Abstract
Behavioral and neurophysiological studies suggest that skill learning can be mediated by discrete, experience-driven changes within specific neural representations subserving the performance of the trained task. We have shown that a few minutes of daily practice on a sequential finger opposition task induced large, incremental performance gains over a few weeks of training. These gains did not generalize to the contralateral hand nor to a matched sequence of identical component movements, suggesting that a lateralized representation of the learned sequence of movements evolved through practice. This interpretation was supported by functional MRI data showing that a more extensive representation of the trained sequence emerged in primary motor cortex after 3 weeks of training. The imaging data, however, also indicated important changes occurring in primary motor cortex during the initial scanning sessions, which we proposed may reflect the setting up of a task-specific motor processing routine. Here we provide behavioral and functional MRI data on experience-dependent changes induced by a limited amount of repetitions within the first imaging session. We show that this limited training experience can be sufficient to trigger performance gains that require time to become evident. We propose that skilled motor performance is acquired in several stages: “fast” learning, an initial, within-session improvement phase, followed by a period of consolidation of several hours duration, and then “slow” learning, consisting of delayed, incremental gains in performance emerging after continued practice. This time course may reflect basic mechanisms of neuronal plasticity in the adult brain that subserve the acquisition and retention of many different skills.
The performance of many tasks improves, throughout life, with repetition and practice. Even in adulthood simple tasks such as reaching to a target or rapidly and accurately tapping a short sequence of finger movements, which appear, when mastered, to be effortlessly performed, often require extensive training before skilled performance develops. What changes occur in the adult brain when a new skill is acquired through practice? When, and after how much practice, do these changes occur? Functional reorganization of adult mammalian sensory and motor cortical representations has been found to occur in many different animal models of brain plasticity in the last two decades, advancing the idea that throughout life the functional properties of central nervous system neurons, as well as the neural circuitry within different brain areas, are malleable and retain a functionally significant degree of plasticity (e.g., refs. 1–4). These representational changes have been shown to be induced not only in response to lesions of peripheral or central sensory input or motor output pathways but also, in normal individuals, as a result of practice and experience. The advent of new brain imaging techniques, especially functional MRI (fMRI) (5), which allows repeated mapping of cortical representations as a consequence of long-term practice, provides a way to examine over an extended time frame the neurobiological correlates of skill learning in the adult human brain.
In this paper we briefly outline two characteristics of skill learning—the specificity and the time course of learning—which, we propose, can provide important constraints on the neural locus and substrates of adult skill learning. By using the learning of sequential finger movements as the main experimental paradigm, we review recent findings, mainly from our own work, suggesting that: (i) the acquisition and retention of motor skills may result in significant experience-related reorganization within specific motor cortical representations in the adult human brain; and (ii) these representational changes occur in several stages and are characterized by a distinct time course. We review our fMRI and behavioral data (6) and recent experimental data (7) from monkeys trained to perform complex motor tasks to demonstrate that long-term training results in highly specific skilled motor performance, paralleled by the emergence of a specific, more extensive representation of a trained sequence of movements in the contralateral primary motor cortex (M1). We then present fMRI as well as behavioral evidence for an important intermediate stage in the acquisition of the skill that is set in motion by a few minutes of practice and continues to evolve after practice has ended. This stage presumably is subserved by neuronal processes that require time to become effective. These processes may underlie the consolidation of motor experience and thus provide a basis for the long-term memory of the skill. Further, they may be related to similar processes that have been described in adult human perceptual skill learning (8, 9). The finding that a similar time course characterizes the learning both of different types of motor skills and of different perceptual skills lends support to the idea that the time course of skill learning is determined by the time constants of a limited repertoire of basic neuronal mechanisms of plasticity subserving procedural memory throughout the adult cortex (10, 11).
Characteristics of Skill Learning
Skills constitute one of two distinct, broad categories of memory (12, 13). Although different taxonomies exist, the dichotomy accounts for deficits of fact and event memories (“what,” declarative knowledge) on the one hand, and the preservation of skills and habits (“how to,” procedural knowledge) on the other, in individuals and nonhuman primates with focal lesions to medial temporal lobe structures (12–15). Many instances of skill learning, both perceptual and motor, are specific for basic parameters of the training experience; that is, learning can be strongly dependent on simple physical attributes of the stimulus presented in training a perceptual task (e.g., refs. 16–18) or on factors such as the specific effector organs’ positions, trajectories and sequence of trajectories experienced in motor training (e.g., refs. 6 and 19–21). For example, training to perform an arm movement aimed at a specific target location against a specific perturbation resulted in learning to compensate for the perturbation in the trained part of the workspace but showed little generalization to the rest of the workspace (19). Similarly, training to overcome a specific perturbation did not generalize to overcoming an identical perturbation in the orthogonal direction (20).
There is considerable anatomical and physiological evidence for a hierarchical organization of information processing in sensory and motor systems in the mammalian brain, such that many physical parameters of a sensory input, or a motor output, are selectively represented only in specific processing stages. The specificity of learning for a given parameter of the training experience implies, therefore, that only a discrete part (or subset of neurons) within a processing stream—that wherein the parameter is differentially represented—has undergone learning related changes. At a level of processing in which neurons respond invariantly, one would expect learning to generalize for that particular parameter. Thus, the finding of specificity in the learning of a given skill has been used to generate predictions on the possible neuronal loci and type of representations affected by the training experience (6, 16–19, 21–24). This is not to say that all skills are specific for low-level parameters of the training experience: indeed, one would predict otherwise whenever the relevant aspects of a task are represented at higher levels within the processing stream (25). Nevertheless, in many instances the degree of specificity has indicated discrete changes in low-level representations as an important locus of learning (25). This interpretation of the human behavioral data is supported by experimental animal studies that have revealed that the details of the representation of the sensory input in low-level processing areas engaged in the performance of a given sensory discrimination task change, so as to reflect, by evolving improved and enlarged representations, the specific behavioral experiences of the animals under study (26–29). Similarly, motor representations have been shown to undergo experience-specific reorganization after long-term training (7), whereas cortical representational maps, often on a much shorter time scale, have been found to be altered by manipulations of their sensory inputs (1, 2) or motor outputs (3, 30).
An important difference between declarative and procedural memory is the time course of learning. Declarative learning can be very fast and may take place even after a single event (13, 31). Procedural learning, in contrast, is slow and often requires many repetitions, usually over several training sessions, to evolve (12, 31). Thus, one may remember the contents of a book after a single reading but the skills of reading evolve over multiple practice sessions and require many repetitions to become established.
Several recent studies have examined the time course of experience-dependent perceptual learning (8, 9, 32–34). In these studies, adult individuals were found to gain an increase in perceptual sensitivity when given practice in basic sensory discrimination tasks. These studies indicate that improved perceptual performance often evolves in two distinct stages (8): first, a fast within-session improvement that can be induced by a limited number of trials on a time scale of minutes (“fast learning”), and second, slowly evolving, incremental performance gains, triggered by practice but taking hours to become effective (“slow learning”). In many instances, most gains in performance evolved in a latent manner not during, but rather a minimum of 6–8 hr after training, that is, between sessions (8, 33–35). Improvements in performance continued to develop over the course of 5–10 daily practice sessions, spaced 1 to 3 days apart, before nearing asymptotic performance. The skill then was retained for months and years (8). Because of the long-term retention and by analogy to the time course described in several paradigms of developmental plasticity (36, 37), the latent phase in human skill learning is thought to reflect a process of consolidation of experience-dependent changes in the adult cortex that is triggered by training but continues to evolve after the training session has ended (8). Furthermore, it was proposed that fast learning reflects the setting up of a task-specific processing routine for solving the perceptual problem whereby those representations that are relevant for task performance are selected. Slow learning, on the other hand, is thought to reflect ongoing, perhaps structural, modifications of basic perceptual modules within the selected representations (8, 25, 32, 38).
Recent studies suggest that a similar time course may characterize the acquisition of some motor skills by human adults (6, 20, 39). Studies conducted in the early decades of this century have described a latent consolidation phase in perceptuomotor tasks under the term reminiscence (see ref. 40). In the monkey, fast, within-session learning, as well as large incremental gains in performance over weeks of daily training sessions—“slow” learning—have been described in both perceptual and motor skill learning paradigms (7, 27, 29). The monkey data further suggest that the long-term changes that can be induced in different brain areas by the learning of motor (7, 41) and perceptual skills (29) may be subserved by similar mechanisms of plasticity. Although the data are limited by the small number of studies and the different time windows examined in each of these studies, the results lend support to the idea that although the nature of the practice-dependent cortical representational changes are determined by the specifics of the training experience, the time course of skill learning may be determined by the time constants of basic mechanisms of neuronal plasticity irrespective of the locus of plasticity.
“Slow” Learning and the Long-Term Reorganization of M1
The learning of many motor skills involves the formation of novel sequences of muscle activity and the reconstruction of existing muscle control architectures (3, 41, 42). A hallmark of such learning is improved speed of motor execution without reciprocal deterioration in accuracy (43), which indicates the acquisition of a new capability of the motor system rather than functional adaptation within the limits of a pre-existing motor gain control mechanism (44). In recent years, the learning of sequential finger movements—related to skills such as writing, typing, or playing musical instruments—has become an important paradigm for the study of the acquisition of motor skills by using imaging techniques (45–52). These studies however, have been confined to relatively short time intervals and were not designed to look at the effects of long-term training. Also, many of these studies were concerned not only with the issue of how the performance of a known sequence of movements becomes fast and accurate through practice (42–44), but also with the issue of how declarative knowledge of a given sequence, embedded in the task unknown to the subject, is acquired through motor performance (45, 46, 49).
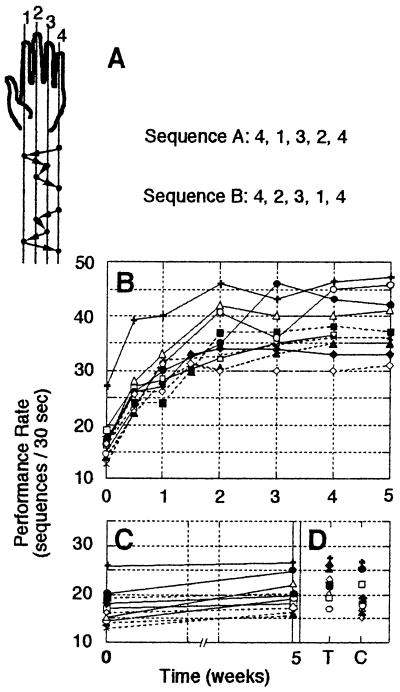
To investigate the effects of long-term training on the performance of a given sequence of movements, we recently have used a simple sequential finger opposition task in which the effects of training in young, healthy adults could be tracked over several weeks by using both behavioral measurements and functional brain imaging (6). In this task, subjects were instructed to oppose the fingers of the nondominant hand to the thumb in one of two given sequences (Fig. 1A). The sequences were composed of five component movements and their mirror-reversed (tapped back to front) counterparts. Subjects were required to tap each sequence, with no visual feedback, as accurately and rapidly as possible. Speed and accuracy were independently scored. The results for speed are reproduced in Fig. 1 B–D. Although initial performance of the two sequences, in terms of speed and accuracy, did not differ (Fig. 1 B and C), 10–20 min of daily practice during which subjects were instructed to repeatedly tap a given sequence (the other sequence served as the unpracticed control) in a rapid self-paced and accurate manner induced large gains in performance. The speed at which the trained sequence could be performed increased across consecutive sessions, nearing asymptote after about 3 weeks of training, with more than doubling of the initial rate (Fig. 1B) and a concurrent gain in accuracy (6). This improvement was specific to the trained hand, with no significant transfer to the untrained hand (Fig. 1D). Moreover, the effects of training did not generalize to the performance of the control sequence (Fig. 1C). These behavioral results suggested that a specific, highly effective representation of the trained sequence of movements (rather than a representation of the individual component opposition movements) had developed as a function of training.
Figure 1.
The effects of long-term practice on sequence performance. (A) The two sequences of finger-to-thumb opposition movements used in our study (6). In sequence A the order of finger movements was 4,1,3,2,4 (numbering the fingers from index to little), and in sequence B the order was 4,2,3,1,4 as indicated by the arrows (matched, mirror-reversed sequences). Practice consisted of tapping the designated training sequence as fast and accurately as possible for 10–20 min a day, a few minutes at a time separated by half-minute rests. (B) Learning curves, trained sequence. Each curve (symbol) depicts the performance of a single subject as a function of time. Pre-training (time point 0), day 3 and 10 of training, and performance on the day of the subsequent weekly imaging sessions is shown for 10 subjects. The number of complete sequences performed in a 30-sec test interval (rate) increased from 17.4 ± 3.9 to 38.4 ± 5.8 (mean, SD; week 0 and 5 weeks of training, respectively; paired t test, P < 0.001). Accuracy improved, too, with the number of sequences that contained errors decreasing from a mean of 2.4 ± 0.9 to 0.5 ± 0.5 (paired t test, P < 0.001). (C) No significant improvement for the control sequence (performance rate 18.1 ± 3.7 to 19.4 ± 4.2; 0 and 5 weeks of training, respectively; paired t test, not significant). (D) There was little or no transfer of the learning effect to the contralateral (dominant) hand. Trained (T) vs. control (C) sequence performance rates, at week 5, were 22.3 ± 2.9 and 19.8 ± 4.0, respectively (paired t test, P = 0.097).
We conjectured that a strongly lateralized representation of finger movements—one in which a discrete population of neurons would encode the movements of one hand exclusively—would be a likely locus for this learning-related plasticity. To test this possibility, a long-term functional brain imaging study of M1 was undertaken (6). We focused on M1 because it contains a well-lateralized representation of finger movements; the cerebellum, which also contains lateralized representations of the hand, has been found to be less active with practice on a given sequence, even within the time frame of a single session (47, 48, 50, 52). Moreover, M1 has been indicated by studies in adult monkeys as a locus of manual skill learning (7, 53), and it is thought to be important in the initiation of voluntary motor actions, especially those associated with fine manipulative abilities (54). Finally, we considered a possible analogy to the results of several basic perceptual tasks in which primary cortical representations were shown to reorganize as a function of training and learning (2).
In the imaging study, six young adults were scanned once a week for 4–6 consecutive weeks—before, and then in parallel to training with one of the above finger opposition sequences, the other serving as the untrained control (6). The motor activity-evoked signal changes were measured by using a 4-T MRI system with a gradient echo, echo planar imaging sequence sensitive to local blood-oxygenation-level-dependent contrast. Each session consisted of 6–10 experimental sets with each set made of two performance intervals of 20-sec duration each (X1 and X2, respectively) separated by 40 sec of rest. In a set, either one sequence of movements was repeated in both performance intervals (X1 and X2) or a different sequence was performed in each activation interval assigned in a random but balanced manner. During all scanning sessions, both the trained and the control sequence were performed at a fixed, comfortable rate of 2 Hz, paced by the magnetic field gradient switch noise. Thus both rate and component movements were matched, and the only difference between sequences during scanning was the difference in practice histories. Data analysis consisted of determining those pixels in which signal intensity changed during each performance interval of a set, relative to the level at rest, and then comparing the two statistical maps generated from each set.
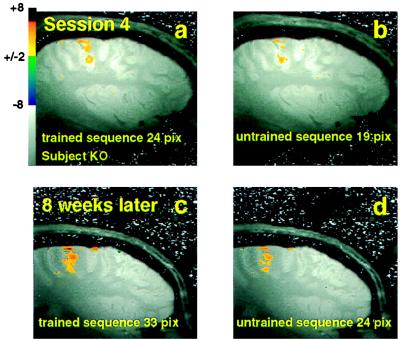
In the first scanning session, performed before any training was given, a comparable extent of the contralateral M1 was activated by the execution of both sequences. However, by session 4, which corresponded to 3 weeks of daily practice on the designated training sequence, and in all subsequent sessions, the extent of activation evoked by the trained sequence in M1 was significantly larger compared with the extent of activation evoked by the performance of the control, untrained sequence (Fig. 2 a and b). The area of evoked response in M1 for the trained sequence was larger in extent irrespective of the order in which the sequences were performed in the set. As in the initial, naive state, the activation in M1 appeared somewhat patchy (but to a lesser degree) and it did not extend beyond the hand representation itself, as indicated by control experiments, in which single-digit and wrist extension-flexion movements, as well as eye (orbicularis oculi) closure were mapped. These results suggest that as the skill was acquired no significant expansion of the total hand representation area occurred. Indeed, the differential activation was accounted for by a subpopulation of pixels, in the hand area, that showed a significant response to the trained sequence, but little or no response to the performance of the untrained sequence (6). The more extensive blood-oxygenation-level-dependent signal evoked in M1 by the trained compared with the untrained sequence, persisted weeks after training was discontinued (Fig. 2 c and d). There was also no significant decrease in performance and, in fact, 1 year after training was stopped there was still significant retention of the skill.
Figure 2.
Differential evoked responses in M1 to the trained vs. the untrained (control) sequence. Training and performance during scanning done with the left (nondominant) hand. (a and b) Emergence of differential activation after 3 weeks of daily practice on the designated training sequence. (c and d) Maintained differential activation 8 weeks later with no additional training in the interval. Sagital sections through the right hemisphere centered ≈35 mm from midline are shown: right, anterior; top, dorsal aspect of the brain. The activity-dependent blood-oxygenation-level-dependent signals evoked by the trained sequence are shown in a and c. Those evoked by the untrained sequence are shown in b and d. Z-score values are indicated by the pseudo-color scale. A surface coil was used, which had the advantage of providing enhanced signal-to-noise ratios, but at the cost of limiting the data to M1 and surrounding areas contralateral to the performing hand. Imaging parameters are given in ref. 6. The comparison is always to the control sequence, performed within the same set. No direct comparison is possible because of different shims and a somewhat different placing of the subject in the magnet and of the surface coil on the subject’s head. The area of evoked signal in M1 was consistently larger in extent for the trained as compared with the untrained sequence by 3 weeks of training and remained so 8 weeks later.
These imaging data suggest that long-term practice results in a gradually evolving, specific, and more extensive representation of the trained sequence of movements in M1. The results are compatible with the idea that motor practice induces the recruitment of additional M1 units into a local network specifically representing the trained motor sequence (6). This interpretation is in agreement with the recent finding, in monkeys, of practice-dependent changes in the functional topography of M1 (7). Nudo et al. (7) found that after a few weeks of training on a task, which developed skilled manipulation, the evoked-movement digit representation, as well as the representation of task-related movement combinations in M1 gradually were expanded. A second important insight gained from our human imaging data is the indication that M1 may code not just single movements, but rather complex movement sequences, including those acquired in adulthood. This indication, too, is supported by the finding in monkeys that following long-term practice, co-contracting muscles used in the task come to be represented together in motor cortex, with those movement combinations that were used more frequently in training, more extensively corepresented (7).
An almost universal finding in animal studies of training-dependent cortical changes is the expansion, through recruitment of additional units, of the specific representation of the input or output that the monkey experiences (3, 30, 53) and, to a much larger degree, of the representation of inputs that are crucial to the performance of a behaviorally meaningful trained task (2, 7, 27–29). The finding of an enlargement of a sensory-motor representation of a body part in the setting of skill acquisition poses the question of how extensive such an enlargement can be. It suggests that the learning of a sequence of movements can in some instances interfere with or limit the learning of other sequences, or even result in an expansion of the representation of a body part, even at the cost of the representation of other, less used parts. Interference with a newly acquired skill may be possible, but only within a very limited time window (20, 39). The specificity of skill learning implies that different subpopulations of neurons within a representational domain participate in the representation of different task conditions, which in turn suggests a potential for many parallel skills within a given representation (38). Nevertheless, Pascual-Leone et al. (55) have found that in Braille readers, the sensory-motor cortical representation of the index finger used in reading was significantly larger, compared both to that of the nondominant index finger in those subjects and to that of the dominant index finger of non-Braille reading control subjects. Similarly, a recent study using magnetic source imaging revealed that the cortical representation of the left hand digits of string players, in primary somatosensory cortex was larger than that in nonmusician controls (56). Our results, on the other hand, as well as Nudo et al.’s monkey data (7) suggest that, rather than an enlargement of a specific effector organ’s representation, training can result in a more extensive representation of a trained sequence of movements, i.e., a specific representation of skilled function rather than body parts. This finding is of importance because the human imaging data support the notion of M1 as a locus of the long-term acquired representation of specific motor skills.
A variety of motor tasks can be conceptualized as consisting of a serial sequence of simple movement components; the skilled generation of a sequence of movements then would be reduced to the problem of choosing the correct components in the proper order, determining the time at which each component movement is initiated and ensuring smooth continuity from one component to the next. Such a scheme, however, may not hold true in all cases of sequence performance (4, 41, 42). For example, in piano playing, a particular key press is subject to modification by succeeding elements of the given, well-rehearsed musical phrase (coarticulation) (57). Such anticipatory kinematic changes may explain why identical component movements are differentially represented in M1 when arranged in a trained sequence vs. an untrained sequence. Furthermore, there is evidence from monkeys showing that fingers do not move independently of each other and that each instructed movement is generated by combined activation of several muscles, many acting on more than one digit (54). Additionally, there is a large body of evidence demonstrating the complex overlapping representations of movements in maps of M1 (54). This evidence, together with the data of Nudo et al. (7) suggesting the training-dependent evolution of corepresentations of temporally correlated joint movements by single M1 units, provides a possible neural basis by which different sequences of individual digit movements can be represented by different patterns of activity in M1. Thus, the implementation of a sequence in M1 may be related to the representation of transitional movements (switching from one digit to the other) and temporally correlated movements (7), which would be dependent on the particular temporal ordering of the component movements in the sequence (41, 58). This order-specific aspect of the representation may be enhanced, extended, and consolidated by practice.
Fast Learning
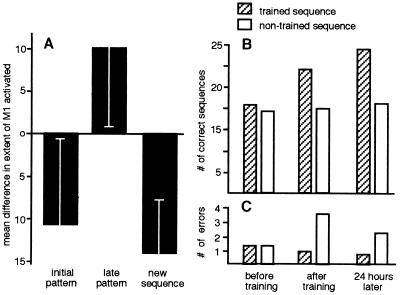
Although the evolution of a sequence-specific, differential pattern of activation in M1 required extended practice over several weeks to be completed, some important changes occurred in the activity of M1 as early as the first imaging session (6). These changes related to the effects of the interval (first, X1, or second, X2) in which a given sequence was performed within a 2-min set, rather than to the sequence itself. We termed these interval-dependent signal modulations the “ordering effects.” The difference in the extent of cortex activated in the two performance intervals of different sets is depicted in Fig. 3A. In the early sets of session 1, before any training was given, a consistent ordering effect was found: the performance of either sequence, irrespective of the sequence type, resulted in a larger area of evoked response when executed first (during interval X1) rather than second (during interval X2) in the set. We interpreted this finding as a habituation-like response across the 40-sec rest interval interposed between X1 and X2 (6). By the latter part of the first session, however, by which time each subject had typically performed six 2-min experimental sets over the course of approximately 30 min, this ordering effect was reversed. A larger extent of M1 was activated by a given sequence when executed second rather than first in the set. The interaction between activation period (X1 vs. X2) and sets (early vs. late), was significant [blocked two-factorial ANOVA, F(1,27) = 4.946, P = 0.035)] (Fig. 3A).
Figure 3.
Cortical and behavioral effects of short-term practice. (A) Cortical effects. The ordering effects during the initial imaging session: The mean difference in the extent of the evoked signal calculated as the difference in the number of pixels in M1 in which the signal changed above a threshold of Z = 2 during the respective activation intervals relative to rest, for each set (X2-X1, see text) during the two activation intervals of the initial and late sets of the session as well as when a new sequence was introduced is shown. Initial pattern: Averaged data from five subjects from the first two sets of each subject showing the initial ordering effect irrespective of sequence type, lesser extent of M1 activated during the second compared with the first interval. Late pattern: Averaged data from the two late sets in the session (sets 6 and 7) of five subjects showing the reversed ordering effect, irrespective of sequence type, larger extent of M1 activated during the second compared with the first interval. New sequence: Averaged data from three subjects (one set each) performing a new sequence, ordering effect reverted to the naive, initial pattern with smaller extent of M1 activated during the second compared with the first interval. (Bars = SD.) (B and C) Behavioral effects. Speed (B) and accuracy (C) of performance recorded during a test interval of 30 sec for two sequences (one randomly assigned to be trained or other the untrained control) before training (before), after a few minutes of externally paced performance of the designated trained sequence (after), and 24 hr later, with no additional training in the interval. Data from 12 subjects. An ANOVA showed that the effects of training, time and the interaction time*training were significant [F(2,55) = 33.06 P < 0.001, F(1,55) = 26.83 P < 0.001, F(2,55) = 3.57 P < 0.03, respectively, for speed; F(2,55) = 29.58 P < 0.001, F(1,55) = 47.73 P < 0.001, F(2,55) = 6.34 P = 0.003, respectively, for errors].
Is the switch in ordering effects a specific effect? That is, is it specific to the sequences that were repeatedly performed during the imaging session? To test this possibility, three subjects were given a new, third sequence, again composed of the same component movements (sequence C: 4, 3, 1, 2, 4; digit numbers as in Fig. 1A). This new sequence was introduced after the switch in ordering effects had occurred, that is when an enhanced response to the second sequence of the set was established for both sequences A and B. In all three subjects, the initial habituation-like pattern of the evoked fMRI responses to repetition returned on performing the new sequence, with a larger extent of activation during the first interval compared with the second interval of the set (Fig. 3A). Thus, the switch in ordering effect reflected the accumulating motor experience gained when subjects repeatedly performed the two sequences during the acquisition of the imaging data, indicating that the switch may represent a learning effect triggered by repetition of a motor sequence at a paced fixed rate.
Consolidation of Motor Experience
If the switch in ordering effect reflects learning, then one would expect a concurrent improvement in performance. We previously have reported that performing the two sequences during the initial imaging session resulted in a significant improvement in both speed and accuracy (6). Moreover, the pattern of enhanced response to repetition (a larger extent of M1 activated in the second compared with the first performance interval) was maintained during the second and third imaging sessions even for the untrained sequence, which was performed only during the weekly scanning sessions. This finding suggests a rather long-lasting effect in M1: the change in processing mode effected during the first scanning session was retained for at least 1 week. Taken together, these findings indicate that the accumulating motor experience gained through the paced tapping of a given sequence during the imaging session was, in itself, sufficient to trigger long-term effects in M1’s representation of the sequence. The purpose of the following experiments was to investigate whether a limited amount of paced motor experience was sufficient to trigger delayed gains in the speed and accuracy of performance of a given sequence of movements. We were specifically interested in exploring the possibility that some performance gains become effective after practice has ended similar to the delayed gains described for perceptual skill learning (6, 25, 32–34).
Twelve young adults (23–42 years old; seven females, five males; all but one right-hand dominant) took part in these experiments. Subjects were instructed to accurately tap, by using their nondominant hand and with no visual feedback, the two five-element sequences of finger-to-thumb opposition movements depicted in Fig. 1A, as in our original study (6). Motor performance was recorded, during both testing and training, with a video camera at a frame length of 40 ms. Performance was tested before, immediately after, and then 24 hr after a single training session. During testing, as in our earlier study (6), subjects were required to tap each sequence as accurately and rapidly as possible over a test interval of 30 sec. Both speed (the number of sequences performed within the test interval) and accuracy (the number of times an out-of-sequence finger opposition movement was executed within the test interval) were scored independently, from the video recordings. In the training session, one of the sequences, randomly chosen, was tapped at a rate of 2 Hz, paced by a metronome, in six short training intervals of 40 sec each, separated by 2–3 min of rest.
Motor performance for the two sequences, before, immediately after, and on the day after training is shown in Fig. 3 B and C. Initial performance of the two sequences, in terms of speed and accuracy, did not differ. Training, however, induced a significant gain in both speed and accuracy for the trained sequence. Moreover, on the next day, with no additional training, a significant gain in both speed and accuracy, compared with the immediate post-training performance level, was found for the trained sequence only (Fig. 3 B and C).
Our results show that not all learning in a sequential finger opposition task is concurrent with practice. A limited amount of paced opposition movements was sufficient practice not only to improve performance during the session but also to initiate significant additional gains that affect performance by the next day; apparently, some gains require time to become effective and continue to develop after motor practice has ended. The concurrent gain in speed and accuracy, is characteristic of the acquisition of a new skill (44).
Delayed neuronal plasticity, which evolves hours after the inducing experience, has been demonstrated in several studies of the developing visual cortex in kittens (36, 37). These studies showed that the changes in neuronal properties induced by brief visual experience became effective, that is, consolidated, only after time, several hours to several days, was allowed to elapse. The notion of consolidation in these studies is consistent with the distinction between the “induction” and the “expression” and maintenance of plasticity suggested by studies of synaptic plasticity at the cellular and biochemical level (10, 11). It is also consistent with the kinetics of memory consolidation, in terms of its resistance to disruption, in animal and cellular models of learning (39). Karni and Sagi (8) have described similar delayed gains in the performance of adults emerging a minimum of 6–8 hr after training in a simple visual detection task. The term consolidation was suggested for the process, presumably initiated during the practice session, which underlies the improvement of performance hours after the training experience was terminated, and results in an enduring memory of the skill. Recently, while training subjects on moving a manipulandum against a force-field, Brashers-Krug et al. (20, 39) found evidence for an ongoing process of consolidation after training for one task condition was terminated. The introduction of a second task condition within a time window of several hours after the initial training disrupted long-term (overnight) improvement on the first task. Moreover, their data show that training not only results in within session (fast) gains, but also, provided enough time was allowed for the consolidation phase, in additional gains that are only apparent by the next day. Similar delayed gains in performance after a latent consolidation phase also have been described for a rotor pursuit task (J. Travis quoted in ref. 40). Altogether, these results indicate that human motor memory continues to evolve after the training session, and with the passage of time is transformed into a long-term trace. Furthermore, the data establish an important parallel between the time course of motor skill learning and perceptual learning and suggest the idea that the time course of skill learning may reflect the time constants of basic neuronal mechanisms of memory storage that are shared by different cortical representations in the adult brain.
Functional Stages in Skill Learning
Although the fractionation of skill learning into only two discrete phases is most likely an oversimplification (11), it provides an important conceptual framework for describing and accounting for the human skill learning data (6, 8, 22, 39). Our imaging data suggest that the acquisition of skilled motor performance occurs in two distinct phases in M1. First, a within-first-session switch in the representation of the repeatedly performed sequences of movements from a habituation-like decrease to an increase in the extent of motor cortex activated by a given sequence of repeated movements; and second, after about 3 weeks of training, the emergence of an enlarged, differential representation of the trained as compared with the untrained sequence of movements. Both stages of sequence learning are experience specific. The switch in ordering effects, or fast learning, occurs only for those sequences that have been repeated a critical number of times in the session, and it is correlated with a specific, significant gain in performance occurring within the session. The emerging, more extensive representation of the trained sequence of movements in M1 was a correlate of highly specific gains in performance that were incrementally acquired over a few weeks of daily practice (slow learning).
The switch in M1 processing mode may constitute an important step in initiating subsequent experience-dependent changes in M1. The imaging data show that the switched ordering effect that occurred in M1 late in the first imaging session was maintained, for the designated control sequence, for at least 1 week during which the sequence was not performed. This is not to say that the switch in M1 processing mode, and much of the behavioral effects that constitute fast learning, are products of major changes principally occurring in M1 within the time frame of a single session. The switch in ordering effects may reflect neural changes occurring in other parts of the distributed motor system (45, 47–52, 59–60). Psychophysical data from perceptual (8) and motor (39) learning tasks suggest that fast learning is mediated, at least in part, by brain regions distinct from those that subserve slow learning. It has been argued, based on electrophysiological data from monkeys, that brain regions active during the acquisition of a motor skill do not necessarily correspond to the regions that eventually will store the memory (4, 61). In humans, there is evidence from functional brain imaging studies that distinct brain areas are differentially activated during initial, naive performance compared with subsequent performance as learning proceeds both within a session [see for example Buckner (66) and Petersen (67) in this issue of the Proceedings] and across consecutive sessions (60, 62).
One should note, that in our study (6), the extent of activation in M1 for either sequence did not increase significantly during the initial scanning session. The learning related changes in M1 that occurred during the first session were related to the ordering effects within a time window of 40 sec. A number of positron emission tomography (PET) studies have examined changes in brain activations occurring within a single session as a consequence of practice in motor and sensory-motor tasks (45, 47–52). Although some studies have suggested that, as learning proceeded within the session, blood flow in M1 increased (47, 49), no significant changes in blood flow have been found in M1 when the rate of movements in the trained and untrained conditions were kept the same (50, 52). A recent PET study in which movement rate was controlled (45), as well as a transcranial magnetic stimulation study (46), found increased activity in M1 as learning progressed but only when subjects had no previous implicit knowledge of the sequence of finger movements. When explicit knowledge of the sequence was allowed to develop no significant learning-related M1 changes were found. However, in contrast to the M1 findings, several PET studies have found a consistent decrease in the activation of the cerebellum and prefrontal cortex (with conflicting observations concerning premotor cortex) as a function of practice within a session (45, 47–52, 60).
As the decrease in activation in areas projecting to M1 occurred over a similar time window as the switch in ordering effects that we observed in M1 within the first session, we proposed that this switch reflects changes in modulatory inputs to M1. This initial phase in the acquisition of the skill may be conceptualized as the setting up of a sequence-specific routine (6). Our working hypothesis is that, initially, the evoked response in M1 relates to the component movements of the sequences, which being identical, exert a smaller, i.e., habituated, response on repetition across a time window of 40 sec. By the end of the session, however, after the two sequences each have been repeated a few tens of times, the switch in ordering effect reflects the fact that a given sequence of movements constitutes a special entity of behavioral significance: it is consistently performed as a sequence rather than as unordered component movements. An experience-dependent change from representation of component movements in an explicit sequence to a representation, rather “automatic” (45, 48, 60), in M1 of the sequence as a unitary motor plan can be related to the decrease of activation in the cerebellum and prefrontal cortex through a decreasing need for movement by movement internal monitoring.
Although important changes occur on a short time scale, our results clearly demonstrate that skilled performance of the trained sequence is not the product of a single training session. Both the imaging and the behavioral data show that the initial changes in ordering effects and the gains in performance acquired during the first session were retained after the session and then consolidated; however, it took about 3 weeks of practice on a daily basis for performance to approach asymptote. The correlate of this acquired proficiency was an enlarged representation of the trained, relative to the untrained, sequence in M1. The emergence of this differential in the evoked fMRI signal corresponded in time to the attainment of maximal near asymptotic performance on the trained sequence. This, however, may be a result of a limitation in the sensitivity of our measurement, and it remains to be seen whether a differential representation of the trained sequence begins to evolve even earlier than the attainment of asymptotic performance. Nevertheless, our results have provided what we believe is direct evidence that long-term motor training can result in significant experience-dependent reorganization in the adult human motor cortex. These data provide an important link with a growing body of data in the nonhuman mammalian brain of representational changes associated with the acquisition of skills.
Two main mechanisms have been proposed for the changes induced in motor and sensory representational maps as a function of experience: (i) the transcription dependent improvement and growth of new connections and synapses (e.g., 34, 63); and (ii) the unmasking, or disinhibition, of previously existing lateral connections between neurons within a representational domain through internal or external modulating inputs (3, 30, 64). The latter mechanism can induce changes on a short time scale and may subserve fast learning; the former has been invoked to explain the delayed, time-dependent nature of developmental cortical plasticity and cortical reorganization compensating for injury and subserving learning. These mechanisms are not mutually exclusive, however, and one may conjecture that the pre-existing lateral connections between local populations of neurons, whose outputs result in different sets of movements, provide a basic network that short-term experience may unmask and subsequent practice may selectively improve (63, 65). Thus, our results support the idea that adult skill motor learning is contingent on the functional architecture of the motor system but, at the same time, modifies it.
Conclusions
The human imaging data together with the behavioral measurements of the effects of training over time lead to three important insights into the neurobiological substrates of skill learning in the adult brain. First, practice can set in motion neural processes that continue to evolve many hours after practice has ended. Thus, even a limited training experience can induce behaviorally significant changes in brain activity, and initiate important long-term effects that may provide the basis for the consolidation of the experience. Second, although many brain areas may be important in the initial stages of acquiring a new skill, an important substrate of skill proficiency can be an enlarged, better representation within the earliest level of processing in which a differential representation of those experience parameters that are critical for the performance of the task is available. This may be a basis for the specificity of procedural knowledge for basic parameters of the training experience. It is very likely the case that different parts of the distributed motor system, including subcortical structures, take part and subsequently represent acquired skills. Nevertheless, the data are consistent with the proposal that local changes in discrete representations subserve the long-term memory of skills. Third, motor skill learning requires time and has two distinct phases, analogous to those subserving perceptual skill learning. An initial, fast improvement phase (“fast learning”) is followed by a slowly evolving, post-training incremental performance gains (“slow learning”). The hypothesis is that fast learning involves processes that select and establish an optimal routine or plan for the performance of the given task. Slow learning, on the other hand, may reflect the ongoing long-term, perhaps structural, modifications of basic motor modules; it may be implemented through time-dependent strengthening of links between motor neurons as a function of correlated activity, and their recruitment into a specific representation of the trained sequence of movements.
ABBREVIATIONS
- fMRI
functional magnetic resonance imaging
- M1
primary motor cortex
References
- 1.Kaas J H. Annu Rev Neurosci. 1991;14:137–167. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- 2.Merzenich M M, Sameshima K. Curr Opin Neurobiol. 1993;3:187–196. doi: 10.1016/0959-4388(93)90209-h. [DOI] [PubMed] [Google Scholar]
- 3.Donoghue J P, Hess G, Sanes J N. In: Acquisition of Motor Behavior in Vertebrates. Bloedel J, Ebner T, Wise S P, editors. Cambridge, MA: MIT Press; 1996. pp. 363–386. [Google Scholar]
- 4.Aizawa H, Inase M, Mushiake H, Shima K, Tanji J. Exp Brain Res. 1993;84:668–671. doi: 10.1007/BF00230980. [DOI] [PubMed] [Google Scholar]
- 5.Le Bihan D, Karni A. Curr Opin Neurobiol. 1995;5:231–237. doi: 10.1016/0959-4388(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 6.Karni A, Meyer G, Jezzard P, Adams M, Turner R, Ungerleider L G. Nature (London) 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 7.Nudo R, Millike G W, Jenkins W M, Merzenich M M. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karni A, Sagi D. Nature (London) 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- 9.Karni A, Tanne D, Rubenstein B S, Askenasy J J M, Sagi D. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 10.Kandel E R, Schwartz J H. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- 11.Dudai Y. Neuron. 1996;17:367–370. doi: 10.1016/s0896-6273(00)80168-3. [DOI] [PubMed] [Google Scholar]
- 12.Mishkin M, Malamut B, Bachevalier J. In: The Neurobiology of Learning and Memory. Lynch G, McGaugh J L, Weinberger N M, editors. New York: Guilford; 1988. pp. 64–88. [Google Scholar]
- 13.Squire L R. Science. 1986;232:1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- 14.Schacter D L, Tulving E T. Memory Systems. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- 15.Weizkrantz L. Philos Trans R Soc London Biol. 1990;329:99–108. doi: 10.1098/rstb.1990.0154. [DOI] [PubMed] [Google Scholar]
- 16.Fiorentini A, Berardi N. Vision Res. 1982;21:1149–1158. doi: 10.1016/0042-6989(81)90017-1. [DOI] [PubMed] [Google Scholar]
- 17.Ball K, Sekuler R. Vision Res. 1987;27:953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- 18.Karni A, Sagi D. Proc Natl Acad Sci USA. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandolfo F, Mussa-Ivaldi F A, Bizzi E. Proc Natl Acad Sci USA. 1996;93:3843–3846. doi: 10.1073/pnas.93.9.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brashers-Krug T, Shadmehr R, Bizzi E. Nature (London) 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- 21.Martin T A, Keating J G, Goodkin H P, Bastian A J, Thatch W T. Brain. 1996;119:1199–1211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- 22.Karni A. Cognit Brain Res. 1996;5:39–48. doi: 10.1016/s0926-6410(96)00039-0. [DOI] [PubMed] [Google Scholar]
- 23.Kitazawa S, Kimura T, Uka T. J Neurosci. 1997;17:1481–1492. doi: 10.1523/JNEUROSCI.17-04-01481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stelmach G E. In: Acquisition of Motor Behavior in Vertebrates. Bloedel J, Ebner T, Wise S P, editors. Cambridge, MA: MIT Press; 1996. pp. 392–407. [Google Scholar]
- 25.Karni A, Bertini G. Curr Opin Neurobiol. 1997;7:530–535. doi: 10.1016/s0959-4388(97)80033-5. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins W M, Merzenich M M, Ochs M T, Allard T, Guic-Robles E. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Recanzone G H, Merzenich M M, Schreiner C E. J Neurophysiol. 1992;67:1071–1091. doi: 10.1152/jn.1992.67.5.1071. [DOI] [PubMed] [Google Scholar]
- 28.Recanzone G H, Schreiner C E, Merzenich M M. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Merzenich M M, Sameshima K, Jenkins W M. Nature (London) 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- 30.Sanes J N, Suner S, Donoghue J P. Exp Brain Res. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- 31.Fitts P M. In: Categories of Human Learning. MeHou A W, editor. New York: Academic; 1964. pp. 243–285. [Google Scholar]
- 32.Fahle M. Perception. 1994;23:411–427. doi: 10.1068/p230411. [DOI] [PubMed] [Google Scholar]
- 33.Schoups A A, Vogels R, Orban G A. J Physiol. 1995;483:797–810. doi: 10.1113/jphysiol.1995.sp020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polat U, Sagi D. Proc Natl Acad Sci USA. 1994;91:1206–1209. doi: 10.1073/pnas.91.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sathian K, Zangaladze A. Percept Psychophys. 1997;59:119–128. doi: 10.3758/bf03206854. [DOI] [PubMed] [Google Scholar]
- 36.Pettigrew J D, Garey L J. Brain Res. 1974;66:160–164. [Google Scholar]
- 37.Peck C K, Blakemore C. Exp Brain Res. 1975;22:57–68. doi: 10.1007/BF00235411. [DOI] [PubMed] [Google Scholar]
- 38.Poggio T, Fahle M, Edelman S. Science. 1992;256:1018–1021. doi: 10.1126/science.1589770. [DOI] [PubMed] [Google Scholar]
- 39.Shadmehr R, Brashers-Krug T. J Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eysenk H J. Br J Psychol. 1965;56:163–181. doi: 10.1111/j.2044-8295.1965.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 41.Hikosaka O, Rand M K, Miyachi K, Miyashita K. J Neurophysiol. 1995;74:1652–1661. doi: 10.1152/jn.1995.74.4.1652. [DOI] [PubMed] [Google Scholar]
- 42.Gordon A M, Casabona A, Soechting J F. J Neurophysiol. 1994;72:1596–1610. doi: 10.1152/jn.1994.72.4.1596. [DOI] [PubMed] [Google Scholar]
- 43.Adams J A. Psychol Bull. 1987;101:41–74. [Google Scholar]
- 44.Hallet M, Pascual-Leone A, Topeka H. In: Acquisition of Motor Behavior in Vertebrates. Bloedel J, Ebner T, Wise S P, editors. Cambridge, MA: MIT Press; 1996. pp. 289–301. [Google Scholar]
- 45.Grafton S T, Hazeltine E, Ivry R. J Cognit Neurosci. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- 46.Pascual-Leone A, Grafman J, Hallet M. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- 47.Grafton S T, Woods R P, Mike T. Hum Brain Map. 1994;1:221–234. doi: 10.1002/hbm.460010307. [DOI] [PubMed] [Google Scholar]
- 48.Seitz R J, Roland P E. Eur J Neurosci. 1992;4:154–165. doi: 10.1111/j.1460-9568.1992.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 49.Hazeltine E, Grafton S T, Ivry R. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- 50.Jenkins I H, Brooks D J, Nixon P D, Frackoviak R S J, Passingham R E. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawashima R, Roland P E, O’Sullivan B T. J Neurosci. 1994;14:3462–3474. doi: 10.1523/JNEUROSCI.14-06-03462.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friston K J, Frith C D, Passingham R E, Liddle P F, Frackoviak R S J. Proc R Soc London B. 1992;1:210–220. [Google Scholar]
- 53.Juliano S L, Ma W, Eslin D. Proc Natl Acad Sci USA. 1991;88:780–784. doi: 10.1073/pnas.88.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schieber M H. J Neurosci. 1995;15:284–297. doi: 10.1523/JNEUROSCI.15-01-00284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pascual-Leone A, Torres F. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- 56.Ebert T, Pantev C, Weinbruch C, Rockstroh B, Taub E. Nature (London) 1995;260:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 57.Soechting J H, Gordon A M, Engel K C. In: Acquisition of Motor Behavior in Vertebrates. Bloedel J, Ebner T, Wise S P, editors. Cambridge, MA: MIT Press; 1996. pp. 344–360. [Google Scholar]
- 58.Tanji J, Shima K. Nature (London) 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- 59.Hikosaka O, Sakai K, Miyauchi B, Takino R, Sasaki Y, Putz B. J Neurophysiol. 1996;76:617–621. doi: 10.1152/jn.1996.76.1.617. [DOI] [PubMed] [Google Scholar]
- 60.Doyon J. Int Rev Neurobiol. 1997;41:273–294. doi: 10.1016/s0074-7742(08)60356-6. [DOI] [PubMed] [Google Scholar]
- 61.Pavlides C, Miyashita E, Asanuma H. J Neurophysiol. 1993;70:733–741. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- 62.Doyon J, Karni A, Song A W, Adams M M, Maisog J M, Ungerleider L G. 3rd International Conference on Functional Mapping of the Human Brain. Copenhagen, Denmark: Organization for Human Brain Mapping; 1997. [Google Scholar]
- 63.Darian S C, Gilbert C D. J Neurosci. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobs K M, Donoghue J P. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- 65.Keller A, Arissian K, Asanuma H. J Neurophysiol. 1992;68:295–308. doi: 10.1152/jn.1992.68.1.295. [DOI] [PubMed] [Google Scholar]
- 66.Buckner R L, Koutstaal W. Proc Natl Acad Sci USA. 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petersen S E, Van Mier H, Fiez J A, Raichie M E. Proc Natl Acad Sci USA. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]