The clinical significance of extracellular signal–related kinase (ERK) was assessed in patients with triple-negative breast cancer versus patients with non–triple negative breast cancer. High ERK-2 levels were correlated with a lower overall survival rate and high phosphorylated mitogen-activated protein kinase levels were correlated with a higher relapse-free survival rate in triple-negative breast cancer patients.
Keywords: Triple-negative breast cancer, ERK, Survival, PEA-15, RPPA
Abstract
The mitogen-activated protein kinase (MAPK) signaling pathway is known to be activated in triple-negative breast cancer (TNBC). Extracellular signal–related kinase (ERK), a member of the MAPK pathway, promotes cell proliferation, angiogenesis, cell differentiation, and cell survival. To assess the prognostic impact of ERK in TNBC patients, relative quantities of ERK (ERK-2 and pMAPK) and direct targets of the ERK pathway (MAPK/ERK kinase 1, phospho-enriched protein in astrocytes [PEA]-15, phosphorylated (p)PEA-15, tuberous sclerosis protein 2, p70S6 kinase, and p27) were measured using reverse-phase protein arrays in tumor tissue from patients with TNBC (n = 97) and non-TNBC (n = 223). Protein levels in patients with TNBC were correlated with clinical and tumor characteristics and outcome. The median age of patients with TNBC was 55 years (range, 27–86 years). Disease stage was I in 21%, II in 60%, and III in 20% of the patients. In a multivariate analysis, among patients with TNBC, those with ERK-2–overexpressing tumors had a lower overall survival rate than those with low ERK-2–expressing tumors (hazard ratio [HR], 2.76; 95% confidence interval [CI], 1.19–6.41). However, high pMAPK levels were associated with a significantly higher relapse-free survival rate (HR, 0.66; 95% CI, 0.46–0.95). In conclusion, ERK-2 and pMAPK are valuable prognostic markers in TNBC. Further studies are justified to elucidate ERK's role in TNBC tumorigenicity and metastasis.
Introduction
Breast tumors that are negative for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor (HER)-2 on immunohistochemistry and/or fluorescence in situ hybridization studies are classified as triple-negative breast cancer (TNBC). Of the four molecular subgroups of breast cancer—luminal, ErbB-2, normal, and basal-type—basal-type is the subgroup to which TNBCs most often belong. Although TNBCs share many characteristics with basal-type tumors, these two groups are not identical: TNBCs comprise about 85% of all basal-like tumors [1]. Currently, TNBC is the only major type of breast cancer for which no specific U.S. Food and Drug Administration–approved targeted therapy is available to improve patient outcomes [2].
In TNBCs, the mitogen-activated protein kinase (MAPK) [3, 4] and Akt [5] pathways are known to be activated. Extracellular signal–related kinase (ERK), a member of the MAPK pathway, plays an important role in cell proliferation and differentiation, promotes epithelial–mesenchymal transition, and facilitates cell migration because of its effects on cell–matrix contacts [6]. Higher levels of active MAPK have been found to be associated with metastasis to the lymph nodes [7]. Another study showed that TNBCs with overexpression of active MAPK (phosphorylated [p]MAPK) and low MAPK scores (which reflect weak immunohistochemical staining and low tumor grade) were associated with a higher recurrence rate [4]. However, because of the small number of TNBCs in that study, the role of the MAPK signaling pathway in TNBC was not well characterized. Likewise, the expression and prognostic significance of the ERK subtypes (isoforms) ERK-1 and ERK-2 in TNBC have not been well defined.
Phospho-enriched protein in astrocytes (PEA)-15 is a novel 15-kDa protein that blocks the activity of ERK by inhibiting the transcription factor Elk-1, which regulates ERK-dependent transcription [8–11]. PEA-15 binding to ERK may be regulated by phosphorylation [9, 10, 12–14]. Akt has been shown to activate mammalian target of rapamycin (mTOR), which phosphorylates and activates p70S6 kinase. Akt also regulates the phosphorylation of PEA-15 at serine 116 (S116) both in vitro and in vivo, stabilizing its antiapoptotic function [15]. The phosphorylation of both S104 and S116 on PEA-15 is required to block the binding of PEA-15 to ERK and thereby regulate the MAPK cascade [16]. Thus, PEA-15's state of phosphorylation may affect its modulation of ERK activity in TNBC cells. Indeed, loss of PEA-15 expression is a marker of the transition from noninvasive (ductal carcinoma in situ) to invasive mammary epithelial tumors [17]. Further, PEA-15 gene therapy inhibited tumor growth in a xenograft model of TNBC, and when PEA-15 was overexpressed in TNBC cells, it inhibited cell proliferation, sequestered ERK in the cytoplasm, and induced apoptosis through the death effector pathway [18].
Tuberous sclerosis protein 2 (TSC-2) is a tumor suppressor and is directly phosphorylated by ERK, resulting in functional inactivation of TSC-2, impairing its ability to inhibit mTOR signaling and cell proliferation [19]. Akt has been shown to activate mTOR, which phosphorylates and activates p70S6 kinase, which is required for cell growth and G1 cell cycle progression. ERK-1 and ERK-2 signaling can also trigger downregulation of p27 KIP1, a cyclin-dependent kinase inhibitor that is required for S-phase entry and cell cycle progression [20].
The objective of the present study was to establish, in more detail and in a larger population, the clinical significance of ERK (by assessing ERK-2 and pMAPK), its direct targets (MAPK/ERK kinase [MEK]-1, PEA-15, pPEA-15, TSC-2, p70S6 kinase, and p27), and other related important targets (Akt and Aktp308) in patients with TNBC versus non-TNBC patients. Further, we attempted to address the significance of PEA-15 and its relationship with ERK in TNBC. We measured the expression of ERK and its direct targets in patients with TNBC and non-TNBC by reverse-phase protein array (RPPA), a method that allows measurement of a protein in hundreds of samples using a robust quantification of protein concentrations over a dynamic range. We correlated the relative expression of ERK and its direct targets with the pathological and clinical variables of patients with TNBC and found that high ERK-2 levels were correlated with a lower overall survival (OS) rate and that high pMAPK levels were correlated with a higher relapse-free survival (RFS) rate.
Materials and Methods
Patient Selection
This retrospective study involved 97 patients with TNBC and 223 patients with non-TNBC. The solid breast tumors were obtained from the breast tissue frozen-tumor banks at The University of Texas MD Anderson Cancer Center and Hospital Clínico Universitario de Valencia. The use of tissue blocks in the creation of the RPPAs, use of RPPAs, and chart review for this study were approved by the institutional review boards of MD Anderson Cancer Center and Hospital Clínico Universitario de Valencia.
Our retrospective analysis included only patients who had invasive ductal or invasive lobular carcinoma and a disease stage of I, II, or III. Patient ages, histological tumor types, tumor grades, and disease stages were compared among patient subgroups defined on the basis of hormone receptor and HER-2 status.
Reverse-Phase Protein Lysate Microarray
Protein was extracted from human breast cancer samples, and lysates were prepared and arrayed as previously described [20–21]. Each array slide was then probed with the following dilutions of validated primary rabbit antibodies: anti-PEA-15 (1:1,000) (Cell Signaling Technology, Danvers, MA), rabbit anti-pPEA-15 (S116) (1:1,000) (Invitrogen, Carlsbad, CA), rabbit anti–ERK-2 (1:250) (Cell Signaling Technology), rabbit anti-pMAPK (1:1,000) (Cell Signaling Technology), rabbit anti–MEK-1 (1:15,000) (Epitomics, Inc., Burlingame, CA), rabbit anti–TSC-2 (1:500) (Epitomics, Inc.), rabbit anti-p70S6 kinase (1:500) (Epitomics, Inc.), rabbit anti-p27 (1:500) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-Akt (1:250) (Cell Signaling Technology), or rabbit anti-Aktp308 (1:250) (Cell Signaling Technology), and the signal was amplified with a DakoCytomation (Dako, Carpinteria, CA) catalyzed system.
Statistical Analysis
The statistical analysis was based on all 320 patients in the study. Descriptive statistics were used to summarize the patients' demographic and clinical characteristics. The mean, standard deviation, median, and range were summarized for continuous variables, and either the two-sample t-test or the analysis of variance method was used to compare the continuous variables among two or more groups. The number of patients in each level and the corresponding frequencies were provided for categorical variables. The Spearman correlation coefficient was used to assess relationships between two biomarkers. We used the Kaplan–Meier method to estimate the median OS and RFS times. For the analysis of OS times, death was counted as an event, and patients who survived were counted as censored at their date of last follow-up. The OS time was calculated as the interval between the diagnosis date and either the date of death or date of last follow-up for those who survived. For the analysis of RFS times, disease relapse and death were considered events. The RFS interval was calculated as the interval between the diagnosis date and the date of disease relapse, date of death, or, for patients who did not experience relapse, date of last follow-up. Cox proportional hazards regression analysis was then used to examine the relationship between potential covariates and OS and RFS times. This modeling was done in a univariate fashion. From this model, we estimated the hazard ratio (HR) and 95% confidence interval (CI) for each potential prognostic factor. All prognostic factors were then included in a saturated model, and backward elimination was used to remove factors from the model on the basis of the likelihood ratio test in the multiple regression analysis to derive the final multivariate model. The statistical software programs used in the analysis were SAS 9.2 (SAS Institute Inc., Cary, NC) and R version 2.11.1 (R Foundation for Statistical Computing).
Results
Clinical and Molecular Characteristics of Patients with TNBC
We first compared the characteristics of the 97 patients with TNBC with those of the 223 patients with non-TNBC (Table 1). The median ages at diagnosis were 55 years (range, 27–86) for patients with TNBC and 67 years (range, 30–89) for patients with non-TNBC (Wilcoxon rank-sum test p < .0001). We subdivided patients with non-TNBC into three groups: hormone receptor positive and HER-2−, hormone receptor negative and HER-2+, and triple positive. Whereas most TNBC tumors had a nuclear grade of 3 (84%), most tumors that were hormone receptor positive and HER-2− had a nuclear grade of 2 (56%). Invasive ductal carcinoma was the most common histological type for both TNBC (97%) and non-TNBC (91%) tumors.
Table 1.
Clinical characteristics of breast cancer patients according to HR status
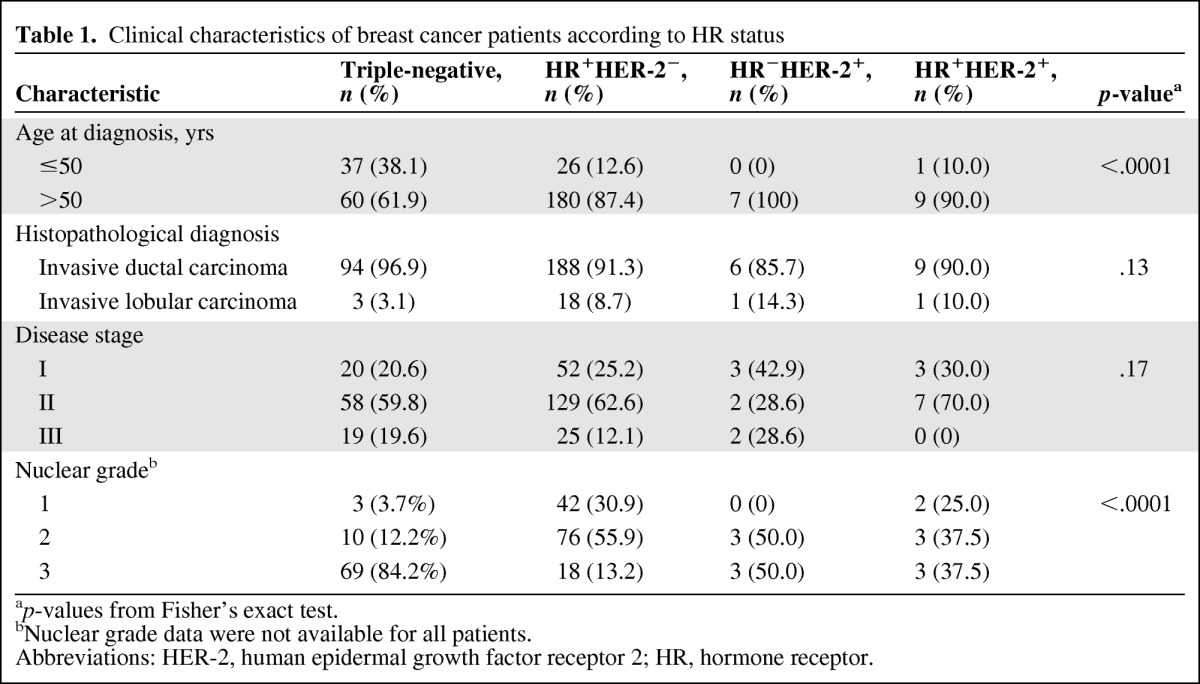
ap-values from Fisher's exact test.
bNuclear grade data were not available for all patients.
Abbreviations: HER-2, human epidermal growth factor receptor 2; HR, hormone receptor.
We observed that, compared with patients with hormone receptor–positive and HER-2− breast cancer, patients with TNBC had significantly lower levels of ERK-2, PEA-15, pPEA-15, and p27 and higher levels of MEK-1, Akt, and Aktp308. The pMAPK level was higher in the TNBC and hormone receptor–positive and HER-2− groups than in the hormone receptor–negative and HER-2+ group. In addition, the mean expression level of PEA-15 was lower in patients with TNBC than in patients with triple-positive breast cancer (Table 2).
Table 2.
Biomarkers in breast cancer patients according to HR status
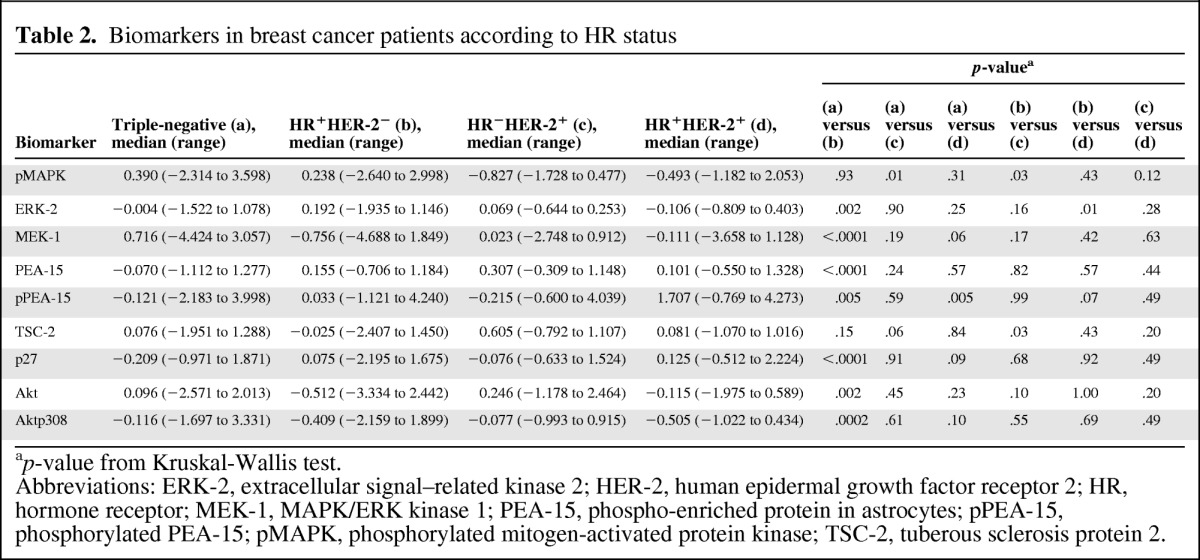
ap-value from Kruskal-Wallis test.
Abbreviations: ERK-2, extracellular signal–related kinase 2; HER-2, human epidermal growth factor receptor 2; HR, hormone receptor; MEK-1, MAPK/ERK kinase 1; PEA-15, phospho-enriched protein in astrocytes; pPEA-15, phosphorylated PEA-15; pMAPK, phosphorylated mitogen-activated protein kinase; TSC-2, tuberous sclerosis protein 2.
To assess the relationship between ERK and PEA-15, pPEA-15, and p70S6 kinase in patients with TNBC, Spearman correlation coefficients were calculated. We observed positive correlations between PEA-15 and ERK-2 (p < .0001) and between PEA-15 and pPEA-15 (S116) (p < .0001) (Table 3) and negative correlations between PEA-15 and p70S6 kinase (p = 0.02) and between pPEA-15 and p70S6 kinase (p < .0001).
Table 3.
MAPK pathway-related biomarker correlation in patients with triple-negative breast cancer
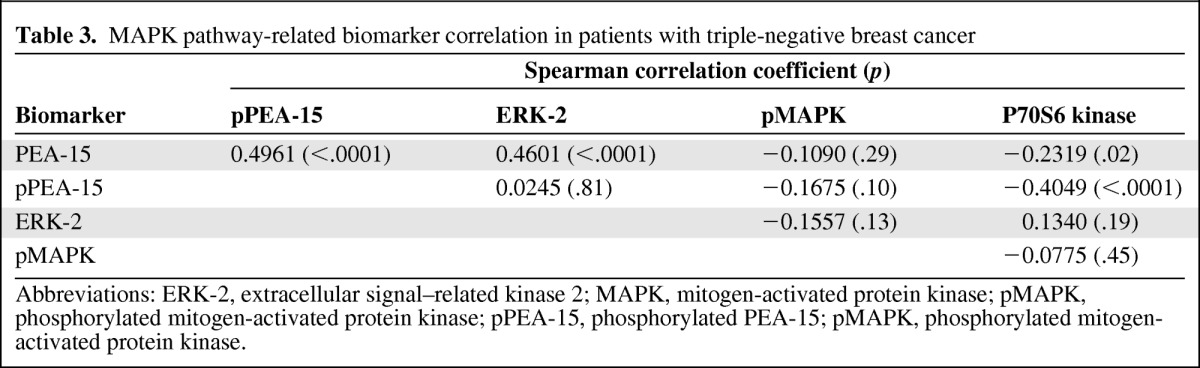
Abbreviations: ERK-2, extracellular signal–related kinase 2; MAPK, mitogen-activated protein kinase; pMAPK, phosphorylated mitogen-activated protein kinase; pPEA-15, phosphorylated PEA-15; pMAPK, phosphorylated mitogen-activated protein kinase.
Outcomes in TNBC and non-TNBC Patients
In the patients with TNBC, the median follow-up time was 2.47 years (range, 0.25–19.45 years). Twenty-four of the 97 patients had died by the time of the analysis. The median OS time was 7.08 years. Thirty-one of the 97 patients with TNBC had relapsed by the time of the analysis. The median RFS interval was 5.38 years (range, 0.25–19.45 years).
On univariate analysis, ERK-2 expression level and disease stage were significantly associated with the OS time (Table 4), and pMAPK levels were marginally significantly associated with the RFS interval (Table 5). Patients with four to nine positive lymph nodes (HR, 6.29; 95% CI, 2.19–18.04; p = .0006) had a higher risk for death than patients with one to three or >10 positive nodes, although the latter observation was based on only seven patients and two deaths.
Table 4.
Univariate Cox proportional hazards model for overall survival time in patients with triple-negative breast cancer
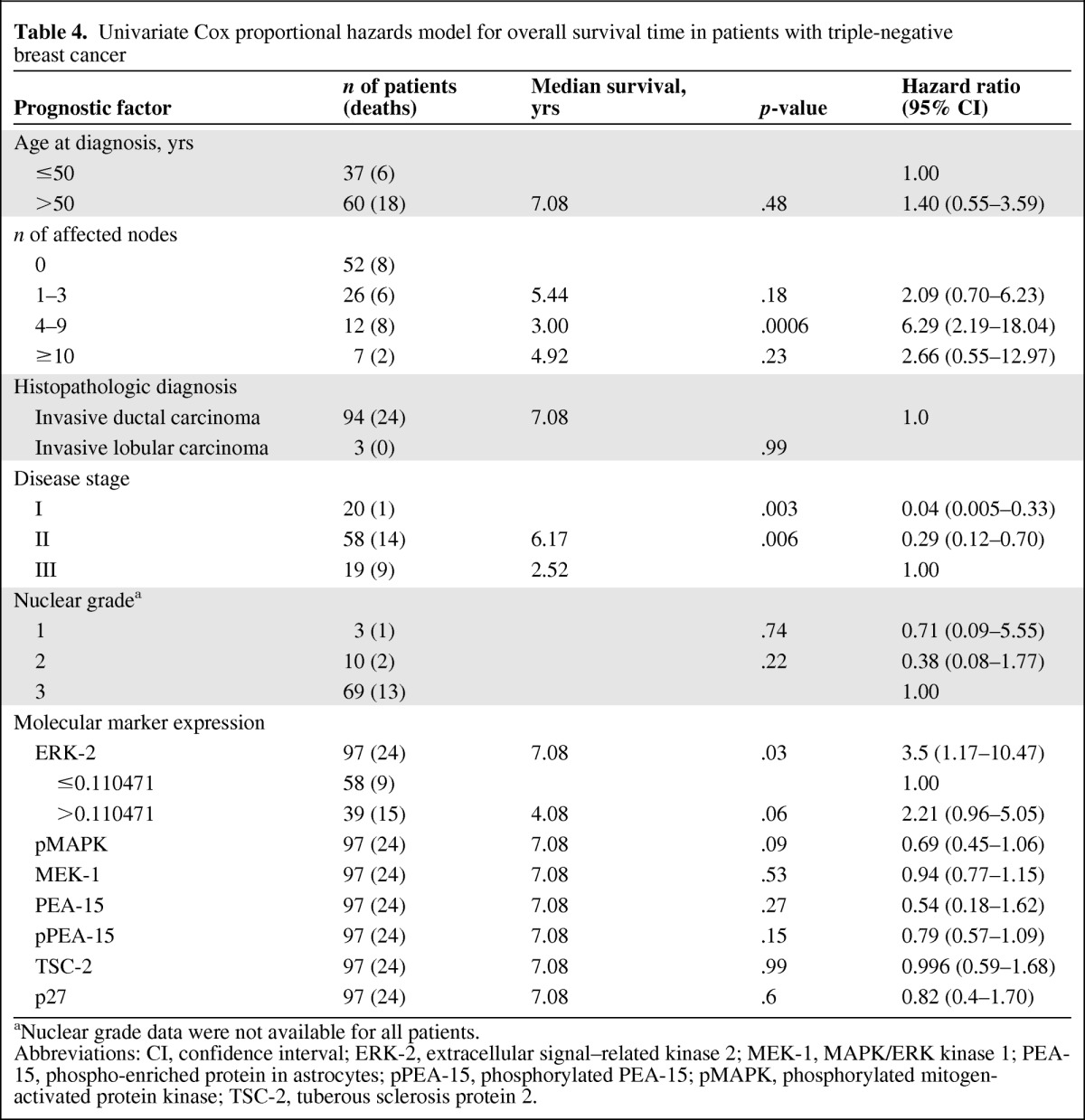
aNuclear grade data were not available for all patients.
Abbreviations: CI, confidence interval; ERK-2, extracellular signal–related kinase 2; MEK-1, MAPK/ERK kinase 1; PEA-15, phospho-enriched protein in astrocytes; pPEA-15, phosphorylated PEA-15; pMAPK, phosphorylated mitogen-activated protein kinase; TSC-2, tuberous sclerosis protein 2.
Table 5.
Univariate Cox proportional hazards model for relapse-free survival time in patients with triple-negative breast cancer
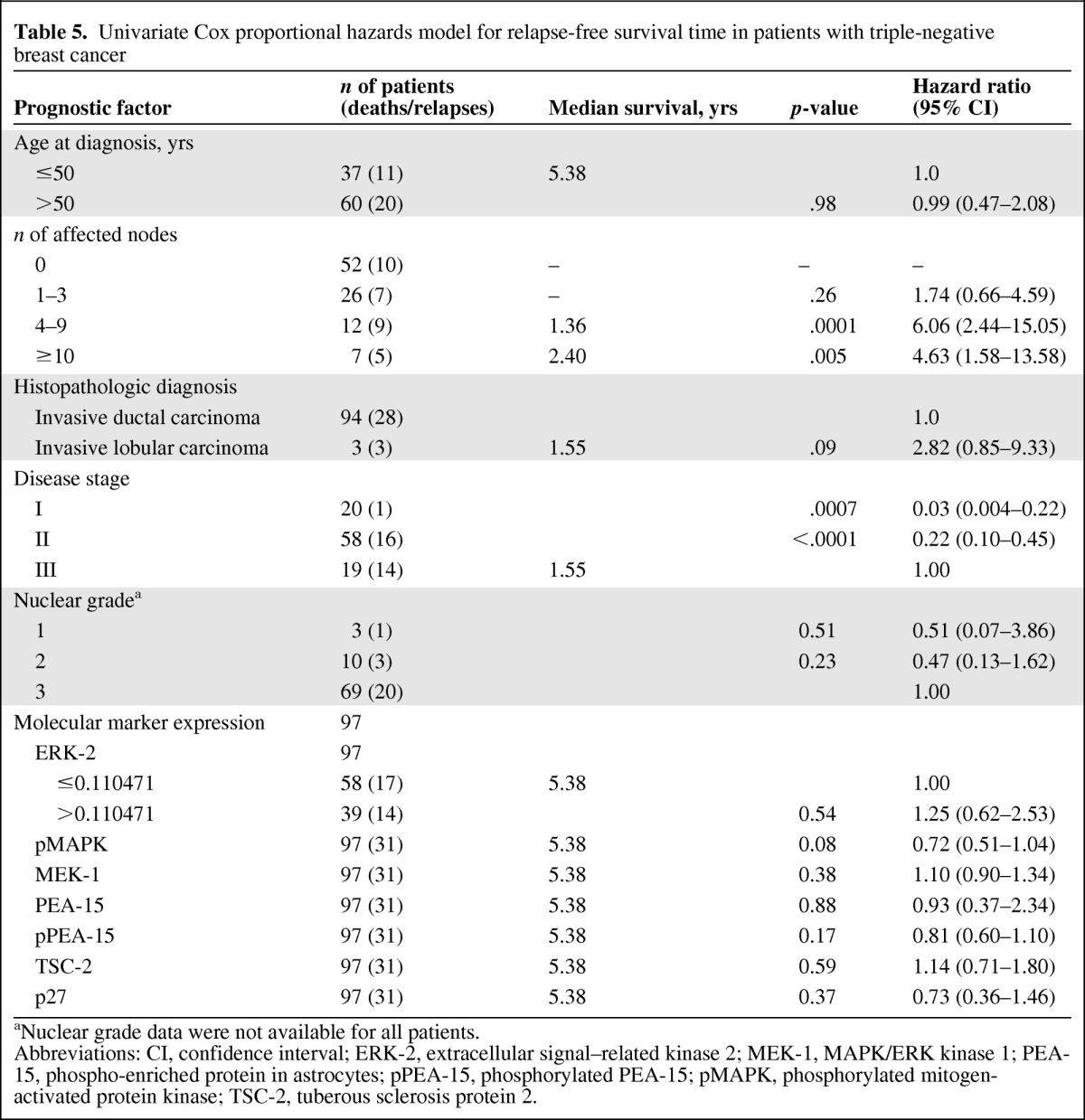
aNuclear grade data were not available for all patients.
Abbreviations: CI, confidence interval; ERK-2, extracellular signal–related kinase 2; MEK-1, MAPK/ERK kinase 1; PEA-15, phospho-enriched protein in astrocytes; pPEA-15, phosphorylated PEA-15; pMAPK, phosphorylated mitogen-activated protein kinase; TSC-2, tuberous sclerosis protein 2.
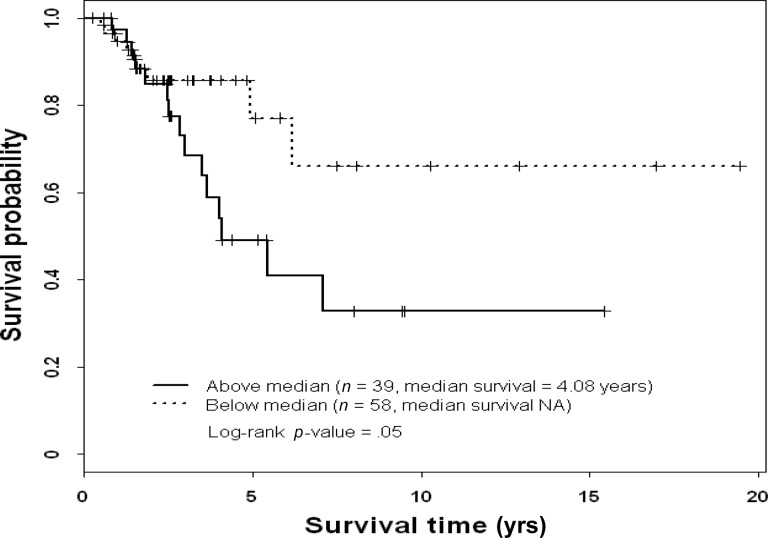
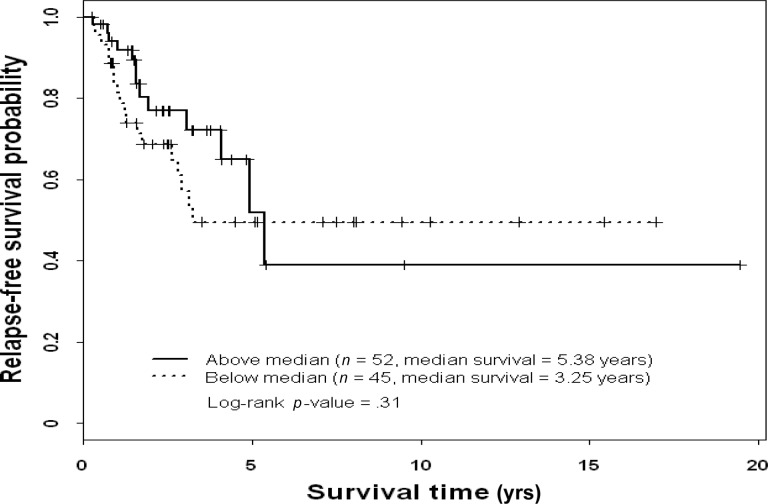
On multivariate analysis, patients with stage I or stage II disease had a lower risk for death during the study period than patients with stage III disease (Table 6), and patients with high ERK-2–expressing tumors had a higher risk for death during the study period than patients with low ERK-2–expressing tumors. On multivariate analysis, high pMAPK levels were associated with a significantly higher RFS rate in patients with TNBC (Table 7). Thus, ERK-2 was a significant predictor of the OS time and pMAPK was a significant predictor of the RFS interval in TNBC patients. Figures 1–4 show the Kaplan–Meier survival curves for OS and RFS probabilities by ERK-2 and pMAPK expression levels for patients with TNBC. The median OS time was 4.08 years among patients with high ERK-2 levels and was not available for patients with low ERK-2 levels (p = .05) (Fig. 1). The 5-year OS rates were 57% among patients with high ERK-2 levels and 79% among patients with low ERK-2 levels. For the direct targets of ERK (MEK-1, PEA-15, pPEA-15, TSC-2, p70S6 kinase, and p27), the differences in OS and RFS outcomes between patients with high expression and patients with low expression were not significant in patients with TNBC.
Table 6.
Multivariate Cox proportional hazards model for overall survival time in triple-negative breast cancer patients (n = 97)
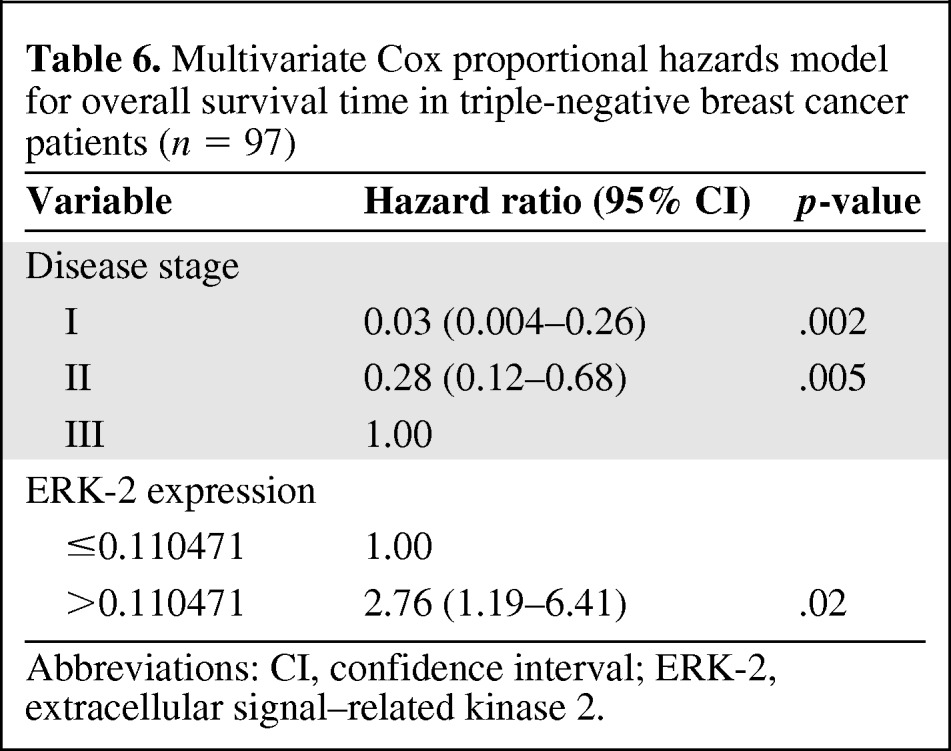
Abbreviations: CI, confidence interval; ERK-2, extracellular signal–related kinase 2.
Table 7.
Multivariate Cox proportional hazards model for relapse-free survival time in triple-negative breast cancer patients (n = 97)
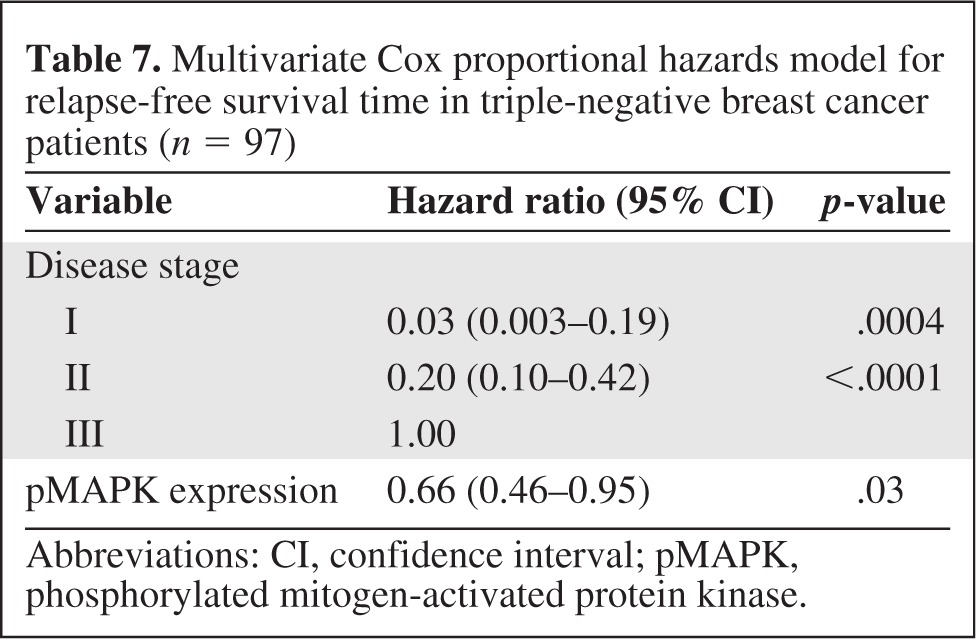
Abbreviations: CI, confidence interval; pMAPK, phosphorylated mitogen-activated protein kinase.
Figure 1.
Overall survival probability by extracellular signal–related kinase 2 status in patients with triple-negative breast cancer.
Abbreviation: NA, not available.
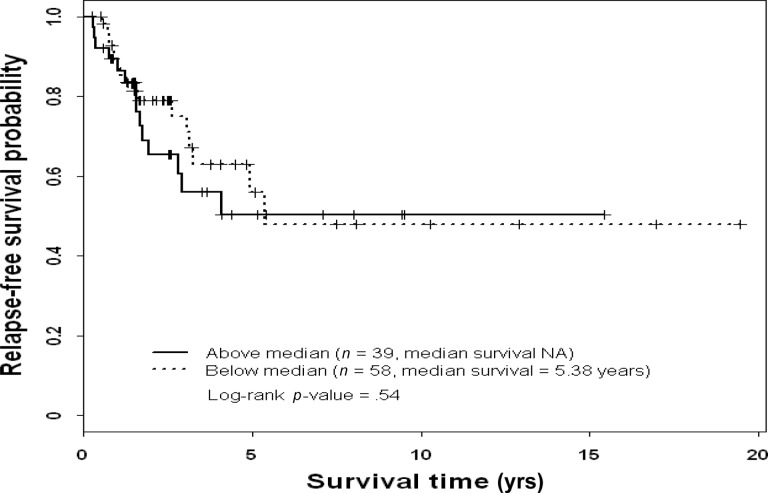
Figure 2.
Relapse-free survival probability by extracellular signal–related kinase 2 status in patients with triple-negative breast cancer.
Abbreviation: NA, not available.
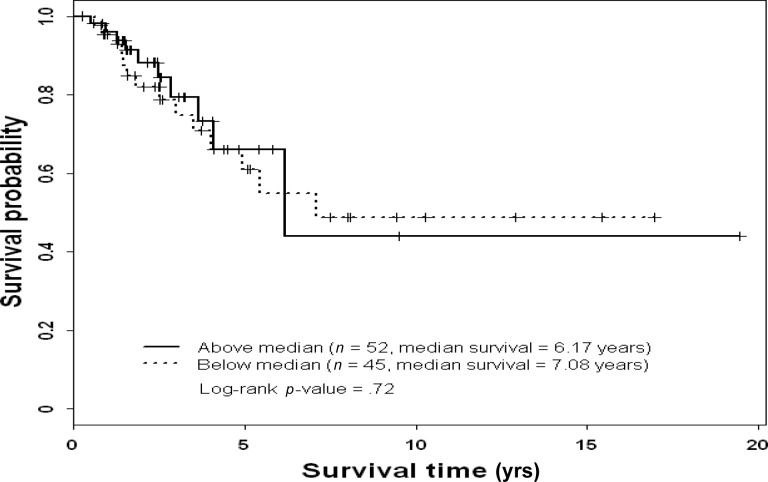
Figure 3.
Overall survival probability by phosphorylated mitogen-activated protein kinase status in patients with triple-negative breast cancer.
Figure 4.
Relapse-free survival probability by phosphorylated mitogen-activated protein kinase status in patients with triple-negative breast cancer.
In patients with non-TNBC, the median OS time was 16.42 years, and the median RFS interval was 14.58 years. Age at diagnosis, disease stage, and normalized p70S6 kinase values were significantly associated with the OS time in patients with non-TNBC. Patients with stage I disease had a lower risk for death during the study period than patients with stage III disease, and patients with higher normalized p70S6 kinase values had a higher risk for death than those with lower normalized p70S6 kinase values. Patients aged >50 years also had a higher risk for death than patients aged <50 years.
Discussion
Our results demonstrate that high ERK (ERK-2 and pMAPK) expression is an important predictor of OS and disease-free survival outcomes in patients with TNBC and suggest that ERK expression may be the driving force in TNBC, in contrast to non-TNBC, in which the driving force is HER-2 or estrogen receptor expression. Our comparison of the mean levels of expression of ERK and its direct targets in TNBC and non-TNBC tumors also confirmed the importance of the ERK pathway in TNBC.
ERK-1 and ERK-2 are dually phosphorylated by MEK on threonine and tyrosine residues. ERK is a downstream target for receptor tyrosine kinases and for Ras, which is mutated in many cancers. The oncogenic potential of ERK was demonstrated in part by the finding that elevated ERK-1 or ERK-2 activity was associated with a shorter disease-free survival interval in patients with breast cancer [7, 22–24]. Oncogenic activation of ERK resulting in persistent activation of the ERK–MAPK cascade occurs primarily through mutationally activated Ras and B-Raf and/or epidermal growth factor receptor (EGFR) and HER-2 overexpression [25, 26]. The chemotherapeutic agent paclitaxel activates the antiapoptotic MEK–ERK signaling pathway, and thus MEK and ERK levels or activation could affect how well paclitaxel induces apoptosis [27, 28]. MAPK overexpression was previously shown to be associated with both a high recurrence rate in patients with TNBC and a low survival rate following relapse [4].
In our study, patients with TNBC with high ERK-2–expressing tumors had a shorter OS duration. However, patients with TNBC with high pMAPK levels had a significantly higher RFS rate. We speculate that the location of ERK and pMAPK may have contributed to physiological responses in breast cancer cells, affecting patient outcomes. The RPPA approach used in our current study did not provide information about whether ERK and pMAPK were located in the cytoplasm or the nucleus. A recent study involving patients with gastric cancer showed that cytoplasmic pmTOR expression was associated with significantly shorter RFS and OS times, whereas nuclear pmTOR expression was associated with longer RFS and OS times, suggesting that the cellular localization of pmTOR may play an important role in patient outcomes [29]. Thus, it would be relevant to perform immunohistochemical studies to determine the cellular localization of ERK.
PEA-15 levels correlated positively with ERK levels, consistent with recent findings that PEA-15 leads to prolonged tyrosine phosphorylation of fibroblast growth factor receptor substrate 2α, resulting in activation of MEK-1 and MEK-2 and ERK-1 and ERK-2 [30]. This is consistent with our study and other studies that have shown that PEA-15 can induce the expression of ERK and pMAPK [13, 31]. Interestingly, we found a negative correlation between PEA-15 and p70S6 kinase and between pPEA-15 (S116) and p70S6 kinase. Overexpression of p70S6 kinase is known to be associated with aggressive disease and a poor prognosis in patients with breast cancer. We previously showed that high PEA-15 expression is associated with a longer OS time in patients with ovarian cancer. Thus, we can hypothesize that patients with high PEA-15 expression express low levels of p70S6kinase. Further studies are needed to confirm this hypothesis.
PEA-15 is not known to impair the activation or the enzymatic activity of ERK, although it can sequester ERK in the cytoplasm, thereby preventing ERK's phosphorylation. PEA-15 may act as a master regulator of ERK by regulating ERK's cellular localization. Furthermore, PEA-15 may control cell proliferation by preventing ERK accumulation in the nucleus. Although certain cancer and normal cells proliferate rapidly while expressing PEA-15 at high levels [32, 33], we previously found that high levels of PEA-15 protein expression in women with ovarian cancer were independently associated with a higher OS rate [31]. In breast cancer patients, low PEA-15 expression was previously linked with aggressive forms of the disease. Similarly, greater PEA-15 expression was associated with less invasion in breast cancer through effects on the ERK pathway [17]. We also found that PEA-15 expression levels were lower in high-grade than in low-grade breast tumors.
The ERK–MAPK pathway could become an important therapeutic target in TNBC for several reasons. First, elevated MAPK activity correlates with shorter survival times in breast cancer patients. Second, aberrant activation of the MAPK pathway in human cancer cells occurs by upstream activation by EGFR and Ras small GTPase. Third, ERK promotes cell proliferation, cell survival, epithelial–mesenchymal transition, angiogenesis, and metastasis. Finally, the MEK–ERK signaling pathway has been shown to play a critical role in the survival and growth of breast cancer cells [34].
Conclusion and Summary
We found, in the current study, that patients with TNBC with high levels of ERK-2 had shorter OS times than patients with TNBC with low levels of ERK-2, and that high pMAPK levels correlated with a higher RFS rate. Our data demonstrate that ERK and its signaling pathway may be an important therapeutic target in TNBC. It would be relevant to develop MAPK pathway signatures in TNBC that can be used for finding novel therapeutic interventions, predicting prognosis, and optimizing treatment options. We are planning further immunohistochemical studies to determine the cellular localization of ERK and pMAPK and are developing a MEK–ERK pathway signature in TNBC cells that could be used for patient selection for treatment with a MEK inhibitor.
Acknowledgments
We thank Bryan Tutt and Sunita Patterson of the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for their expert editorial assistance. We also are grateful to Dr. Naoto Ueno for critical review of the manuscript.
This work was supported by National Cancer Institute grant 1K99CA139006-01A1 (C.B.) and the Nellie B. Connally Breast Cancer Research Fund (Breast Cancer Translational Research Laboratory).
This work was presented in part at the American Association for Cancer Research meeting, Washington, DC, April 2010.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Chandra Bartholomeusz
Provision of study material or patients: Ana M. Gonzalez-Angulo, Ana Lluch, Jaime Ferrer-Lozano
Collection and/or assembly of data: Ana M. Gonzalez-Angulo, Ana Lluch, Jaime Ferrer-Lozano
Data analysis and interpretation: Chandra Bartholomeusz, Ping Liu, Naoki Hayashi
Manuscript writing: Chandra Bartholomeusz, Gabriel N. Hortobágyi
Final approval of manuscript: Chandra Bartholomeusz, Ana M. Gonzalez-Angulo, Ana Lluch, Ping Liu, Gabriel N. Hortobágyi, Jaime Ferrer-Lozano, Naoki Hayashi
References
- 1.Petrelli F, Cabiddu M, Ghilardi M, et al. Current data of targeted therapies for the treatment of triple-negative advanced breast cancer: Empiricism or evidence-based? Expert Opin Investig Drugs. 2009;18:1467–1477. doi: 10.1517/13543780903222268. [DOI] [PubMed] [Google Scholar]
- 2.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: A critical review. J Clin Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 3.Corkery B, Crown J, Clynes M, et al. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol. 2009;5:862–867. doi: 10.1093/annonc/mdn710. [DOI] [PubMed] [Google Scholar]
- 4.Eralp Y, Derin D, Ozluk Y, et al. MAPK overexpression is associated with anthracycline resistance and increased risk for recurrence in patients with triple-negative breast cancer. Ann Oncol. 2008;19:669–674. doi: 10.1093/annonc/mdm522. [DOI] [PubMed] [Google Scholar]
- 5.Umemura S, Yoshida S, Ohta Y, et al. Increased phosphorylation of Akt in triple-negative breast cancers. Cancer Sci. 2007;98:1889–1892. doi: 10.1111/j.1349-7006.2007.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCawley LJ, Li S, Wattenberg EV, et al. Sustained activation of the mitogen-activated protein kinase pathway. A mechanism underlying receptor tyrosine kinase specificity for matrix metalloproteinase-9 induction and cell migration. J Biol Chem. 1999;274:4347–4353. doi: 10.1074/jbc.274.7.4347. [DOI] [PubMed] [Google Scholar]
- 7.Adeyinka A, Nui Y, Cherlet T, et al. Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin Cancer Res. 2002;8:1747–1753. [PubMed] [Google Scholar]
- 8.Bartholomeusz C, Itamochi H, Nitta M, et al. Antitumor effect of E1A in ovarian cancer by cytoplasmic sequestration of activated ERK by PEA15. Oncogene. 2006;25:79–90. doi: 10.1038/sj.onc.1209014. [DOI] [PubMed] [Google Scholar]
- 9.Formstecher E, Ramos JW, Fauquet M, et al. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev Cell. 2001;1:239–250. doi: 10.1016/s1534-5807(01)00035-1. [DOI] [PubMed] [Google Scholar]
- 10.Hill JM, Vaidyanathan H, Ramos JW, et al. Recognition of ERK MAP kinase by PEA-15 reveals a common docking site within the death domain and death effector domain. EMBO J. 2002;21:6494–6504. doi: 10.1093/emboj/cdf641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitehurst AW, Robinson FL, Moore MS, et al. The death effector domain protein PEA-15 prevents nuclear entry of ERK2 by inhibiting required interactions. J Biol Chem. 2004;279:12840–12847. doi: 10.1074/jbc.M310031200. [DOI] [PubMed] [Google Scholar]
- 12.Ramos JW, Kojima TK, Hughes PE, et al. The death effector domain of PEA-15 is involved in its regulation of integrin activation. J Biol Chem. 1998;273:33897–33900. doi: 10.1074/jbc.273.51.33897. [DOI] [PubMed] [Google Scholar]
- 13.Ramos JW, Hughes PE, Renshaw MW, et al. Death effector domain protein PEA-15 potentiates Ras activation of extracellular signal receptor-activated kinase by an adhesion- independent mechanism. Mol Biol Cell. 2000;11:2863–2872. doi: 10.1091/mbc.11.9.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou FL, Hill JM, Hsieh JC, et al. PEA-15 binding to ERK1/2 MAPKs is required for its modulation of integrin activation. J Biol Chem. 2003;278:52587–52597. doi: 10.1074/jbc.M309322200. [DOI] [PubMed] [Google Scholar]
- 15.Trencia A, Perfetti A, Cassese A, et al. Protein kinase B/Akt binds and phosphorylates PED/PEA-15, stabilizing its antiapoptotic action. Mol Cell Biol. 2003;23:4511–4521. doi: 10.1128/MCB.23.13.4511-4521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krueger J, Chou FL, Glading A, et al. Phosphorylation of phosphoprotein enriched in astrocytes (PEA-15) regulates extracellular signal-regulated kinase-dependent transcription and cell proliferation. Mol Biol Cell. 2005;16:3552–3561. doi: 10.1091/mbc.E04-11-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glading A, Koziol JA, Krueger J, et al. PEA-15 inhibits tumor cell invasion by binding to extracellular signal-regulated kinase 1/2. Cancer Res. 2007;67:1536–1544. doi: 10.1158/0008-5472.CAN-06-1378. [DOI] [PubMed] [Google Scholar]
- 18.Bartholomeusz C, Gonzalez-Angulo AM, Kazansky A, et al. PEA-15 inhibits tumorigenesis in an MDA-MB-468 triple-negative breast cancer xenograft model through increased cytoplasmic localization of activated extracellular signal-regulated kinase. Clin Cancer Res. 2010;16:1802–1811. doi: 10.1158/1078-0432.CCR-09-1456. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Chen Z, Erdjument-Bromage H, et al. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Rivard N, Boucher MJ, Asselin C, et al. MAP kinase cascade is required for p27 downregulation and S phase entry in fibroblasts and epithelial cells. Am J Physiol. 1999;277:C652–C664. doi: 10.1152/ajpcell.1999.277.4.C652. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–2478. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 22.Gee JM, Robertson JF, Ellis IO, et al. Phosphorylation of ERK1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int J Cancer. 2001;95:247–254. doi: 10.1002/1097-0215(20010720)95:4<247::aid-ijc1042>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 23.McClelland RA, Barrow D, Madden TA, et al. Enhanced epidermal growth factor receptor signaling in MCF7 breast cancer cells after long-term culture in the presence of the pure antiestrogen ICI 182,780 (Faslodex) Endocrinology. 2001;142:2776–2788. doi: 10.1210/endo.142.7.8259. [DOI] [PubMed] [Google Scholar]
- 24.Mueller H, Flury N, Eppenberger-Castori S, et al. Potential prognostic value of mitogen-activated protein kinase activity for disease-free survival of primary breast cancer patients. Int J Cancer. 2000;89:384–388. doi: 10.1002/1097-0215(20000720)89:4<384::aid-ijc11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 25.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 26.Stephens P, Hunter C, Bignell G, et al. Lung cancer: Intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–526. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 27.McDaid HM, Horwitz SB. Selective potentiation of paclitaxel (Taxol)-induced cell death by mitogen-activated protein kinase kinase inhibition in human cancer cell lines. Mol Pharmacol. 2001;60:290–301. doi: 10.1124/mol.60.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKeigan JP, Collins TS, Ting JP. MEK inhibition enhances paclitaxel-induced tumor apoptosis. J Biol Chem. 2000;275:38953–38956. doi: 10.1074/jbc.C000684200. [DOI] [PubMed] [Google Scholar]
- 29.Murayama T, Inokuchi M, Takagi Y, et al. Relation between outcomes and localisation of p-mTOR expression in gastric cancer. Br J Cancer. 2009;100:782–788. doi: 10.1038/sj.bjc.6604915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haling JR, Wang F, Ginsberg MH. Phosphoprotein enriched in astrocytes 15 kDa (PEA-15) reprograms growth factor signaling by inhibiting threonine phos-phorylation of fibroblast receptor substrate 2α. Mol Biol Cell. 2010;21:664–673. doi: 10.1091/mbc.E09-08-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartholomeusz C, Rosen D, Wei C, et al. PEA-15 induces autophagy in human ovarian cancer cells and is associated with prolonged overall survival. Cancer Res. 2008;15;68:9302–9310. doi: 10.1158/0008-5472.CAN-08-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araujo H, Danziger N, Cordier J, et al. Characterization of PEA-15, a major substrate for protein kinase C in astrocytes. J Biol Chem. 1993;268:5911–5920. [PubMed] [Google Scholar]
- 33.Estellés A, Charlton CA, Blau HM. The phosphoprotein protein PEA-15 inhibits Fas- but increases TNF-R1-mediated caspase-8 activity and apoptosis. Dev Biol. 1999;216:16–28. doi: 10.1006/dbio.1999.9510. [DOI] [PubMed] [Google Scholar]
- 34.Ripple MO, Kalmadi S, Eastman A. Inhibition of either phosphatidylinositol 3-kinase/Akt or the mitogen/extracellular-regulated kinase, MEK/ERK, signaling pathways suppress growth of breast cancer cell lines, but MEK/ERK signaling is critical for cell survival. Breast Cancer Res Treat. 2005;93:177–188. doi: 10.1007/s10549-005-4794-6. [DOI] [PubMed] [Google Scholar]