euvolemic hyponatremia may also be associated with hypothyroidism (in patients with myxedema or panhypopituitarism) or adrenal insufficiency, and therefore assessment of thyroid and adrenal function may be considered in the differential diagnosis [3, 28, 46, 49]. Hypovolemic hyponatremia can be caused by either renal or extrarenal sodium loss; the former may be a result of diuretic therapy, cerebral salt wasting, or mineralocorticoid deficiency, whereas the latter may reflect gastrointestinal sodium loss caused by vomiting or diarrhea, third-space losses caused by bowel obstruction, pancreatitis, muscle trauma, or burns, or excessive sweating during endurance exercising. Hypervolemic hyponatremia is associated with congestive heart failure, liver cirrhosis, nephrotic syndrome, and acute and chronic renal failure.
Treatment Options
Formalized guidelines for the management of hyponatremia in oncology patients have not been established. In general terms, the treatment of hyponatremia depends on whether or not symptoms are present, their severity and time of onset, and the extracellular volume status of the patient [1, 3, 28]. Symptomatic patients require prompt attention in order to prevent serious complications. However, the adaptive mechanisms that control brain swelling during the development of chronic hyponatremia also make the brain susceptible to osmotic demyelination if serum sodium is corrected in an overly rapid manner. Therefore, the serum sodium level should be raised in a controlled manner: the rate of correction should be kept <12 mEq/L in 24 hours and <18 mEq/L in 48 hours [28]. Patients with acute onset of hyponatremia are less susceptible to osmotic demyelination with rapid serum sodium increases because the initial adaptive mechanisms are not fully established. However, when it is difficult to pinpoint the timing of onset, it is prudent to assume a chronic course and correct serum sodium levels in a controlled manner.
The correction of severely symptomatic hyponatremia in patients with SIADH or other euvolemic states or hypervolemia is achieved by administration of hypertonic (3%) saline via a continuous infusion or bolus [28]. Symptoms of severe hyponatremia, such as seizures, impaired mental status, or coma, are usually seen with strenuous exercise, use of 3,4-methylenedioxymethamphetamine (ecstasy), primary polydipsia, and postoperatively in patients with known intracerebral pathology. Treatment with hypertonic saline should be stopped once symptoms resolve, a safe serum sodium concentration is achieved, or the maximum sodium correction limits are approached. The treatment approach to salt wasting and other hypovolemic conditions differs: isotonic (0.9%) saline is typically administered until the volume deficit is corrected and the patient returns to a euvolemic state [28].
Asymptomatic patients with euvolemic or hypervolemic hyponatremia are usually managed initially by fluid restriction, with the goal of achieving a negative water balance [3, 28]. Fluid (but not sodium) should be restricted to approximately 500 ml below the average daily urine output, and any drug known to cause SIADH should be discontinued whenever possible and replaced with another agent that does not cause hyponatremia. Fluid restriction usually takes several days before significant increases in serum sodium levels are achieved. Fluid restriction poses a particular challenge in oncology patients requiring urgent treatment with cisplatin because patients must be adequately hydrated when receiving this agent. One potential approach is to correct the hyponatremia as rapidly as possible and then proceed with cisplatin therapy (a more likely scenario in patients with severe hyponatremia). A second option is to treat the cancer first—which could also yield improvement in the sodium status—while monitoring carefully for hyponatremia (a likely scenario if cancer therapy is deemed to be more urgent). The approach to these patients should be dictated by the specific clinical scenario and by the treating physician's best medical judgment.
Patient compliance with fluid restriction is often poor, and therefore pharmacological interventions may be considered in patients with euvolemic or hypervolemic hyponatremia who do not respond adequately. When SIADH is caused by a tumor, pharmacological treatment should be avoided initially because successful treatment of the malignancy may eliminate or reduce the inappropriate AVP secretion [28]. Nevertheless, pharmacological intervention may be necessary, and several options are available.
Older medications such as demeclocycline, urea, and lithium are limited by variable efficacy, poor palatability, and/or toxicity. The tetracycline derivative demeclocycline induces nephrogenic diabetes insipidus, but it has an unpredictable onset and only works in ∼60% of patients [28, 50]. Monitoring of renal function is necessary because demeclocycline causes reversible azotemia and nephrotoxicity, particularly in patients with, or at risk for, renal compromise (e.g., those with cirrhosis or congestive heart failure). Lithium also induces nephrogenic diabetes insipidus, but it works in a smaller proportion of patients than demeclocycline [50]. Lithium toxicity includes gastrointestinal and CNS side effects, renal toxicity, hypothyroidism, and antianabolic effects. Urea is an osmotic diuretic that increases water excretion and decreases urinary sodium excretion [28]. Although it is generally effective, urea has the potential to cause azotemia, liver failure, and hypersensitivity. A convenient dosage form is unavailable, and because of its unpleasant taste, it needs to be dissolved in a strongly flavored liquid. Urea is currently used only in some European countries where palatability is not deemed to be an issue.
The limitations of fluid restriction and these older pharmacological interventions suggest the need for newer, more effective treatment strategies. The V2 receptor on renal collecting duct cells represents an attractive molecular target because AVP is elevated in patients with euvolemic and hypervolemic hyponatremia despite the presence of low plasma osmolality, and AVP activates the V2 receptor to stimulate water reabsorption, with consequent dilution of serum sodium concentrations [51]. The use of vasopressin receptor antagonists to address hyponatremia in cancer patients has two potential benefits: (a) patients can undergo chemotherapy with platinum-based regimens without concerns for further hyponatremia and (b) in patients who will not be treated with chemotherapy, these agents may reduce the risks and mitigate the symptoms associated with hyponatremia.
Three vasopressin antagonists (conivaptan, tolvaptan, mozavaptan) have been introduced into clinical practice and others (e.g., lixivaptan, satavaptan) have undergone clinical testing. Mozavaptan is an oral vasopressin V2-receptor antagonist approved in Japan as an orphan drug for use in cancer patients with ectopic ADH syndrome (i.e., excessive AVP secretion by cancer cells leading to hyponatremia). Short-term (7-day) treatment of 16 patients with ectopic ADH syndrome yielded significant increases in sodium concentrations, resulting in improvements in hyponatremia symptoms [52]. Conivaptan is an i.v. administered vasopressin V1A- and V2-receptor antagonist that has been shown to increase serum sodium levels in hospitalized patients with euvolemic or hypervolemic hyponatremia [53], and it is approved for use in this patient population in the U.S. Treatment with this agent is limited to 2–4 days. Tolvaptan, which is approved in Europe for the treatment of hyponatremia secondary to SIADH, in the U.S. for treatment of euvolemic or hypervolemic hyponatremia, and in Japan for treatment of excess water retention in patients with cardiac failure, is an oral vasopressin V2-receptor antagonist that is administered once daily and can be continued after patients are discharged from the hospital [54]; accordingly, it may be of more practical value for treating hyponatremia in cancer patients. The following section reviews the clinical profile and practical use of tolvaptan.
Tolvaptan
Tolvaptan increases urine water excretion by blocking the effects of endogenous AVP at V2 receptors in the renal collecting duct, thereby increasing free water clearance (aquaresis), reducing urine osmolality, and consequently raising serum sodium concentrations [55, 56]. In the Study of Ascending Levels of Tolvaptan in Hyponatremia-1 (SALT-1) and SALT-2 trials (n = 448), tolvaptan (starting dose, 15 mg/day; maximum dose, 60 mg/day) was significantly better at increasing serum sodium levels than placebo in patients with euvolemic or hypervolemic hyponatremia during the first 4 days of treatment and during the entire 30-day study period (both p < .001) [57]. Significantly more patients achieved normal serum sodium concentrations with tolvaptan than with placebo on day 4 (40% versus 13% in the SALT-1 trial and 55% versus 11% in the SALT-2 trial; both p < .001) and on day 30 (53% versus 25% and 58% versus 25%, respectively; both p < .001). Importantly, correction of the serum sodium level by tolvaptan was achieved without the use of fluid restriction during the first 24 hours of treatment, and it was brought about in a controlled manner: only four of 223 patients (1.8%) had an overly rapid serum sodium correction on day 1 and four of 223 patients (1.8%) had a serum sodium level >146 mEq/L at some point during the study period. Tolvaptan was generally well tolerated: thirst (14% versus 5%), dry mouth (13% versus 4%), and increased urination (7% versus 3%) were the most common adverse events that occurred more frequently with tolvaptan than with placebo. Tolvaptan was discontinued at the end of the 30-day study period. When measured 7 days later, serum sodium levels had declined to levels found in placebo-treated patients.
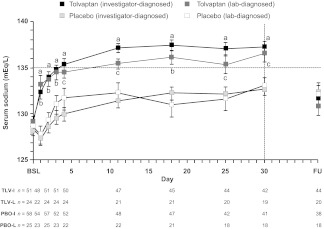
The SALT trials enrolled patients with hyponatremia resulting from a variety of underlying causes, including SIADH, heart failure, and liver cirrhosis. In each of these subsets, as well as in the subgroups with baseline serum sodium levels <130 mEq/L or <125 mEq/L, the efficacy of tolvaptan was comparable to that observed in the entire study population [54, 58, 59]. As shown in Figure 2, tolvaptan was significantly better at improving serum sodium levels than placebo over the first 4 days and during the entire 30-day treatment period (both p < .0001) in the subset of 110 patients with a primary diagnosis of SIADH [58]. Higher rates of normalized serum sodium were observed at both time points (day 4, 60% versus 11.5%; day 30, 66.6% versus 26.8%; both p < .05). The inclusion criteria for the SALT trials did not exclude patients with oncology-induced SIADH; however, results in this subpopulation have not been reported. Prospective studies are needed to confirm the hypothesis that improving hyponatremia leads to better outcomes.
Figure 2.
Serum sodium levels in SIADH patients during treatment with tolvaptan or placebo in the SALT trials. Investigator-diagnosed patients received a primary diagnosis of SIADH from the investigator; lab-diagnosed patients received a primary diagnosis of SIADH from the investigator and had a urine sodium concentration >20 mEq/L during the first day of treatment.
ap < .0001, tolvaptan (investigator-diagnosed) versus placebo (investigator-diagnosed).
bp < .001, tolvaptan (lab-diagnosed) versus placebo (lab-diagnosed).
cp < .029, tolvaptan (lab-diagnosed) versus placebo (lab-diagnosed).
Error bars are ± standard error of the mean.
Abbreviations: BSL, baseline; FU, 7-day follow-up visit; PBO-I, placebo (investigator-diagnosed); PBO-L, placebo (lab-diagnosed), TLV-I; tolvaptan (investigator-diagnosed); TLV-L, tolvaptan (lab-diagnosed); SALT, Study of Ascending Levels of Tolvaptan in Hyponatremia; SIADH, syndrome of inappropriate antidiuretic hormone.
Reproduced with permission from Verbalis JG, Adler S, Schrier RW et al. Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur J Endocrinol 2011;164:725–732. ©Society of the European Journal of Endocrinology (2011).
Tolvaptan is indicated by the European Medicines Agency for the treatment of hyponatremia secondary to SIADH [60], whereas it is indicated for the treatment of clinically significant hypervolemic or euvolemic hyponatremia (serum sodium <125 mEq/L or less marked hyponatremia that is symptomatic and has resisted correction with fluid restriction) in the U.S. [54]. It is contraindicated in patients with hypovolemic hyponatremia, volume depletion, and anuria, and in those who cannot perceive or respond appropriately to thirst, and it should not be used in patients whose serum sodium levels need to be urgently raised [54, 60]. Tolvaptan is also contraindicated in Europe in women who are pregnant or breastfeeding, whereas it carries pregnancy category C labeling in the U.S. [54, 60].
Tolvaptan therapy should be started while patients are in a hospital to allow monitoring of the therapeutic response and ensure that serum sodium is corrected in a controlled manner [54, 60]. The starting dose is 15 mg once daily, which can be given without regard to the timing of meals. Dose increases to 30 mg once daily and subsequently to a maximum of 60 mg once daily may be made at 24-hour intervals if serum sodium is not raised to the desired level. Patients should be provided with access to sufficient amounts of water to ensure that they do not become overly dehydrated. Dose adjustments based on age, gender, race, mild or moderate renal impairment, and mild or moderate hepatic impairment are not necessary. If the serum sodium level rises at an overly rapid rate (>12 mEq/L in 24 hours), tolvaptan should be discontinued and treatment with hypotonic fluid should be considered. Following completion of tolvaptan therapy, fluid restriction should be resumed, and changes in serum sodium and volume status should be monitored [54]. If additional tolvaptan is needed, treatment should be restarted in a hospital setting in order to monitor the therapeutic response. In an open-label extension of the SALT trials, reinitiation of tolvaptan raised serum sodium levels to levels comparable to those seen to initial therapy, and these levels were maintained by continued daily therapy for ≥1 year [61].
Conclusions
Hyponatremia is a negative prognostic factor in cancer patients, and it is commonly caused by SIADH. In the oncology setting, SIADH may be a result of ectopic AVP production by tumor cells or may be a result of stimulation of AVP secretion or potentiation of AVP effects by anticancer drugs or palliative medications. Distinguishing SIADH from other underlying causes of hyponatremia, particularly salt wasting syndromes and other hypovolemic states, is important for selecting appropriate treatment and ensuring that the serum sodium abnormality is not worsened. Laboratory assessments, including measurements of plasma osmolality, urine osmolality, and urine sodium, and clinical assessment of extracellular volume status are critical to the differential diagnosis of hyponatremia. Symptomatic patients with hyponatremia caused by SIADH are treated initially with hypertonic saline, whereas asymptomatic patients are generally managed with fluid restriction. However, fluid restriction is associated with poor patient compliance and is less likely to be effective with greater elevations in urine osmolality (indicative of higher plasma AVP levels) [28]. Older pharmacological medications, such as demeclocycline, urea, and lithium, are limited by their variable efficacy, poor palatability, and significant toxicity, thus underscoring the need for new treatment approaches. By selectively blocking V2 receptors in the renal collecting duct, tolvaptan provides a mechanism-based approach for treating hyponatremia secondary to SIADH, including in patients for whom fluid restriction was ineffective. In the oncologic setting, the use of vasopressin receptor antagonists could improve hyponatremia and allow patients to receive adequate therapies or palliate symptoms. Further studies are needed to evaluate the prognostic value of hyponatremia and its treatment in cancer patients.
Acknowledgments
Eric Justice of BioScience Communications, New York, NY, provided writing and editing support, copyediting support, and editorial and production assistance for the development of this manuscript (supported by Otsuka America Pharmaceutical, Inc., Rockville, MD).
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Jorge J. Castillo, Marc Vincent
Collection and/or assembly of data: Jorge J. Castillo, Marc Vincent, Eric Justice
Data analysis and interpretation: Jorge J. Castillo, Marc Vincent
Manuscript writing: Eric Justice
Final approval of manuscript: Jorge J. Castillo, Marc Vincent, Eric Justice
References
- 1.Palmer BF, Gates JR, Lader M. Causes and management of hyponatremia. Ann Pharmacother. 2003;37:1694–1702. doi: 10.1345/aph.1D105. [DOI] [PubMed] [Google Scholar]
- 2.Onitilo AA, Kio E, Doi SA. Tumor-related hyponatremia. Clin Med Res. 2007;5:228–237. doi: 10.3121/cmr.2007.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–1589. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 4.Lassen U, Østerlind K, Hansen M, et al. Long-term survival in small-cell lung cancer: Posttreatment characteristics in patients surviving 5 to 18+ years—an analysis of 1,714 consecutive patients. J Clin Oncol. 1995;13:1215–1220. doi: 10.1200/JCO.1995.13.5.1215. [DOI] [PubMed] [Google Scholar]
- 5.Østerlind K, Andersen PK. Prognostic factors in small cell lung cancer: Multivariate model based on 778 patients treated with chemotherapy with or without irradiation. Cancer Res. 1986;46:4189–4194. [PubMed] [Google Scholar]
- 6.Allan SG, Stewart ME, Love S, et al. Prognosis at presentation of small cell carcinoma of the lung. Eur J Cancer. 1990;26:703–705. doi: 10.1016/0277-5379(90)90121-9. [DOI] [PubMed] [Google Scholar]
- 7.Hansen O, Sr̸ensen P, Hansen KH. The occurrence of hyponatremia in SCLC and the influence on prognosis: A retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer. 2010;68:111–114. doi: 10.1016/j.lungcan.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Harper PG, Souhami RL, Spiro SG, et al. Tumor size, response rate, and prognosis in small cell carcinoma of the bronchus treated with combination chemotherapy. Cancer Treat Rep. 1982;66:463–470. [PubMed] [Google Scholar]
- 9.Gross AJ, Steinberg SM, Reilly JG, et al. Atrial natriuretic factor and arginine vasopressin production in tumor cell lines from patients with lung cancer and their relationship to serum sodium. Cancer Res. 1993;53:67–74. [PubMed] [Google Scholar]
- 10.Raftopoulos H. Diagnosis and management of hyponatremia in cancer patients. Support Care Cancer. 2007;15:1341–1347. doi: 10.1007/s00520-007-0309-9. [DOI] [PubMed] [Google Scholar]
- 11.Souhami RL, Bradbury I, Geddes DM, et al. Prognostic significance of laboratory parameters measured at diagnosis in small cell carcinoma of the lung. Cancer Res. 1985;45:2878–2882. [PubMed] [Google Scholar]
- 12.Cerny T, Blair V, Anderson H, et al. Pretreatment prognostic factors and scoring system in 407 small-cell lung cancer patients. Int J Cancer. 1987;39:146–149. doi: 10.1002/ijc.2910390204. [DOI] [PubMed] [Google Scholar]
- 13.Rawson NS, Peto J. An overview of prognostic factors in small cell lung cancer. A report from the Subcommittee for the Management of Lung Cancer of the United Kingdom Coordinating Committee on Cancer Research. Br J Cancer. 1990;61:597–604. doi: 10.1038/bjc.1990.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawahara M, Fukuoka M, Saijo N, et al. Prognostic factors and prognostic staging system for small cell lung cancer. Jpn J Clin Oncol. 1997;27:158–165. doi: 10.1093/jjco/27.3.158. [DOI] [PubMed] [Google Scholar]
- 15.Maestu I, Pastor M, Gómez-Codina J, et al. Pretreatment prognostic factors for survival in small-cell lung cancer: A new prognostic index and validation of three known prognostic indices on 341 patients. Ann Oncol. 1997;8:547–553. doi: 10.1023/a:1008212826956. [DOI] [PubMed] [Google Scholar]
- 16.Ray P, Quantin X, Grenìer J, et al. Predictive factors of tumor response and prognostic factors of survival during lung cancer chemotherapy. Cancer Detect Prev. 1998;22:293–304. doi: 10.1046/j.1525-1500.1998.cdoa43.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohan A, Goyal A, Singh P, et al. Survival in small cell lung cancer in India: Prognostic utility of clinical features, laboratory parameters and response to treatment. Indian J Cancer. 2006;43:67–74. doi: 10.4103/0019-509x.25887. [DOI] [PubMed] [Google Scholar]
- 18.Kimura T, Kudoh S, Hirata K, et al. Prognostic factors in elderly patients with unresectable non-small cell lung cancer. Anticancer Res. 2001;21:1379–1383. [PubMed] [Google Scholar]
- 19.Jacot W, Colinet B, Bertrand D, et al. OncoLR Health Network. Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Ann Oncol. 2008;19:1458–1464. doi: 10.1093/annonc/mdn064. [DOI] [PubMed] [Google Scholar]
- 20.Vasudev NS, Brown JE, Brown SR, et al. Prognostic factors in renal cell carcinoma: Association of preoperative sodium concentration with survival. Clin Cancer Res. 2008;14:1775–1781. doi: 10.1158/1078-0432.CCR-07-1721. [DOI] [PubMed] [Google Scholar]
- 21.Jeppesen AN, Jensen HK, Donskov F, et al. Hyponatremia as a prognostic and predictive factor in metastatic renal cell carcinoma. Br J Cancer. 2010;102:867–872. doi: 10.1038/sj.bjc.6605563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HS, Yi SY, Jun HJ, et al. Clinical outcome of gastric cancer patients with bone marrow metastases. Oncology. 2007;73:192–197. doi: 10.1159/000127386. [DOI] [PubMed] [Google Scholar]
- 23.Aggerholm-Pedersen N, Rasmussen P, Dybdahl H, et al. Serum natrium determines outcome of treatment of advanced GIST with imatinib: A retrospective study of 80 patients from a single institution. ISRN Oncol. 2011;2011:523915. doi: 10.5402/2011/523915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhaliwal HS, Rohatiner AZ, Gregory W, et al. Combination therapy for intermediate and high grade non-Hodgkin's lymphoma. Br J Cancer. 1993;68:767–774. doi: 10.1038/bjc.1993.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampshire PA, Welch CA, McCrossan LA, et al. Admission factors associated with hospital mortality in patients with haematological malignancy admitted to UK adult, general critical care units: A secondary analysis of the ICNARC Case Mix Programme Database. Crit Care. 2009;13:R137. doi: 10.1186/cc8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122:857–865. doi: 10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doshi SM, Shah P, Lei X, et al. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am J Kidney Dis. 2012;59:222–228. doi: 10.1053/j.ajkd.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Verbalis JG, Goldsmith SR, Greenberg A, et al. Hyponatremia treatment guidelines 2007: Expert panel recommendations. Am J Med. 2007;120(suppl 1):S1–S21. doi: 10.1016/j.amjmed.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Schrier RW. Vasopressin and aquaporin 2 in clinical disorders of water homeostasis. Semin Nephrol. 2008;28:289–296. doi: 10.1016/j.semnephrol.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson BE, Chute JP, Rushin J, et al. A prospective study of patients with lung cancer and hyponatremia of malignancy. Am J Respir Crit Care Med. 1997;156:1669–1678. doi: 10.1164/ajrccm.156.5.96-10075. [DOI] [PubMed] [Google Scholar]
- 31.Chute JP, Taylor E, Williams J, et al. A metabolic study of patients with lung cancer and hyponatremia of malignancy. Clin Cancer Res. 2006;12:888–896. doi: 10.1158/1078-0432.CCR-05-1536. [DOI] [PubMed] [Google Scholar]
- 32.List AF, Hainsworth JD, Davis BW, et al. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol. 1986;4:1191–1198. doi: 10.1200/JCO.1986.4.8.1191. [DOI] [PubMed] [Google Scholar]
- 33.Sørensen JB, Andersen MK, Hansen HH. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in malignant disease. J Intern Med. 1995;238:97–110. doi: 10.1111/j.1365-2796.1995.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 34.Talmi YP, Hoffman HT, McCabe BF. Syndrome of inappropriate secretion of arginine vasopressin in patients with cancer of the head and neck. Ann Otol Rhinol Laryngol. 1992;101:946–949. doi: 10.1177/000348949210101111. [DOI] [PubMed] [Google Scholar]
- 35.Ferlito A, Rinaldo A, Devaney KO. Syndrome of inappropriate antidiuretic hormone secretion associated with head and neck cancers: Review of the literature. Ann Otol Rhinol Laryngol. 1997;106:878–883. doi: 10.1177/000348949710601014. [DOI] [PubMed] [Google Scholar]
- 36.Robertson GL, Bhoopalam N, Zelkowitz LJ. Vincristine neurotoxicity and abnormal secretion of antidiuretic hormone. Arch Intern Med. 1973;132:717–720. [PubMed] [Google Scholar]
- 37.Stuart MJ, Cuaso C, Miller M, et al. Syndrome of recurrent increased secretion of antidiuretic hormone following multiple doses of vincristine. Blood. 1975;45:315–320. [PubMed] [Google Scholar]
- 38.Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis. 2008;52:144–153. doi: 10.1053/j.ajkd.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Harlow PJ, DeClerck YA, Shore NA, et al. A fatal case of inappropriate ADH secretion induced by cyclophosphamide therapy. Cancer. 1979;44:896–898. doi: 10.1002/1097-0142(197909)44:3<896::aid-cncr2820440316>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Bressler RB, Huston DP. Water intoxication following moderate-dose intravenous cyclophosphamide. Arch Intern Med. 1985;145:548–549. [PubMed] [Google Scholar]
- 41.Hamdi T, Latta S, Jallad B, et al. Cisplatin-induced renal salt wasting syndrome. South Med J. 2010;103:793–799. doi: 10.1097/SMJ.0b013e3181e63682. [DOI] [PubMed] [Google Scholar]
- 42.Momi J, Tang CM, Abcar AC, et al. Hyponatremia—what is cerebral salt wasting? Permanente J. 2010;14:62–65. doi: 10.7812/tpp/08-066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterns RH, Silver SM. Brain volume regulation in response to hypo-osmolality and its correction. Am J Med. 2006;119(suppl 1):S12–S16. doi: 10.1016/j.amjmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Adrogué HJ. Consequences of inadequate management of hyponatremia. Am J Nephrol. 2005;25:240–249. doi: 10.1159/000086019. [DOI] [PubMed] [Google Scholar]
- 45.Douglas I. Hyponatremia: Why it matters, how it presents, how we can manage it. Clev Clin J Med. 2006;73(suppl 3):S4–S12. doi: 10.3949/ccjm.73.suppl_3.s4. [DOI] [PubMed] [Google Scholar]
- 46.Zenenberg RD, Carluccio AL, Merlin MA. Hyponatremia: Evaluation and management. Hosp Pract (Minneap) 2010;38:89–96. doi: 10.3810/hp.2010.02.283. [DOI] [PubMed] [Google Scholar]
- 47.Rose BD, editor. Clinical Physiology of Acid-Base and Electrolyte Disorders. Fourth Edition. New York: McGraw-Hill; 1994. Hyponatremia and hyperkalemia in adrenal insufficiency; pp. 672–675. [Google Scholar]
- 48.Graber M, Corish D. The electrolytes in hyponatremia. Am J Kidney Dis. 1991;18:527–545. doi: 10.1016/s0272-6386(12)80647-0. [DOI] [PubMed] [Google Scholar]
- 49.Rachoin JS, Cerceo EA. Four nephrology myths debunked. J Hosp Med. 2011;6:E1–E5. doi: 10.1002/jhm.703. [DOI] [PubMed] [Google Scholar]
- 50.Sherlock M, Thompson CJ. The syndrome of inappropriate antidiuretic hormone: Current and future management options. Eur J Endocrinol. 2010;162(suppl 1):S13–S18. doi: 10.1530/EJE-09-1057. [DOI] [PubMed] [Google Scholar]
- 51.Verbalis JG. AVP receptor antagonists as aquaretics: Review and assessment of clinical data. Cleve Clin J Med. 2006;73(suppl 3):S24–S33. doi: 10.3949/ccjm.73.suppl_3.s24. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi K, Shijubo N, Kodama T, et al. Ectopic ADH Syndrome Therapeutic Research Group. Clinical implication of the antidiuretic hormone (ADH) receptor antagonist mozavaptan hydrochloride in patients with ectopic ADH syndrome. Jpn J Clin Oncol. 2011;41:148–152. doi: 10.1093/jjco/hyq170. [DOI] [PubMed] [Google Scholar]
- 53.Ghali JK, Farah JO, Daifallah S, et al. Conivaptan and its role in the treatment of hyponatremia. Drug Des Devel Ther. 2009;3:253–268. doi: 10.2147/dddt.s4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2009. Samsca™ (tolvaptan) tablets for oral use [prescrib-ing information] [Google Scholar]
- 55.Yamamura Y, Nakamura S, Itoh S, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: Pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287:860–867. [PubMed] [Google Scholar]
- 56.Costello-Boerrigter LC, Smith WB, Boerrigter G, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol. 2006;290:F273–F278. doi: 10.1152/ajprenal.00195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schrier RW, Gross P, Gheorghiade M, et al. SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 58.Verbalis JG, Adler S, Schrier RW, et al. Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur J Endocrinol. 2011;164:725–732. doi: 10.1530/EJE-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cárdenas A, Ginès P, Marotta P, et al. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol. 2012;56:571–578. doi: 10.1016/j.jhep.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 60.Samsca™ (tolvaptan) Tablets (2009) Annex I. Summary of Product Characteristics. [accessed June 14, 2011]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000980/WC500048716.pdf.
- 61.Berl T, Quittnat-Pelletier F, Verbalis JG, et al. SALTWATER Investigators. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21:705–712. doi: 10.1681/ASN.2009080857. [DOI] [PMC free article] [PubMed] [Google Scholar]