This article explores the perception of collaborative language as used by physicians during shared decision-making for pediatric patients with cancer. Interviews of patients, parents/guardians and grandparents, and family members were conducted prior to allogeneic blood and marrow transplantation.
Keywords: Shared decision-making, Ethics, Pediatric bone marrow transplant
Learning Objectives:
After completing this course, the reader will be able to:
When the alternative to medical treatment is likely death, ask parents and children whether they agree to the medical plan rather than suggesting they are sharing in “a decision.”
Use the model of shared decision in appropriate settings, that is, those in which a bona fide choice exists.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Introduction.
Shared decision-making between health care professionals, patients, parents, and guardians is widely recommended today. However, it is unclear what happens when collaborative language is used by physicians in clinical situations for which patients and parents/guardians believe there is no decision to be made.
Methods.
We conducted a qualitative study of decision-making for pediatric allogeneic blood and marrow transplantation by interviewing patients, parents, grandparents, donor siblings, and nondonor children after the decision to proceed to transplant but before the transplantation. Each interview was audio recorded, transcribed, and coded for major themes.
Results.
In total, 107 members of 30 families at four sites were interviewed, including 15 patients, 22 mothers, 2 stepmothers, 1 grandmother, 19 fathers, 3 stepfathers, 1 grandfather, 13 sibling donors, and 31 nondonor children (siblings, half-siblings, and cousins). In all, 81% of parents/guardians, 73% of patients, 31% of donors, and 29% of other children reported there was no decision to be made. Almost all (88%) parents/guardians indicated that the physician's recommendation was a large determinant in their agreement to go forward with the transplantation. All parents/guardians reported that “agreeing to a plan” was a better description of what their consent entailed.
Conclusions.
To be respectful of patients and parents/guardians, we suggest that “agreeing to a plan” may be a better description for what parents/guardians must consider when the alternative to a transplantation is likely death. In this clinical context, the shared decision-making model with a focus on “a decision to be made” may be misleading.
Introduction
The art of oncology has evolved dramatically in treatment decision-making from paternalism (the physician making treatment decisions alone) to autonomy (the patient or surrogate making the decision) and now to shared decision-making (a collaborative approach in which the physician's expertise is melded with the patient and/or surrogate's perspectives) [1–6]. The shared decision-making approach is reportedly preferred by some patients [7, 8], and physicians and other health care providers are being schooled in the language and methods of the collaborative approach for treatment decision-making [9]. However, this approach and the language of shared decision-making may not be the best practice in certain clinical care contexts. It is unclear what happens when collaborative language is used by physicians in clinical situations for which patients and parents/guardians believe there is no decision to be made.
We conducted a qualitative grounded theory study of decision-making for pediatric allogeneic blood and marrow transplantation, which is an ideal laboratory for studying decision-making given its complexities: anxiety and stress resulting from having a child with a life-threatening illness, time-sensitive pressure to make a decision, complexity of treatment plans, treatment often offered on randomized clinical trials, and a need for parental permission and child assent. Following grounded theory [10], our goal was to create a substantive theory of family decision-making regarding pediatric allogeneic transplantation for the treatment of childhood cancer by gathering the perspective of each member of the family; thus, we were not testing a specific hypothesis.
We consecutively approached all eligible families (namely, those who had a child who was being offered an allogeneic stem cell transfer) at each of four geographically diverse sites: Atlanta; Philadelphia; Kansas City, MO; and Calgary, AB, Canada. Depending on the site, a nurse practitioner, research assistant, or a social worker identified all eligible families. All children between the ages of 9 and 22 years old who were considered by the parent(s) to be part of the family, including half-siblings and cousins, were eligible to be interviewed. After the first seven families, we selectively sampled families with eligible siblings. We qualitatively interviewed family members after the decision to proceed to transplant had been made but before the stem cell transfer occurred. The parents or guardians within each family and all eligible children were individually interviewed in the location most convenient for them, although we encouraged interviews in their homes. Each interview was audio recorded, transcribed, and coded for major themes.
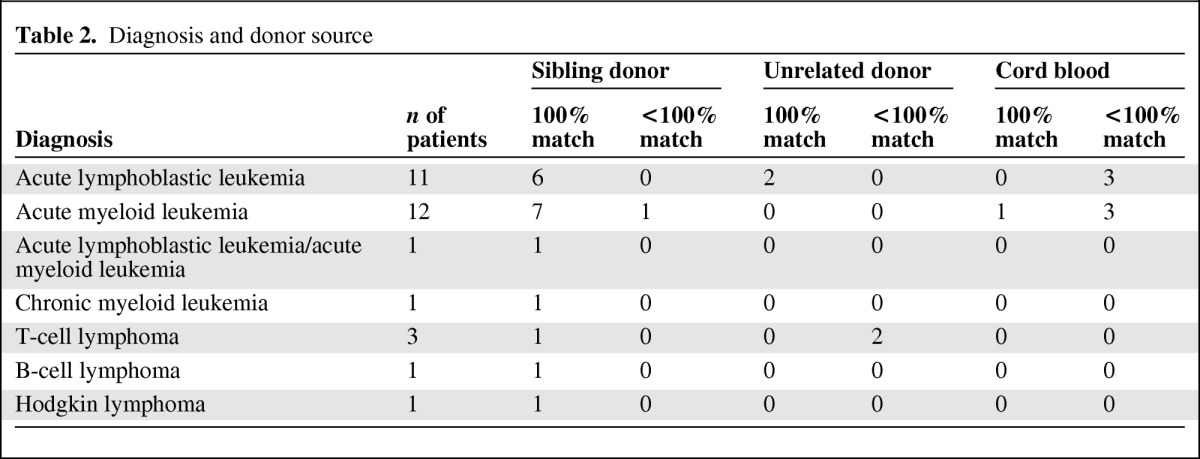
We interviewed 107 members of 30 families: 15 patients, 22 mothers, 2 stepmothers, 1 grandmother, 19 fathers, 3 stepfathers, 1 grandfather, 13 sibling donors, and 31 nondonor children (siblings, half-siblings, and cousins). Basic demographics are provided in Table 1. Diagnosis, stem cell source, and match are detailed in Table 2. All members of the families consented/assented to the study following guidance from the local sites' institutional review board or research ethics board.
Table 1.
Participant demographics
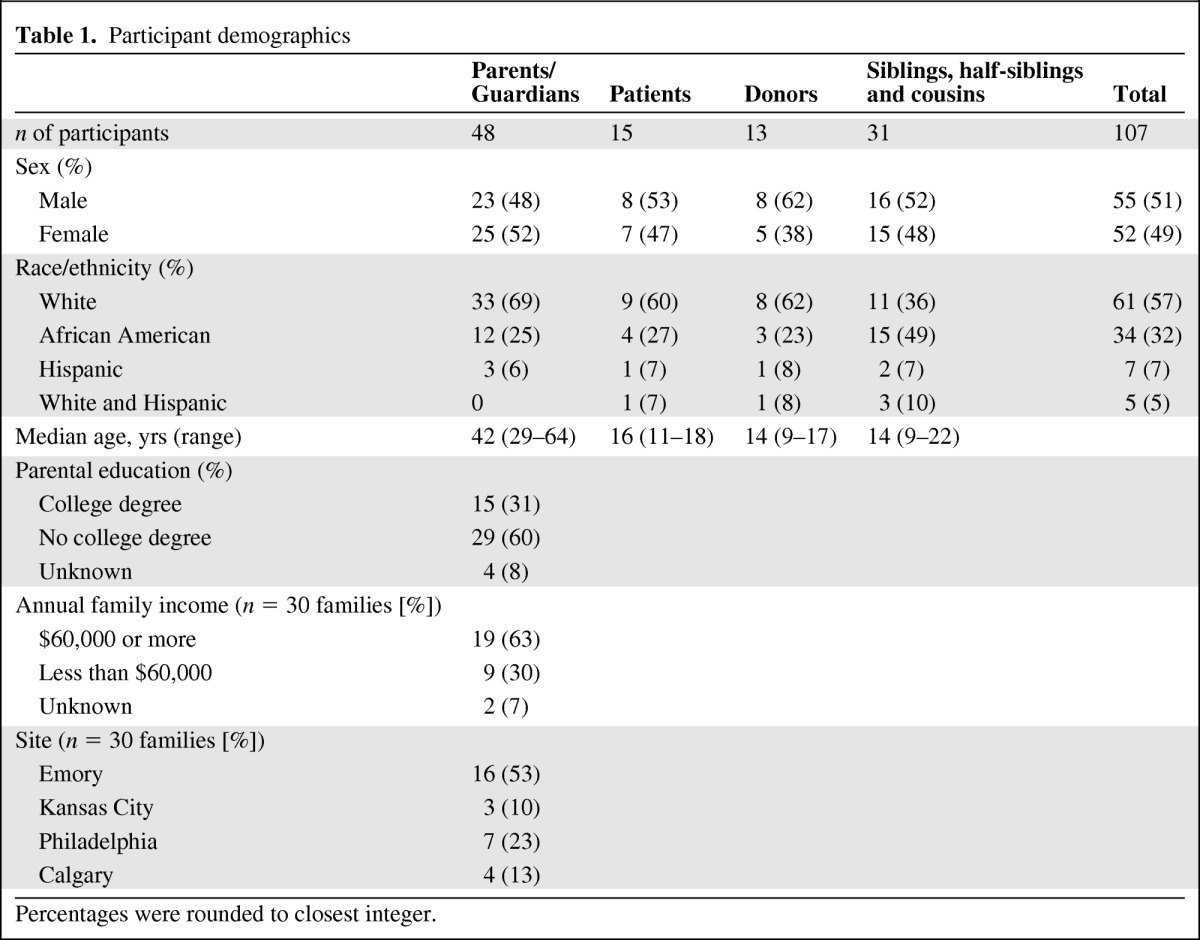
Percentages were rounded to closest integer.
Table 2.
Diagnosis and donor source
The responses were not at all what we expected given our assumption that a family decision-making theory could be constructed. A single theme emerged. Most of the parents, siblings, donors, and patients looked at us quizzically or even laughed when we asked our basic question: “Recently, you and your family made an important decision about [patient's name] getting a transplant. Would you please tell me about how your family made that decision?” The answer again and again was a variation of the following: “You can call it a decision but it wasn't a decision. It was either get the transplant or let my child/brother/sister die.”
A few quotes suffice:
Father of 3-year-old child with bilineage leukemia: “They have to say you have a choice, but, they are telling you, you really don't have a choice.”
Father of 16-year-old child with acute myeloid leukemia: “There really wasn't much of a decision. I don't see any alternative.”
Mother of 8-year-old with acute lymphoblastic leukemia: “I guess we didn't really see that we had a decision … This was really the only choice … We kept being told we were going to come up here and talk it over with the doctor and then leave and then come back and tell them our decision. And we would look at each other in the car and kind of laugh at that like, ‘Ha, we have a decision?’”
17-year-old patient with acute lymphoblastic leukemia: “There's not really that much decision-making. The transplant was the ‘choice to live.’ There really wasn't anything else they could do for me.”
In total, 81% (39/48) of parents/guardians, 73% (11/15) of patients, 31% (4/13) of donors, and 29% (9/31) of nondonor children indicated there was no decision to be made. A total of 52% (16/31) of the nondonor siblings, half-siblings, and cousins stated they did not know if a decision had been made, which was a rarer response among donors (3/13) and patients (0/15). None of the cousins reported that they knew if a decision had been made. The results are summarized in Table 3.
Table 3.
Interview responses: Was transplantation a decision?
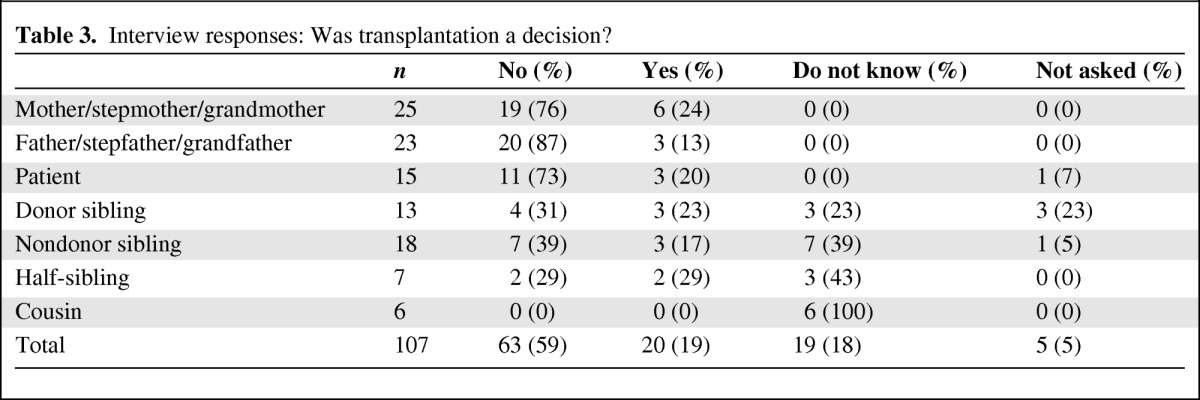
Some of the nondonor siblings within the family speculated that the adults made the decision and were responsible for it. As a 16-year-old sibling explained: “I think it is [my mother's] decision because she is the main guardian of my sister, basically. She is always with my sister and makes the decisions for the most part. I don't really know that much.”
Most parental/guardian pairs (72%, 13/18) agreed that proceeding to transplantation was not a decision. The one family headed by grandparents agreed that there was a decision (6%; 1/18); in fact, every member of that family concurred that the teenaged children within the family made all of their own health care decisions. In 3 of 18 families (17%), the mother thought there was a decision and the father thought there was no decision (because it was a “no brainer,” a quote echoed by two of the other fathers). In the final family, the stepfather was not involved in decisions about the patient; he thought his wife (the patient's mother) may have made the decision, whereas his wife indicated there was no decision to be made. In the remaining 12 families, only one parent/guardian was available to be interviewed.
Eight of the patients received their transplant as part of a research protocol. Although we did not directly ask the families about research, at least one member of five of these families (four mothers and two fathers) spontaneously mentioned the research protocol while describing the decision-making process. All but one of the parents of the patients treated on protocol stated that the transplant was not a decision, even though all were offered transplant off protocol. However, because we do not know how the choice between on-protocol and off-protocol transplantation was presented (i.e., if it was presented as a bona fide choice), we do not know whether the parents realized that participation in research was a choice.
Most parents/guardians (88%) indicated that the physician's recommendation was key in their thinking and a large determinant in their agreement to go forward with transplantation. One father of a 2-year-old child with acute myeloid leukemia, who was firm that there was no decision to be made, explained his reliance on the physician's recommendation:
“The only way you would not go with a decision that the doctors recommend is because you are reckless or you truly don't believe that the doctor has your child's best interest in mind … There is no way that I can educate myself beyond his opinion … His opinion is based on high instincts and exact data … The doctor inspires confidence and hope.”
Two mothers confirmed this:
Mother of 9-year-old child with large cell lymphoma: “You always really default to the doctor with the experience and keep your daughter's best interest at hand.”
Mother of 12-year-old child with acute lymphoblastic leukemia: “I guess the doctors decided it was the best option for her treatment. [They are] the experts in the field.”
Members of 20 of the families explained that the doctor had stated that transplantation was the “best choice,” “best route,” or “needed to be done.” These family members perceived that the physician's recommendation to proceed with transplantation was clear even if other options, such as continued chemotherapy, were mentioned. There was no site variability in these comments; they were made by families from all four sites. The comments also were made both by families for whom transplant was part of the original plan at diagnosis and by those for whom it was not.
A limitation of our family-centered study is that we do not have information on how the physicians perceived the strength of their recommendation or their presentation of options.
Conclusions
Although shared decision-making is often an excellent model, it is important to be sensitive to the treatment context. The clear ethical advantage of shared decision-making is that both the recommendations of the experts and the values and priorities of the patients, parents, and guardians are factored into the final decision. However, language is important. Parents' frustration with “choice” and “decision” language hinted that they perceived some disingenuousness from their physicians. Some parents even stated that the physician had to say there was a choice, but everyone knew that there was not a choice because the only other alternative—the child's eventual death—was not acceptable.
In pediatrics, most clinicians recognize that it is disrespectful and untrustworthy to tell a child there is a choice if a “wrong” choice will be overridden by the parents, the physicians, or even the courts. It is unclear if there are similar parental diminutions in trust and respect if choice language is used when there is no viable option. In extreme cases, parents who refuse a strongly medically indicated transplant for their child may also be overridden by the court. However, even if that is not the case, using decision language can seem less than honest and not transparent. One father of a 2-year-old child with acute myeloid leukemia mused that the physician was quite artful in this ruse:
“The doctors are good at making you believe that you have a decision. I truly believe that you don't have a decision … I know for sure, looking back, that Dr. L. knew that we would be doing exactly what we are doing today. When he presented it to us, he presented it as options. There was no alternate in his mind.”
Furthermore, decision language may imply a level of responsibility that is not there. An early study reported that mothers, who are often overwhelmed and traumatized by the cancer diagnosis and medical recommendation to proceed to transplantation [11], state that they have no choice, yet some later regretted “their decision” (although this study focused on participation in a clinical trial). Decision language may also have been misleading to the children in the family who were not as well informed, such as nondonor siblings and cousins. These children may have assumed that their parents were choosing transplantation instead of another treatment, and therefore the parents were in part responsible for the outcome.
There have been other reports of parents' perceived lack of choice [12] and reliance on the physician's recommendation [13, 14]. Studies of shared decision-making have found that certain classes of patients do not wish to participate in the decision: those with more serious and life-threatening illnesses [15, 16], those for whom there are no alternative treatments [17], or those for whom evidence is lacking for the next treatment [18]. These shared decision-making discussions frame the issue as a decision that one could refuse to participate in or be excluded from. We found, on the contrary, that in certain health care contexts there is no decision to be made at all because there is no acceptable alternative. Physicians may already sense this situation; the lack of alternative treatment options is the largest factor in whether physicians attempt shared-decision-making [19] or think it is advisable [20].
As we talked to more families, it became clear that “choice” and “decision” were not the most apt words to use in this context. Therefore, near the conclusion of the study, we began to ask families if “agreeing to a plan” proposed by the physicians was a preferable description; all of the eight parents who were asked agreed that it was. This description accords with Benedict et al [12], who found that 15 of 20 parents thought there was no choice; they also found that parents understood there were alternatives such as palliation and did not feel coerced to agree to have their child undergo a bone marrow transplantation. It also accords with the many statements by family members that the physician proposed the transplant as the “best thing to do” and that, even though other options were mentioned, they were not presented as the best plan.
The advantage of describing consent in this situation as “agreeing to a plan” is that it provides an important role for the parent; their permission or agreement is sought and must be documented on the informed consent document before transplantation can proceed. However, the responsibility for creating the plan lies squarely on the expert health care professional. We do note a caution: if this language is used it must be clear that families can disagree with the plan. Further, it is important to distinguish this consent for standard of care (which we argue can be best described as agreeing to plan) from consent to participate in a research protocol; research is voluntary and does require a decision.
To be respectful of patients and parents, we must use the language that is the most honest description of the situation confronting them. We suggest that “agreeing to a plan” may be a better description for what parents must consider when the alternative to transplantation is likely death. In this context, the shared decision-making model with a focus on “the decision to be made” may be misleading—or worse, less than honest.
Acknowledgments
This work was supported by the National Cancer Institute (grant 1R21CA131875-01A1).
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Rebecca D. Pentz, Wendy Pelletier, Melissa A. Alderfer, Pamela S. Hinds
Provision of study material or patients: Rebecca D. Pentz, Wendy Pelletier, Melissa A. Alderfer, Kristin Stegenga, Pamela S. Hinds
Collection and/or assembly of data: Rebecca D. Pentz, Wendy Pelletier, Melissa A. Alderfer, Kristin Stegenga
Data analysis and interpretation: Rebecca D. Pentz, Wendy Pelletier, Melissa A. Alderfer, Kristin Stegenga, Diane L. Fairclough, Pamela S. Hinds
Manuscript writing: Rebecca D. Pentz, Wendy Pelletier, Kristin Stegenga, Pamela S. Hinds
Final approval of manuscript: Rebecca D. Pentz, Wendy Pelletier, Melissa A. Alderfer, Kristin Stegenga, Diane L. Fairclough, Pamela S. Hinds
References
- 1.Shama WI. The experience and preparation of pediatric sibling bone marrow donors. Soc Work Health Care. 1998;27:89–99. doi: 10.1300/J010v27n01_06. [DOI] [PubMed] [Google Scholar]
- 2.Davidson JE, Powers K, Hedayat KM, et al. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004–2005. Crit Care Med. 2007;35:605–622. doi: 10.1097/01.CCM.0000254067.14607.EB. [DOI] [PubMed] [Google Scholar]
- 3.Mercurio MR, Forman EN, Ladd RE, et al. American Academy of Pediatrics policy statements on bioethics: Summaries and commentaries: Part 3. Pediatr Rev. 2008;29:e28–34. doi: 10.1542/pir.29-5-e28. [DOI] [PubMed] [Google Scholar]
- 4.Carlet J, Thijs LG, Antonelli M, et al. Challenges in end-of-life care in the ICU. Statement of the 5th International Consensus Conference in Critical Care: Brussels, Belgium, April 2003. Intensive Care Med. 2004;30:770–784. doi: 10.1007/s00134-004-2241-5. [DOI] [PubMed] [Google Scholar]
- 5.American Medical Association. D-373.999 Informed patient choice and shared decision making. [Accessed March 2, 2012]. Available at https://ssl3.ama-assn.org/apps/ecomm/PolicyFinderForm.pl?site=www.ama-assn.org&uri=/ama1/pub/upload/mm/PolicyFinder/policyfiles/DIR/D-373.999.HTM.
- 6.Committee on Comparative Effectiveness Research Prioritization. Washington, DC: Institute of Medicine; 2009. Initial National Priorities for Comparative Effectiveness Research. [Google Scholar]
- 7.Shields CG, Morrow GR, Griggs J, et al. Decision-making role preferences of patients receiving adjuvant cancer treatment: a university of Rochester cancer center community clinical oncology program. Support Cancer Ther. 2004;1:119–126. doi: 10.3816/SCT.2004.n.005. [DOI] [PubMed] [Google Scholar]
- 8.Murray E, Pollack L, White M, et al. Clinical decision-making: Patients' preferences and experiences. Patient Educ Couns. 2007;65:189–196. doi: 10.1016/j.pec.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Braddock CH. The emerging importance and relevance of shared decision making to clinical practice. Med Decis Making. 2010;30:5S–7S. doi: 10.1177/0272989X10381344. [DOI] [PubMed] [Google Scholar]
- 10.Glaser BG, Strauss AL. New Brunswick, NJ: Aldine Transaction; 1967. The Discovery of Grounded Theory: Strategies for Qualitative Research. [Google Scholar]
- 11.Stevens PE, Pletsch PK. Ethical issues of informed consent: Mothers' experiences enrolling their children in bone marrow transplantation research. Cancer Nurs. 2002;25:81–87. doi: 10.1097/00002820-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Benedict JM, Simpson C, Fernandez CV. Validity and consequence of informed consent in pediatric bone marrow transplantation: The parental experience. Pediatr Blood Cancer. 2007;49:846–851. doi: 10.1002/pbc.21073. [DOI] [PubMed] [Google Scholar]
- 13.McKneally MF, Martin DK. An entrustment model of consent for surgical treatment of life-threatening illness: Perspective of patients requiring esophagectomy. J Thorac Cardiovasc Surg. 2000;120:264–269. doi: 10.1067/mtc.2000.106525. [DOI] [PubMed] [Google Scholar]
- 14.Taylor B. Parental autonomy and consent to treatment. J Adv Nurs. 1999;29:570–576. doi: 10.1046/j.1365-2648.1999.00924.x. [DOI] [PubMed] [Google Scholar]
- 15.Arora NK, McHorney CA. Patient preferences for medical decision making: Who really wants to participate? Med Care. 2000;38:335–341. doi: 10.1097/00005650-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Siminoff LA, Fetting JH. Factors affecting treatment decisions for a life-threatening illness: The case of medical treatment of breast cancer. Soc Sci Med. 1991;32:813–818. doi: 10.1016/0277-9536(91)90307-x. [DOI] [PubMed] [Google Scholar]
- 17.Mandelblatt J, Kreling B, Figeuriedo M, Feng S. What is the impact of shared decision making on treatment and outcomes for older women with breast cancer? J Clin Oncol. 2006;24:4908–4913. doi: 10.1200/JCO.2006.07.1159. [DOI] [PubMed] [Google Scholar]
- 18.Keating NL, Beth Landrum M, Arora NK, et al. Cancer patients' roles in treatment decisions: Do characteristics of the decision influence roles? J Clin Oncol. 2010;28:4364–4370. doi: 10.1200/JCO.2009.26.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shepherd HL, Butow PN, Tattersall MH. Factors which motivate cancer doctors to involve their patients in reaching treatment decisions. Patient Educ Couns. 2011;84:229–235. doi: 10.1016/j.pec.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 20.McGuire AL, McCullough LB, Weller SC, Whitney SN. Missed expectations? Physicians' views of patients' participation in medical decision-making. Med Care. 2005;43:466–470. doi: 10.1097/01.mlr.0000160415.08497.11. [DOI] [PubMed] [Google Scholar]


