The relationship among young age (≤40 years), the likelihood of a delay in diagnosis, and stage was examined in breast cancer patients using a National Comprehensive Cancer Network database. Young age was not an independent predictor of a delay in diagnosis and only modestly predicted higher disease stage.
Keywords: Breast cancer, Young age, Delay in diagnosis, Breast cancer screening
Abstract
Background.
Young women with breast cancer are more likely to present with more advanced disease and are more likely to die as a result of breast cancer than their older counterparts. We sought to examine the relationship among young age (≤40 years), the likelihood of a delay in diagnosis, and stage.
Methods.
We examined data from women with newly diagnosed stage I–IV breast cancer presenting to one of eight National Comprehensive Cancer Network centers in January 2000 to December 2007. Delay in diagnosis was defined as time from initial sign or symptom to breast cancer diagnosis >60 days.
Results.
Among 21,818 women with breast cancer eligible for analysis, 2,445 were aged ≤40 years at diagnosis. Young women were not more likely to have a delay in diagnosis >60 days (odds ratio [OR], 1.08; 95% confidence interval [CI], 0.98–1.19) after adjustment for type of initial sign or symptom. Young women were only modestly more likely to present with higher stage disease after a similar adjustment (OR, 1.18; 95% CI, 1.07–1.31). Women presenting with symptomatic disease, more common in younger women, were more likely to have a delay in diagnosis (OR, 3.31; 95% CI, 3.08–3.56) and higher stage (OR, 4.31; 95% CI 4.05–4.58).
Conclusion.
Young age is not an independent predictor of delay in diagnosis of breast cancer and only modestly is associated with higher stage disease. Presenting with symptoms of breast cancer predicts delay and higher stage at diagnosis.
Introduction
Breast cancer diagnosed in women aged ≤40 years is a relatively rare disease. Women in this age group account for ∼7% of women with breast cancer. This translates to >14,000 young women diagnosed annually with invasive or noninvasive breast cancer in the U.S. alone, with thousands more diagnosed worldwide [1–3]. Despite the relative infrequency of the disease in young women, breast cancer is the leading cause of cancer-related death in women aged ≤40 years, and survival rates for young women with breast cancer are lower than those of their older counterparts [4]. Research has repeatedly revealed that younger women are more likely to present with larger sized tumors and a greater number of involved lymph nodes [5, 6]. Factors that could contribute to these women presenting with larger and higher stage cancers could include the lack of effective screening and awareness in these women, biologic factors associated with more aggressive disease, and delays in diagnosis related to the relative infrequency of breast cancer in younger women and the common nature of symptoms related to benign breast disorders in this age group. The literature is mixed with regard to the relationship between age and delay in diagnosis as well as the potential effect of a delay on patient outcomes, though some studies suggest that a delay >2–3 months affects patient outcomes [7–12]. Few studies have included a substantial proportion of young women or women of ethnically diverse groups, and few have evaluated the delay prior to seeking medical attention or clinical outcomes.
We evaluated the effect of age on breast cancer presentation and time from initial presenting sign or symptom to diagnosis using an ongoing national longitudinal cohort study, the National Comprehensive Cancer Network (NCCN) Breast Cancer Outcomes Database Project.
Methods
Participants
The study cohort consisted of women with newly diagnosed stage I, II, III, and IV (American Joint Committee on Cancer Staging Manual, Fifth and Sixth Editions) breast cancer who received their primary cancer care at one of the eight institutions participating in the NCCN Breast Cancer Outcomes Database Project in January 1, 2000 to December 31, 2007. The eight institutions are: The Ohio State University Comprehensive Cancer Center–James Cancer Hospital and Solove Research Institute, Columbus, OH; City of Hope Comprehensive Cancer Center, Duarte, CA; Dana-Farber Cancer Institute, Boston, MA; Fox Chase Cancer Center, Philadelphia, PA; H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; The University of Texas M.D. Anderson Cancer Center, Houston, TX; Roswell Park Cancer Institute, Buffalo, NY; and the University of Michigan Comprehensive Cancer Center, Ann Arbor, MI. Each center is an academic comprehensive cancer center at which the majority of surgical and medical oncologists treating breast cancer devote most or all of their clinical effort to breast cancer care. The institutional review board at each center approved the study, data collection process, data transmission methods, and data storage protocols. Patients presenting for second opinions and those receiving no primary therapy at the NCCN institution are excluded from the database.
We identified 25,131 stage I, II, III, and IV patients who presented with a new diagnosis of breast cancer and received primary therapy at one of the NCCN institutions in January 1, 2000 to December 31, 2007 (Fig. 1). We excluded those who had a previous cancer diagnosis (n = 1,913) and patients for whom the date of the initial symptom or sign of breast cancer was missing, estimated, or listed after the date of the patient's diagnosis of breast cancer (n = 434). We also excluded patients for whom specific information on the initial symptom or sign was not available or who indicated “other” for initial sign or symptom (n = 966). Our final sample included 21,818 women.
Figure 1.
Flow diagram of participants.
Data Sources
The NCCN Breast Cancer Outcomes Database Project has been collecting prospective data on patient and tumor characteristics, sociodemographic information, treatment, and outcomes for newly diagnosed breast cancer patients at participating NCCN institutions since 1997. Clinical and treatment information is gathered from tumor registries, chart review, and inpatient and outpatient records [13–17]. In addition, patient-reported elements are collected via a survey at first presentation at most centers. The survey elicits information about the first indication of breast cancer (with response categories that include screening mammogram, lump detected by a physician, self-discovered lump, bloody nipple discharge, axillary mass, inverted nipple, breast pain or discomfort, and other). The survey also collects information on employment status at diagnosis, educational status at presentation to the NCCN center (defined as highest level of education completed), and menopausal status. For patients with an incomplete or missing survey, dedicated study abstractors complete the missing elements based on chart review.
Rigorous data quality assurance processes are in place for the study, including initial and follow-up data management training, online edit checking during Web-based data entry, programmed logic checks against the pooled data repository, routine quality assurance reports to the centers for rectification by data managers, and on-site audits of a random sample of source documents against the submitted data within the first few months of data collection and annually thereafter.
Variable Definitions
The initial symptom or sign was dichotomized for this analysis as either abnormal mammography screen or clinical sign or symptom. Delay in diagnosis was defined as a time from initial symptom or sign to breast cancer diagnosis >60 days based on prior research suggesting that a delay >2–3 months in seeking medical attention for breast symptoms is associated with a worse prognosis [8, 11, 12, 18]. Secondary analyses included a delay definition of >180 days based on prior literature suggesting that a delay >6 months is associated with higher stage disease [10]. When evaluating the effect of a delay on stage at diagnosis, we grouped women with stage II, III, and IV disease as high stage, compared with women having stage I disease, given the large difference in survival outcomes (and the need for adjuvant therapy) between stage I and higher stage disease.
Analyses
We used multivariate logistic regression models to examine: (a) the effect of age on delay in diagnosis and (b) the effect of a delay in diagnosis on stage of disease at diagnosis as a surrogate for clinical outcome, adjusting for potential confounders to determine an odds ratio (OR) as an estimate of the relative risk and the 95% confidence interval (CI). Covariates were considered potential confounders if there was a priori evidence in the published literature that the factor was related to a delay in breast cancer diagnosis. The following covariates were included in multivariate models as potential confounders: self-designated patient race or ethnicity (white, Hispanic, African American, Asian or Pacific Islander, American Indian, or other), employment status (employed or student, homemaker or retired, or other), education (grade school through high school, some college, college graduate, graduate school, or unknown), insurance status (managed care, Medicaid, Indemnity, Medicare, or other), and NCCN center. Inclusion of height and weight made no appreciable differences in risk estimates and they were not included in the final models.
We evaluated the statistical interactions between age at diagnosis and delay in diagnosis and type of initial sign or symptom. Statistical interaction was assessed by conducting a likelihood ratio test comparing models with the main effects and interaction terms with the model with the main effects only [19]. All statistical tests were two-sided, and p-values <.05 were considered statistically significant. All analyses were conducted using SAS, version 9.1 (SAS Institute Inc., Cary, NC).
Results
Patient characteristics and presenting features by age at diagnosis are presented in Table 1. Among the 21,818 women with breast cancer eligible for analysis, 2,445 were aged ≤40 years at diagnosis, with an average age of 35 years; the average age of women aged >40 years was 57 years. Compared with older women, younger women were more likely to be nonwhite, premenopausal, more educated, and employed or in school and to be diagnosed with higher stage disease. Young women were also more likely to present with symptomatic rather than screening-detected disease: 89.5% of young women compared with 52.1% of older women presented with initially symptomatic disease. Younger women were more likely to have a delay in diagnosis >60 days (35% versus 25%; p < .0001) and >180 days (12.7% versus 8.4%; p < .0001).
Table 1.
Descriptive characteristics of 21,818 stage I, II, III, and IV breast cancer patients according to age at diagnosis
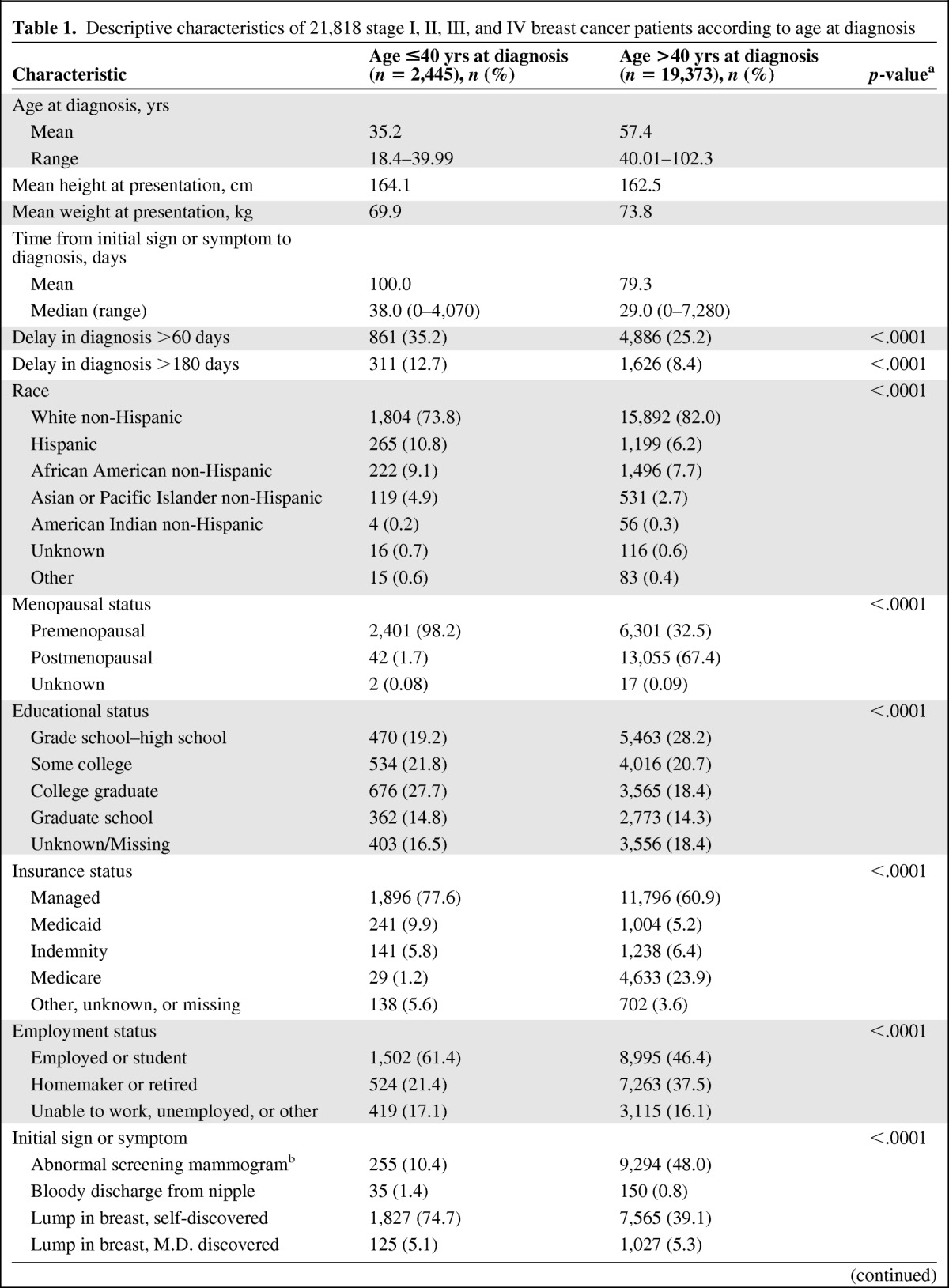
Table 1.
(Continued)
aStatistical tests for categorical variables were performed using the X2 test.
bNo data are available regarding prior screening practices.
Relationship Between Age and Delay in Breast Cancer Diagnosis
In a multivariate model, we found that women aged ≤40 years were 50% (OR, 1.52; 95% CI, 1.39–1.67; p < .0001) more likely to have a delay >60 days in breast cancer diagnosis than older women (Table 2). However, when we additionally adjusted for initial sign or symptom, this association was substantially attenuated (OR, 1.08; 95% CI, 0.98–1.19; p = .11). When evaluating the relationship between initial symptoms and delay in diagnosis, presenting for any reason other than an abnormal screening mammogram was associated with a 3.31 times higher (95% CI, 3.08–3.56; p < .0001) risk for a delay in diagnosis > 60 days, adjusted for age. These associations were also seen when we evaluated a delay ≥180 days (data not shown). We also examined whether or not the relationship between age and delay in diagnosis varied by type of presentation (sign or symptom versus screening); however, we did not find a significant interaction (p = .39).
Table 2.
Associations between delay in diagnosis >60 days and age at diagnosis and initial sign or symptom of breast cancer
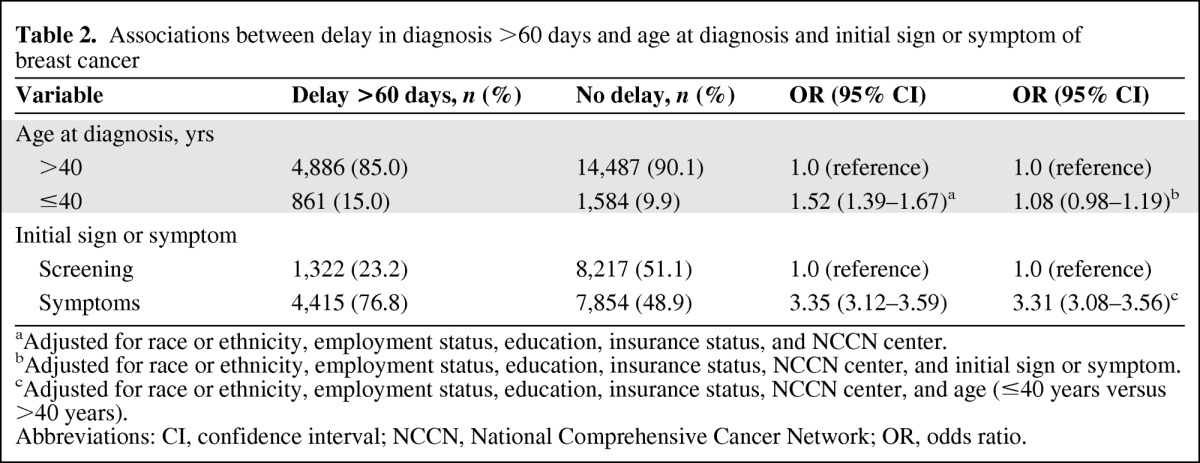
aAdjusted for race or ethnicity, employment status, education, insurance status, and NCCN center.
bAdjusted for race or ethnicity, employment status, education, insurance status, NCCN center, and initial sign or symptom.
cAdjusted for race or ethnicity, employment status, education, insurance status, NCCN center, and age (≤40 years versus >40 years).
Abbreviations: CI, confidence interval; NCCN, National Comprehensive Cancer Network; OR, odds ratio.
Relationship Between Delay in Diagnosis and Stage at Diagnosis
We examined the association between delay in diagnosis and stage at diagnosis. We categorized women with stage II, III, and IV disease as high stage, compared with women with stage I disease (Table 3). In a multivariate analysis, delay in diagnosis was associated with high stage breast cancer (OR, 1.52; 95% CI, 1.43–1.62; p < .0001). Younger age (OR, 1.96; 95% CI, 1.78–2.15; p < .0001) and nonscreening-detected presentation (OR, 4.36; 95% CI, 4.10–4.63) were also associated with high stage. However, in a mutually adjusted model controlling for delay, age, and initial sign or symptom, the associations between a delay in diagnosis (OR, 1.06; 95% CI, 0.99–1.14; p = .11) and age (OR, 1.18; 95% CI, 1.07–1.31; p = .001) and stage at diagnosis were substantially attenuated, although a nonscreening-detected presentation (OR, 4.31; 95% CI, 4.05–4.58) remained highly significant (p < .0001).
Table 3.
Associations between having high stage (stage II, III, and IV) breast cancer and delay in diagnosis, age at diagnosis, and initial sign or symptom of breast cancer
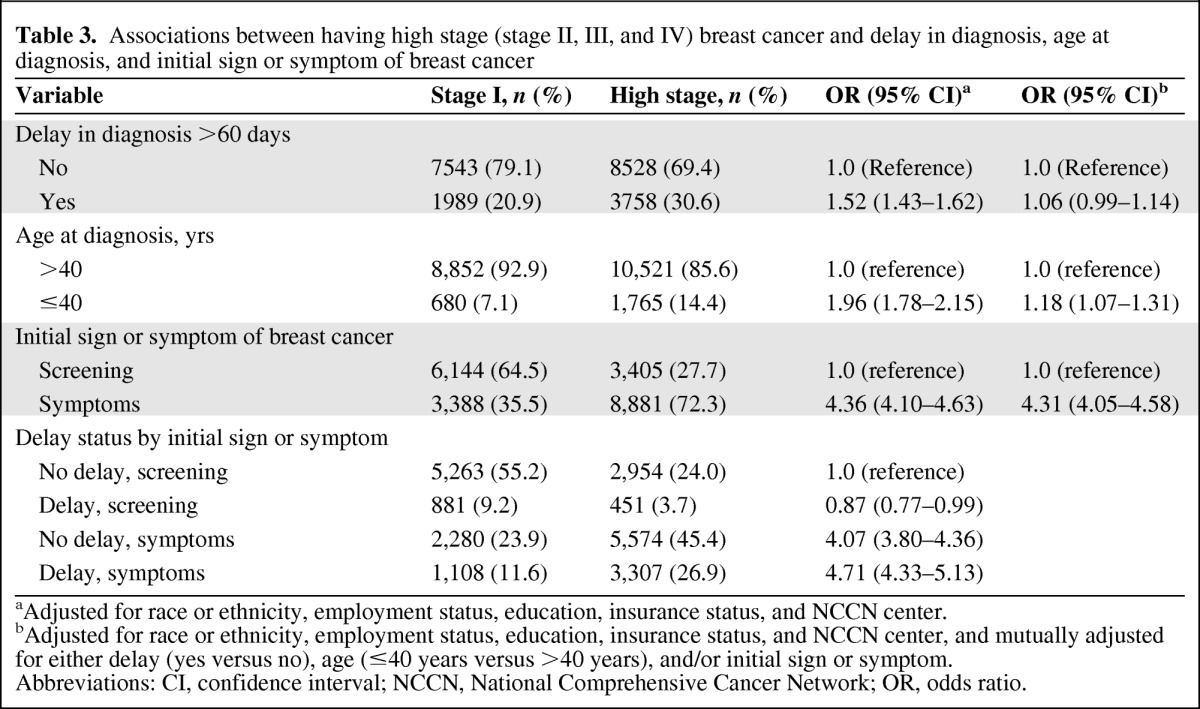
aAdjusted for race or ethnicity, employment status, education, insurance status, and NCCN center.
bAdjusted for race or ethnicity, employment status, education, insurance status, and NCCN center, and mutually adjusted for either delay (yes versus no), age (≤40 years versus >40 years), and/or initial sign or symptom.
Abbreviations: CI, confidence interval; NCCN, National Comprehensive Cancer Network; OR, odds ratio.
In an effort to clarify the association among a delay in diagnosis, reason for presentation, and stage at diagnosis, we conducted an analysis stratified by age (Table 4). Delays in both older and younger women were associated with a modest, nonsignificant higher risk for high stage (p for heterogeneity = .41). Among younger women, nonscreening detection was associated with a 3.10 times higher (95% CI, 2.36–4.06) risk for high stage whereas, among older women, a nonscreening detection was associated with a 4.42 times higher (95% CI, 4.15–4.71) risk for high stage (p for heterogeneity = .03), indicating that older women who present with signs or symptoms have a greater risk for having high-stage disease than younger women, irrespective of delay. A secondary analysis, using a different cutoff point for high stage (stage I and II versus stage III and IV as high stage) was conducted to evaluate the robustness of our original definition of high stage and demonstrated similar effects (data not shown).
Table 4.
Associations between having high stage (stages II, III, and IV) breast cancer and initial sign or symptom and delay in diagnosis stratified by age at diagnosis
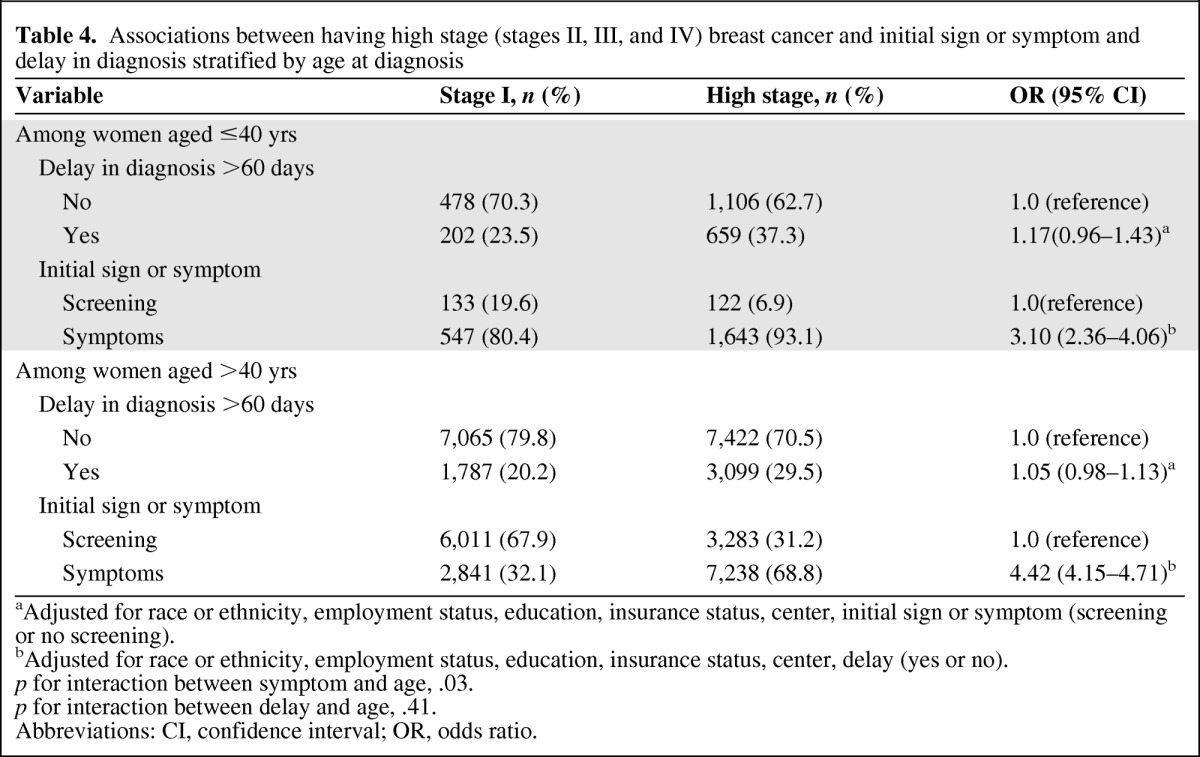
aAdjusted for race or ethnicity, employment status, education, insurance status, center, initial sign or symptom (screening or no screening).
bAdjusted for race or ethnicity, employment status, education, insurance status, center, delay (yes or no).
p for interaction between symptom and age, .03.
p for interaction between delay and age, .41.
Abbreviations: CI, confidence interval; OR, odds ratio.
Table 5 shows that women with screening-detected tumors were more likely to have lower stage disease (Mantel Haenszel χ2 p-value < .0001). For example, 64% of women with a screening-detected tumor had stage I disease, compared with 28% of women with a sign or symptom as the first triggering event. It is notable that a substantial proportion of symptom-detected tumors were lower stage, with a more modest proportion of screening-detected tumors found to be high stage.
Table 5.
Frequency of stage at diagnosis according to initial sign or symptom
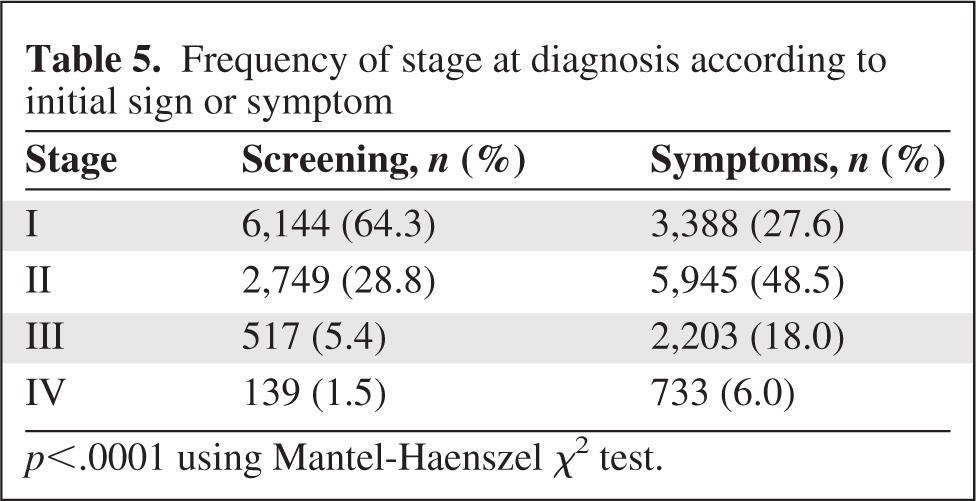
p<.0001 using Mantel-Haenszel χ2 test.
Discussion
Our study demonstrates that young age is not an independent predictor of a delay in diagnosis of breast cancer, but diagnosis based on signs or symptoms is associated with a delay in both younger and older women, and younger women are more likely than older women to present with symptoms. This analysis helps to elucidate the likely mechanisms underlying the historical association of younger age with a delay in breast cancer diagnosis, higher stage of disease, and symptomatic presentation. The finding that a delay had only a modest effect on a surrogate for outcome, stage at presentation, as well as the fact that the majority of younger and older women do not have a substantial delay in diagnosis, may have important potential implications for public health recommendations and future research.
Many prior studies have evaluated delays in diagnosis and delays in treatment of women with breast cancer [8–12, 20–29]. Results have been mixed with regard to the relationship between age and delay and the effect of a delay on patient outcomes. Studies have been limited by small sample sizes, limited heterogeneity of the population studied, and little, if any, information about clinical presentation. Few studies have focused on whether or not younger age is associated with a delay in diagnosis. In a cross-sectional survey of 124 women with breast symptoms presenting to a county general hospital outpatient clinic, Friedman et al. [8] found that younger age (p < .05), less education (p < .01), the absence of a lump (p < .05), lower perceived risk (p < .001), less spirituality (p < .01), cost (p < .001), and not wanting to think about breast symptom(s) (p < .05) were related to a delay. In that study, there was no evaluation of the effect of a delay in diagnosis on stage of disease or disease-free or overall survival outcomes.
In an evaluation of sociodemographic factors and the components of a diagnostic delay for six cancers, including breast cancer, using the National Survey of National Health Service Patients–Cancer database (including a total of 65,192 patients), Neal and Allgar did not find an effect of age on the risk for a delay in diagnosis and reported that the only significant factors associated with a delay were marital status and ethnic group [9]. Further, they did not assess the impact of the initial sign or symptom or the effect on stage or disease outcome.
Love and colleagues evaluated the relationship among age, delay, and outcome in 550 premenopausal women participating in adjuvant hormonal therapy in Vietnam and China [10]. They found that women with a >6-month delay in diagnosis had larger tumors clinically and pathologically (p = .0006 and p = .004, respectively), more frequent axillary node involvement (p = .008), and shorter, but not statistically different, disease-free and overall survival intervals from the time of diagnosis (p = .09 and .35, respectively). Using the same trial data, a multivariate model also revealed that young age was an independent adverse prognostic factor for survival (p = .005, with age as a continuous variable). However, in that analysis, the investigators did not control for delay nor did they control for tumor subtype, and younger women are more likely to have more aggressive subtypes of breast cancer than older women.
This is the largest comprehensive evaluation to assess the relationship among patient age, delay in diagnosis, and stage of disease at presentation among women with breast cancer. A major strength of this study was the availability of detailed information on initial symptoms and the time from initial symptom or sign to diagnosis in a large, diverse, multicenter observational cohort of women with breast cancer. However, our results should be considered in light of the limitations of this research. We relied on patient recall for the baseline survey combined with physician medical record reporting and chart extraction for the timing and presentation of the breast cancer. This variable may be subject to inaccuracies, including differences in ascertainment by center and recall bias on the part of patients, although women are typically surveyed within weeks to months after their diagnosis and confirmatory data were sought in the medical record. Although we controlled for NCCN center in our analysis, there is potential for misclassification, but we would expect this to be nondifferential and thus bias our results toward the null. An additional concern is that we did not have information on whether or not women presenting with symptomatic disease were engaged in regular breast cancer screening. Thus, we do not know whether or not these cancers are interval cancers in a screened population. We also do not have information about the use of screening magnetic resonance imaging (MRI) in this population, although the number of participants having breast MRI screening would be expected to be low. Finally, the NCCN database enrolls patients only from large comprehensive cancer centers and is not population based, and therefore the experiences of women in this study may not be generalizable to all women with breast cancer.
Nevertheless, this study supports the assertion that future research to evaluate the relationship among age at diagnosis, delay in diagnosis, the risk for cancer recurrence, and survival outcomes is warranted. Symptomatic presentation of breast cancer is more common in younger than older women. Regardless of age group, symptomatic presentation predicts delay and higher stage at diagnosis. Young age is not an independent predictor of a delay in diagnosis of breast cancer and only modestly predicts higher stage disease. Our results suggest that research into enhancing recognition of symptomatic presentation among women and health care providers may result in more women of all ages, but young women in particular, presenting for care with earlier stage, and thus lower risk, breast cancer.
See the accompanying commentary on pages 744–746 of this issue.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Ann H. Partridge, Melissa E. Hughes, Joyce C. Niland, Eric P. Winer, Jane C. Weeks, Rulla M. Tamimi
Provision of study material or patients: Ann H. Partridge, Melissa E. Hughes, Yu-Ning Wong, Stephen B. Edge, Richard L. Theriault, Douglas W. Blayney, Joyce C. Niland, Eric P. Winer, Jane C. Weeks
Collection and/or assembly of data: Ann H. Partridge, Melissa E. Hughes, Yu-Ning Wong, Stephen B. Edge, Richard L. Theriault, Douglas W. Blayney, Joyce C. Niland, Jane C. Weeks, Rulla M. Tamimi
Data analysis and interpretation: Ann H. Partridge, Melissa E. Hughes, Rebecca A. Ottesen, Yu-Ning Wong, Stephen B. Edge, Richard L. Theriault, Douglas W. Blayney, Joyce C. Niland, Eric P. Winer, Jane C. Weeks, Rulla M. Tamimi
Manuscript writing: Ann H. Partridge, Melissa E. Hughes, Rebecca A. Ottesen, Yu-Ning Wong, Stephen B. Edge, Richard L. Theriault, Douglas W. Blayney, Joyce C. Niland, Eric P. Winer, Jane C. Weeks, Rulla M. Tamimi
Final approval of manuscript: Ann H. Partridge, Melissa E. Hughes, Rebecca A. Ottesen, Yu-Ning Wong, Stephen B. Edge, Richard L. Theriault, Douglas W. Blayney, Joyce C. Niland, Eric P. Winer, Jane C. Weeks, Rulla M. Tamimi
References
- 1.Hankey BF, Miller B, Curtis R, et al. Trends in breast cancer in younger women in contrast to older women. J Natl Cancer Inst Monogr. 1994;(16):7–14. [PubMed] [Google Scholar]
- 2.American Cancer Society. Atlanta: American Cancer Society, Inc.; 2007. Breast Cancer Facts and Figures 2007–2008; pp. 1–32. [Google Scholar]
- 3.American Cancer Society. Atlanta: American Cancer Society, Inc.; 2006. Breast Cancer Facts and Figures 2005–2006; pp. 1–28. [Google Scholar]
- 4.Adami HO, Malker B, Holmberg L, et al. The relation between survival and age at diagnosis in breast cancer. N Engl J Med. 1986;315:559–563. doi: 10.1056/NEJM198608283150906. [DOI] [PubMed] [Google Scholar]
- 5.Zabicki K, Colbert JA, Dominguez FJ, et al. Breast cancer diagnosis in women ≤ 40 versus 50 to 60 years: Increasing size and stage disparity compared with older women over time. Ann Surg Oncol. 2006;13:1072–1077. doi: 10.1245/ASO.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 6.Ahn SH, Son BH, Kim SW, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: Nationwide survival data in Korea—a report from the Korean Breast Cancer Society. J Clin Oncol. 2007;25:2360–2368. doi: 10.1200/JCO.2006.10.3754. [DOI] [PubMed] [Google Scholar]
- 7.Gorin SS, Heck JE, Cheng B, et al. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166:2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 8.Friedman LC, Kalidas M, Elledge R, et al. Medical and psychosocial predictors of delay in seeking medical consultation for breast symptoms in women in a public sector setting. J Behav Med. 2006;29:327–334. doi: 10.1007/s10865-006-9059-2. [DOI] [PubMed] [Google Scholar]
- 9.Neal RD, Allgar VL. Sociodemographic factors and delays in the diagnosis of six cancers: Analysis of data from the “National Survey of NHS Patients: Cancer.”. Br J Cancer. 2005;92:1971–1975. doi: 10.1038/sj.bjc.6602623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love RR, Duc NB, Baumann LC, et al. Duration of signs and survival in premenopausal women with breast cancer. Breast Cancer Res Treat. 2004;86:117–124. doi: 10.1023/b:brea.0000032980.55245.c3. [DOI] [PubMed] [Google Scholar]
- 11.Facione NC. Delay versus help seeking for breast cancer symptoms: A critical review of the literature on patient and provider delay. Soc Sci Med. 1993;36:1521–1534. doi: 10.1016/0277-9536(93)90340-a. [DOI] [PubMed] [Google Scholar]
- 12.Richards MA, Westcombe AM, Love SB, et al. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 13.Fleming ID, Cooper JS, Henson DE, et al., editors. AJCC Cancer Staging Handbook. Fifth Edition. Philadelphia: Lipincott, Williams, and Wilkins; 1997. American Joint Committee on Cancer; pp. 1–294. [Google Scholar]
- 14.Weeks JC. Outcomes assessment in the NCCN. Oncology (Williston Park) 1997;11:137–140. [PubMed] [Google Scholar]
- 15.Weeks J. Outcomes assessment in the NCCN: 1998 update. National Comprehensive Cancer Network. Oncology (Williston Park) 1999;13:69–71. [PubMed] [Google Scholar]
- 16.Niland JC. NCCN Internet-based data system for the conduct of outcomes research. Oncology (Williston Park) 1998;12:142–146. [PubMed] [Google Scholar]
- 17.Niland JC. NCCN outcomes research database: Data collection via the Internet. Oncology (Williston Park) 2000;14:100–103. [PubMed] [Google Scholar]
- 18.Meechan G, Collins J, Petrie KJ. The relationship of symptoms and psychological factors to delay in seeking medical care for breast symptoms. Prev Med. 2003;36:374–378. doi: 10.1016/s0091-7435(02)00053-1. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer D, Lemeshow S. Applied Logistic Regression, Second Edition. Hoboken, NJ: John Wiley & Sons, Inc.; 2000. pp. 1–373. [Google Scholar]
- 20.Bish A, Ramirez A, Burgess C, et al. Understanding why women delay in seeking help for breast cancer symptoms. J Psychosom Res. 2005;58:321–326. doi: 10.1016/j.jpsychores.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Hébert-Croteau N, Freeman CR, Latreille J, et al. A population-based study of the impact of delaying radiotherapy after conservative surgery for breast cancer. Breast Cancer Res Treat. 2004;88:187–196. doi: 10.1007/s10549-004-0594-7. [DOI] [PubMed] [Google Scholar]
- 22.Kothari A, Fentiman IS. 22. Diagnostic delays in breast cancer and impact on survival. Int J Clin Pract. 2003;57:200–203. [PubMed] [Google Scholar]
- 23.Montella M, Crispo A, D'Aiuto G, et al. Determinant factors for diagnostic delay in operable breast cancer patients. Eur J Cancer Prev. 2001;10:53–59. doi: 10.1097/00008469-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Petticrew MP, Sowden AJ, Lister-Sharp D, et al. False-negative results in screening programmes: Systematic review of impact and implications. Health Technol Assess. 2000;4:1–120. [PubMed] [Google Scholar]
- 25.Brenner RJ. False-negative mammograms. Medical, legal, and risk management implications. Radiol Clin North Am. 2000;38:741–757. doi: 10.1016/s0033-8389(05)70198-6. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez AJ, Westcombe AM, Burgess CC, et al. Factors predicting delayed presentation of symptomatic breast cancer: A systematic review. Lancet. 1999;353:1127–1131. doi: 10.1016/s0140-6736(99)02142-x. [DOI] [PubMed] [Google Scholar]
- 27.Kern KA. Medicolegal analysis of the delayed diagnosis of cancer in 338 cases in the United States. Arch Surg. 1994;129:397–403. doi: 10.1001/archsurg.1994.01420280073009. discussion 403–404. [DOI] [PubMed] [Google Scholar]
- 28.Hunter CP, Redmond CK, Chen VW, et al. Breast cancer: Factors associated with stage at diagnosis in black and white women. Black/White Cancer Survival Study Group. J Natl Cancer Inst. 1993;85:1129–1137. doi: 10.1093/jnci/85.14.1129. [DOI] [PubMed] [Google Scholar]
- 29.Caplan LS, Helzlsouer KJ. Delay in breast cancer: A review of the literature. Public Health Rev. 1992–1993;20:187–214. [PubMed] [Google Scholar]



