Abstract
The incidence of extreme precipitation has increased with the exacerbation of worldwide climate disruption. We hypothesize an association between precipitation and the distribution patterns that would affect the endemic burden of 8 infectious diseases in Taiwan, including water- and vector-borne infectious diseases. A database integrating daily precipitation and temperature, along with the infectious disease case registry for all 352 townships in the main island of Taiwan was analysed for the period from 1994 to 2008. Four precipitation levels, <130 mm, 130–200 mm, 200–350 mm and >350 mm, were categorized to represent quantitative differences, and their associations with each specific disease was investigated using the Generalized Additive Mixed Model and afterwards mapped on to the Geographical Information System. Daily precipitation levels were significantly correlated with all 8 mandatory-notified infectious diseases in Taiwan. For water-borne infections, extreme torrential precipitation (>350 mm/day) was found to result in the highest relative risk for bacillary dysentery and enterovirus infections when compared to ordinary rain (<130 mm/day). Yet, for vector-borne diseases, the relative risk of dengue fever and Japanese encephalitis increased with greater precipitation only up to 350 mm. Differential lag effects following precipitation were statistically associated with increased risk for contracting individual infectious diseases. This study’s findings can help health resource sector management better allocate medical resources and be better prepared to deal with infectious disease outbreaks following future extreme precipitation events.
Introduction
Global climate disruption appears to have increased the intensity of tropical cyclones, including typhoons in Southeast Asia, hurricanes in Central America and the Southeast United States, consequently resulting in much greater probability of extreme precipitation [1]–[5]. Observational and projection studies also suggest that turbulent atmospheric activity has and will intensify the regional precipitation in the past, present and future [4]–[6].
Extreme precipitation events, increasing the amount of regional precipitation and flooding, have heightened the risk and concern over the transmission of various infectious diseases which may affect their distribution and chances of becoming epidemics [6]–[12]. During periods of heavy precipitation, local water quality can be seriously compromised via diverse means, a significant one being the cross-contamination of water sources due to infiltration and inflow between sewage and water pipes [11]. Contaminated water sources for drinking and recreation, due mostly to flooding after extreme precipitation, have been associated with water-borne diseases outbreaks and epidemics [7]–[8], [10]–[16]. Extreme precipitation also can leave pools of stagnant water that create optimal breeding grounds and growth environments for vectors and hosts, including mosquitoes, mites, rodents and insects [9]–[10], [12].
Significant associations between increased precipitation and greater incidences of contracting water-borne infectious diseases were found in various geographic regions [13], [15]–[25]. When data on 548 water-borne disease epidemics and outbreaks reported by the U.S. Environmental Protection Agency (EPA) between 1948 and 1994 were analysed; a statistical relationship was identified between precipitation and water-borne disease, in which 51% of waterborne disease outbreaks were linked to precipitation levels above the 90th percentile, and 68% to levels above the 80th percentile [16]. In Canada, precipitation was implicated with water-borne disease outbreaks [15], [20]. Precipitation of greater than the 93rd percentile increases the risk of water-borne disease outbreaks by a factor of 2.283 [15]. Cholera and leptospirosis epidemics, in Bangladesh and India, were reported to be associated with heavy precipitation [25]. Outbreaks of gastrointestinal infections usually result when people are exposed to increased amounts and diverse types of pathogens, including Giardia lamblia, Cryptosporidium [21], and enterovirus [23]–[24] after heavy precipitation. Furthermore, previous studies indicated the impact of precipitation on mosquito-borne, tick-borne, and rodent-borne diseases such as dengue fever, West Nile fever, Japanese encephalitis, Chikungunya fever, malaria, Lyme borreliosis, and hantavirus infection, especially when there was the concurrent presence of high temperatures [19], [26]–[29].
As global climate change is predicted to increase the trend of extreme precipitation events occurring is more likely, so determining the role of weather in the incidence of infectious disease is a public health priority [13]. A better understanding of the relationship between disease and extreme precipitation can be an important first step toward finding ways to mitigate the risk of diseases and the diseases’ impact on communities [11]. However, only a limited number of studies have explored the relationship between daily precipitation and climate-related infectious diseases, performed in such places as the United Kingdom [22] and North America [11], [13]–[16]. Most of these observations were based on case reports and some localized databases, so only examined the relationship in specific areas [11], [13], [16], [22]. In this study, a database, using the Geographic Information System (GIS) as the analysis platform, integrated nationwide Taiwanese weather and health surveillance data. Poisson regression using a generalized additive mixed model was applied to examine the relationships between daily extreme precipitation and the number of reported cases for 8 climate-related infectious diseases, including water-borne infectious diseases (hepatitis A, enteroviruses, bacillary dysentery, leptospirosis and melioidosis), and vector-borne infectious diseases (scrub typhus, dengue fever, Japanese Encephalitis).
Results
The Pearson product-moment correlation identified that extreme precipitation events were associated with the occurrence of 8 infectious diseases with lags of 0–70 days. Table 1 provides descriptive statistics for the registered cases of 8 infectious diseases, including time periods, disease categories, incubation periods, case numbers, and specifically significant lag days following precipitation.
Table 1. Characteristics of climate-related infectious diseases in Taiwan, 1994–2008.
| Water-borneDiseases | Vector-borne Diseases | |||||||
| Hepatitis A | Enterovirusesa | Bacillary dysentery | Leptospirosis | Melioidosis | Scrub typhus | Dengue fever | Japanese encephalitis | |
| ICD-9 | 701 | 749 | 004 | 100 | 025 | 812 | 061 | 620 |
| Period | 1994–2008 | 1994–2008 | 1994–2008 | 2006–2008 | 2007–2008 | 1994–2008 | 1994–2008 | 1994–2008 |
| Incubation | 15–50 days | 02–10 days | 1 week | 2–30 days | 2 days+ | 06–21 days | 3–14 days | 5–15 days |
| No. of cases | ||||||||
| Total | 3358 | 1970 | 4828 | 116 | 44 | 3110 | 11178 | 314 |
| <130 mm | 3347 (99.67%) | 1940 (98.48%) | 4745 (98.28%) | 110 (94.83%) | 42 (95.45%) | 3074 (98.84%) | 11072 (99.05%) | 306 (97.45%) |
| 130–200 mm | 0009 (00.27%) | 0021 (01.07%) | 0015 (00.31%) | 002 (01.72%) | 01 (02.27%) | 017 (00.55%) | 00062 (00.55%) | 003 (00.96%) |
| 201–350 mm | 0001 (00.03%) | 0007 (00.36%) | 0037 (00.77%) | 004 (03.45%) | 01 (02.27%) | 016 (00.51%) | 00044 (00.39%) | 005 (01.59%) |
| >350 mm | 0001 (00.03%) | 0002 (00.10%) | 0021 (00.43%) | 000 (00.00%) | 00 (00.00%) | 0003 (00.10%) | 00000 (00.00%) | 000 (00.00%) |
| Lag day | lag28 | lag07 | lag07 | lag14 | lag14 | lag21 | lag70 | lag14 |
With severe complications, as adopted by the Taiwan Center for Disease Control.
Adjusted relative risks for the extreme precipitation categories, as they relate to the baseline control precipitation category (<130 mm), are presented in Table 2. Heavy precipitation (130–200 mm) was a significant risk factor for enterovirus infections (RR = 2.45, 1.59–3.78) and dengue fever (RR = 1.96, 1.53–2.52). The results further indicated that torrential rain events (201–350 mm) were a critical determinant for the reporting of most infectious diseases except for Hepatitis A and enterovirus infections. Extreme torrential rain (>350 mm) impacted enterovirus infections (RR = 5.981, 1.474–23.760) and bacillary dysentery (RR = 7.703, 5.008–11.849). The trend tests demonstrated noteworthy associations between precipitation levels and the reporting of enterovirus infection and Japanese encephalitis (Ptrend<0.001), and even stronger linear relationships between precipitation and bacillary dysentery, dengue fever, and leptospirosis (Ptrend<0.0001).
Table 2. Relative risks (RRs) and 95% CIs for 8 infectious diseases in Taiwan, 1994–2008a.
| Regular <130 mm | Heavy 131–200 mm | Torrential 201–350 mm | Extreme torrential >350 mm | Ptrend | |
| Hepatitis A (lag28) | 1.000 | 1.135 (0.589, 02.187) | 0.208 (0.029, 01.481) | 1.533 (0.216, 10.904) | 0.1697 |
| Enteroviruses b (lag 7) | 1.000 | 2.452** (1.592, 03.777) | 1.429 (0.679, 03.006) | 5.981* (1.474, 23.760) | 0.0002 |
| Bacillary dysentery(lag 7) | 1.000 | 0.751 (0.452, 01.248) | 2.851** (2.060, 03.946) | 7.703** (5.008, 11.849) | <0.0001 |
| Leptospirosis (lag14) | 1.000 | 2.958 (0.720, 12.143) | 8.541** (3.083, 23.685) | N.A. | <0.0001 |
| Melioidosis (lag14) | 1.000 | 10.010* (1.303, 78.899) | 10.010* (1.303, 78.899) | N.A. | 0.1062 |
| Scrub typhus (lag21) | 1.000 | 1.233 (0.765, 01.989) | 1.782* (1.089, 02.915) | 1.842 (0.593, 05.722) | 0.0011 |
| Dengue (lag70) | 1.000 | 1.962** (1.527, 02.522) | 2.094** (1.555, 02.819) | N.A. | <0.0001 |
| Jap. encephalitis (lag14) | 1.000 | 1.546 (0.494, 04.834) | 4.258* (1.749, 10.363) | N.A. | 0.0038 |
Note. CI = confidence interval. N.A.: non-available.
p<0.05.
p<0.001.
The multivariable mixed generalized additive model was adjusted for parameters of calendar month and location (352 townships), which was adjusted for loss function of the multiple-lag effects of temperature.
With severe complications adopted by Taiwan CDC.
A relative risk measure for each disease was created individually for each township. This allowed for determination of the specific townships at risk for particular precipitation-disease outbreaks. Figure 1 shows the vulnerable townships that had precipitation related disease outbreaks, categorized based on precipitation and the 8 climate-related infectious diseases. Figure 2 is a specific case situation highlighting the intensity of precipitation and bacillary dysentery with a 7-day lag after Typhoon Nari, 2001.
Figure 1. Risk maps of the 8 climate-related infectious diseases following extreme precipitation events were generated when the analysis was integrated with the GIS system.
The townships that had a significant association between the outbreak of the specified disease with the category of extreme precipitation events are marked on each map. Townships that had significant associations with heavy precipitation are marked in light purple, with torrential precipitation are marked in purple, while those that only had significant associations with extreme torrential precipitation are marked in dark purple.
Figure 2. Distribution of daily mean precipitation (in mm after Typhoon Nari) and the individual cases of bacillary dysentery with a 7-day lag after Typhoon Nari, 2001.
From the 12 socioeconomic, demographic and geographical characteristics examined, the proportion of natives were significantly higher (+15.5%; Pt-test = 0.0042) along with the average elevation of a township (+339 m; Pt-test<0.0001) in those townships that had a significant relationship between extreme precipitation and the occurrence of Bacillary dysentery as compared to townships that did not have this particular significant relationship. In addition, the relative number of elderly living alone was higher (+27.4%; Pt-test = 0.0182) in those townships at risk for Scrub Typhus following extreme precipitation events. Furthermore, townships with higher population densities were the ones associated with extreme precipitation related enterovirus infections (+94888.0; Pt-test = 0.0037) and dengue fever (+59253.6; Pt-test = 0.0212).
Discussion
Our study is the first to demonstrate the quantitative risk of the relationship between extreme precipitation events during the last 15 years and 8 climate-related infectious diseases at the national level of a subtropical country. A statistically significant association was found between different forms of extreme precipitation and diseases, including enterovirus infection, Japan encephalitis, scrub typhus, dengue fever, and leptospirosis in Taiwan. The maps further display at the regional level the specific townships (of the 352 total townships in mainland Taiwan) that are impacted by extreme precipitation-mediated outbreaks of each infectious disease.
For intestinal infectious diseases, the positive relationships between precipitation and enterovirus infection and bacillary dysentery were similar to previous reports [7], [23]–[24], [30]–[32]. Enteroviruses were more likely to be flushed from unsaturated zones and contaminate groundwater after a short spell of heavy rain [23]. Studies in China found that precipitation may affect the transmission and incidence of food-borne diseases like bacillary dysentery [30], [32]. The results indicated that precipitation may influence pathogens, contamination of drinking water, and then influencing the diseases epidemic [30].
For vector-borne diseases, higher precipitation has been associated with dengue fever, scrub typhus, and Japanese encephalitis [26], [29], [33]. An important study indicated that mosquito population dynamics, and so subsequent disease transmission, would be susceptible to precipitation frequency [34]. When the frequency of precipitation event occurrences match the natural frequency of the mosquito reproductive cycle, then quick phase locking and resonance can result allowing mosquito populations to grow most efficiently and exponentially [34]. However, our study suggested that precipitation >350 mm/day probably destroyed the vector habitats, and there may be an upper threshold value for the positive effect of precipitation on disease vectors. It can be speculated that the emphasis on controlling vector-borne infectious diseases can probably be shifted away from it and to focusing on the prevention of outbreaks of water-borne infections after extreme torrential precipitation events.
Although precipitation events usually last on the order of days, in preceding studies, the analytic lags of precipitation effects were mostly performed on the weekly or monthly scale [13], [28], [35]. Even though studies have demonstrated a daily lag, nevertheless, they were often for short-term periods or limited locations [11], [13], [36]. In our study which the data covers a 15 year period, the specific lag effects between showed significant correlations between daily precipitation and registered case dates. The eruption of 7 infectious diseases appeared within 7–28 days, while dengue fever seemed to appear 70 days after an extreme precipitation event (The 70 day lag effect we observed for dengue is not unexpected, since the imago of Aedes Aegypti (the main vector of dengue fever in Taiwan) is about 9 days, its lifespan is about 30 days, and the incubation period is 3 to 14 days).
In Australia, the United States, Germany, India, Malaysia, and Thailand, it has been reported that high-intensity precipitation is associated with reports of leptospirosis or melioidosis [36]–[44]. Our results could not support a distinct quantity of risk for leptospirosis and melioidosis because mandated reporting did not begin until 2005. But, several Taiwanese publications reported a melioidosis outbreak approximately 2 weeks after typhoon Haitang (maximum precipitation was over 1000 mm/day) in 2005 [45]–[46]. A field survey found that 97.5% of cases were reported in flooded areas (Figure 3). Super typhoon Morakot brought extreme precipitation in 2009; the accumulated precipitation in southern Taiwan in the typhoon Morakot period was nearly 3000 mm over 3 days [47]–[48]. Leptospirosis and melioidosis outbreaks in flood zones have also been reported after extreme precipitation [49]–[50]. Consequently, we presume that higher precipitation has relationships with occurrences of higher risk for leptospirosis and melioidosis outbreaks.
Figure 3. Case distribution of melioidosis after Typhoon Haitang, 2005.
There were 40 cases in southern Taiwan after Typhoon Haitang. 97.5% cases resided in the flooded areas and 70% of cases were in proximate contact of mud or flooding waters.
The results provide quantitative risk values (Table 2) and identify the geographic distributions (Figure 1) of 8 infectious diseases as preliminary evidence linking precipitation with those diseases occurrence. However, the occurrence or spread of a disease was also affected by other factors such as public health services, population density and demographics, land use changes, and socioeconomic conditions. This study tried to explore the basic relationships between socioeconomic factors in precipitation-related vulnerable townships. The proportion of native Taiwanese, elevation and proportion of elderly living alone were indicators of those regions vulnerable to disease outbreaks due to precipitation related events. It is important to link climatic impact to these other factors so adequate medical plans, health promotion policies and prevention campaigns can be properly implemented to prevent or at least minimize disease outbreaks in the future.
From 1961 to 2005 the incidence of intense precipitation (defined as the top 10% precipitation events) had doubled [51]. A previous study also provided independent evidence in support for significant increases in the number and/or size of strong global tropical cyclones [52]. This means extreme precipitation and water-related catastrophes such as floods, mudflows and landslides may become more frequent in the future. [2], [6], [51]–[52]. Extreme weather conditions are a global phenomenon making extreme precipitation an important issue not only in Taiwan but also in many other regions worldwide.
In general, for regions vulnerable to extreme precipitation related disease outbreaks regional risk assessments should be performed, as has been done for Taiwan in this study, in order to determine what local areas are at greatest risk, and identify those specific associated characteristics that contribute to the outbreak. This analysis provides decision makers with essential information to guide their proper implementation of technology- and knowledge-based strategies to best manage precipitation related infectious disease outbreaks. Technology-based approaches include creating better, more efficient monitoring and prediction systems, improving emergency medical aid, promoting environmental conservation, and improving emergency alert systems [19]. Knowledge-based approaches include determining the most appropriate distribution of medical resources, and educating the public to increase awareness of hygiene and health and how to protect oneself as this pertains to epidemic control under extreme weather conditions [28].
The infrequent occurrence of the most extreme torrential precipitation in Taiwan limited the statistical power of the analysis in this study; it is possible that more significant associations between extreme precipitation and the occurrence of the diseases we studied exist. This study did not specifically identify the particular vulnerable clusters, for each infectious disease, present within the entire general population. The population characteristics addressed by the t-test comparisons suggest what particular characteristics define each specific cluster. In our future studies, using a cohort follow-up design, the particular vulnerable clusters for infectious disease in a region will be identified for one of the specific infectious diseases examined in this study. This information would be helpful to medical agencies that want better targeted interventions [53]. Future studies will also identify the mechanisms, such as drinking water contamination, responsible for extreme precipitation associations with infectious disease outbreaks.
Previous studies have demonstrated that extreme precipitation has an impact on the occurrence of infectious diseases. The model presented here successfully demonstrated relationships between extreme levels of precipitation and the incidences of water-borne and vector-borne infectious diseases. This is the first study to use nationwide records to conduct spatial statistical analysis focusing on high-spatial-resolution. Our study exhibits the first systematic and quantitative risk assessment of the relationship between daily extreme precipitation and 8 infectious diseases at national and regional levels. There were significant correlations between specific infectious diseases and the occurrences of extreme precipitation events in Taiwan. Results also highlight the local characteristics that may exacerbate infectious disease outbreaks following extreme precipitation conditions. This study’s findings provide critical information that aid in the creation of effective health policies, and the establishment of relevant technology- and knowledge-based strategies so Taiwan is better prepared for the climatic emergencies of the future.
Methods
Island-wide Meteorological Data
Hourly data from 33 fixed–site monitoring stations, and approximately 300 auto-monitoring stations from the CWB (Taiwan’s Central Weather Bureau), that had complete precipitation and temperature records from 1994 to 2008, were obtained. Since the weather monitoring stations in Taiwan are mostly clustered in urban areas along the east coastline, we could not obtain direct measures of precipitation distribution throughout the entire island, especially from rural and mountain areas. In order to generate representative daily precipitation and temperature measures for each of the 352 total townships in Taiwan, we used the Ordinary Kriging geo-statistical method as part of the ArcGIS 9.3 system. This method is useful to generate optimal spatial-linear temperature and precipitation data, by interpolating the temperature and precipitation at an unobserved location using the actual values from nearby locations. We extrapolated precipitation and temperature data in a stepwise procedure at a resolution of 1×1 square kilometres since this was the smallest geographical unit for disease notification [54]–[56]. This approach allowed us to obtain the best-possible representative precipitation and temperature for each township.
Definition of Extreme Precipitation
As defined by the Taiwan CWB, precipitation events were categorized as follows: regular: daily accumulated precipitation below 130 mm; heavy rain: at 130–200 mm; torrential rain: at 201–350 mm; and extreme torrential rain: above 351 mm [57]. Based on these definitions, the probability of extreme precipitation occurring was much less than 1% since the averaged 99th percentile of precipitation for all 352 townships in Taiwan over the 15 year period was 92.80 mm/day (Lower quartile: 0.04 mm/day, upper quartile: 4.63 mm/day). The heavy rain events in Taiwan were 17.4 days, torrential rain events were 11.7 days and extreme torrential rain events were 1.8 days of each township happened in the 15 years.
Case Definition of Infectious Disease
The computerized database, with recorded daily registry of the occurrence of the 8 mandatory-notified infectious diseases, along with age, gender, township of residence, and the time of disease onset for each case, was retrieved from the Taiwan CDC (Center Disease Control) for the period from 1994–2008, with a signed agreement for confidentiality; all diagnoses were confirmed by the reference laboratory at the Research and Diagnostic Center of the Taiwan CDC. Only the “indigenous” cases for each disease, after verification of travel history, disease onset, and virus subtype classification by the National Virus Diagnosis Laboratory in Taiwan, were analysed [58].
Demographic and Socioeconomic Factors
Twelve selected socioeconomic and demographic characteristics for each township, including medical resources (the number of clinics and doctors) and demographic characteristics (percentage of native Taiwanese, elderly, elderly living alone, and disabled persons), type of employment (either service; industry; agricultural; other), and socioeconomic conditions (household ownership, uneducated population, unemployment rate, and percentage of labourers working outside their county of residence) were estimated using the health statistics obtained from the Department of Health, as determined from the national census taken in 2000 (performed once per decade) [54].
Statistical Analysis
The database included the aggregated daily case registry of 8 infectious diseases and weather monitoring data of precipitation and temperature from 1994 to 2008 for each township. Pearson product-moment correlation analyses assessed the specific lag effects (at weekly intervals) between the number of case occurrences and the direct measures of precipitation amount and temperature levels. Only weekly lag intervals were considered, since these are easier to interpret and act upon from a disease management standpoint. Determination of the length of lag time designated for analysis took into account the incubation period of the pathogen by only considering lag times in excess of the minimum incubation period such as the time needed for vector-associated infection to occur and biologically mature. The number of daily registered cases was a dependent variable (count data), and the daily accumulated precipitation level was the major independent variable. Poisson regression, using a generalized additive model (GAM), has been the suggested method of analysis in time-series studies [59]. GAM allows a Poisson regression to be fit as a sum of nonparametric smooth functions of predictor variables. The purpose of GAM is to maximize the predictive quality of a dependent variable, “Y,” from various distributions by estimating archetypical functions of the predictor variables that are connected to the dependent variable.
Poisson regression, using a Generalized Additive Mixed Model (GAMM), was used to evaluate the multiple-lag effects of stratified precipitation levels on specific diseases [59]. Furthermore, all models were adjusted for the multiple-lag effects of daily temperature, month, and township for evaluating the associations between categorized extreme precipitation and diseases, with further trend tests performed to examine linear associations between levels of precipitation and outbreaks of each disease.
The functions are as follows:
Overall statistical function
Function 1: analysis averaged over all of Taiwan
Function 2: analysis performed on each township
where  is the vector of daily case counts for the i = 1, 2,…, 8 infectious diseases and j = 1, 2,…, 352 townships of Taiwan.
is the vector of daily case counts for the i = 1, 2,…, 8 infectious diseases and j = 1, 2,…, 352 townships of Taiwan.  represented the smoothing functions, which have to satisfy standardized conditions such as
represented the smoothing functions, which have to satisfy standardized conditions such as 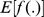 = 0.
= 0.  were parameters that included categorized precipitation amounts, month, and townships, which showed the multiple-level effects of precipitation, monthly patterns and geographical location [59]–[60]. Function 2 was performed individually on our data for each township. We mapped those townships that had significant associations between extreme precipitation events and disease outbreaks to the GIS system and formulated a risk map. In addition, t-tests were used to contrast the differences in basic socioeconomic characteristics in the precipitation-related vulnerable townships to the townships that did not show a significant relationship between precipitation and disease outbreaks.
were parameters that included categorized precipitation amounts, month, and townships, which showed the multiple-level effects of precipitation, monthly patterns and geographical location [59]–[60]. Function 2 was performed individually on our data for each township. We mapped those townships that had significant associations between extreme precipitation events and disease outbreaks to the GIS system and formulated a risk map. In addition, t-tests were used to contrast the differences in basic socioeconomic characteristics in the precipitation-related vulnerable townships to the townships that did not show a significant relationship between precipitation and disease outbreaks.
Acknowledgments
The authors deeply appreciate Dr. Paul Saunders’ help with his editing of this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This supplement received financial support from the Taiwan National Science Council [http://web1.nsc.gov.tw/] (NSC95-2625-Z-006-018, NSC96-2625-Z-006-016, NSC97-2625-Z-006-016, NSC99-2621-M-006-008) and Taiwan’s Centers for Diseases Control [http://www.cdc.gov.tw/mp.asp?mp=1] (DOH98-DC-1002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Allan RP, Soden BJ. Atmospheric Warming and the Amplification of Precipitation Extremes. Science. 2008;321(5895):1481–1484. doi: 10.1126/science.1160787. [DOI] [PubMed] [Google Scholar]
- 2.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, et al. Climate extremes: observations, modeling, and impacts. Science. 2000;289(5487):2068–2074. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- 3.Elsner JB, Kossin JP, Jagger TH. The increasing intensity of the strongest tropical cyclones. Nature. 2008;455(7209):92–95. doi: 10.1038/nature07234. [DOI] [PubMed] [Google Scholar]
- 4.Vecchi GA, Soden BJ. Effect of remote sea surface temperature change on tropical cyclone potential intensity. Nature. 2007;450(7172):1066–1070. doi: 10.1038/nature06423. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly JP, Woodruff JD. Intense hurricane activity over the past 5,000 years controlled by El Nino and the West African monsoon. Nature. 2007;447(7143):465–468. doi: 10.1038/nature05834. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Zwiers FW, Hegerl GC, Lambert FH, Gillett NP, et al. Detection of human influence on twentieth-century precipitation trends. Nature. 2007;448(7152):461–465. doi: 10.1038/nature06025. [DOI] [PubMed] [Google Scholar]
- 7.Greenough G, McGeehin M, Bernard SM, Trtanj J, Riad J, et al. The potential impacts of climate variability and change on health impacts of extreme weather events in the United States. Environ Health Perspect. 2001;109:191–198. doi: 10.1289/ehp.109-1240666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Confalonieri U, Menne B, Akhtar R, Ebi KL, Hauengue K, et al. Parry ML, Canziani OF, Palutikof JP, Linden PJvd, Hanson CE, editors. Human health. Climate Change 2007: Impacts, Adaptation and Vulnerability. 2007. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. UK: Cambridge University Press, Cambridge 391–431.
- 9.McMichael AJ, Neira M, Heymann DL. World Health Assembly 2008: climate change and health. Lancet. 2008;371(9628):1895–1896. doi: 10.1016/S0140-6736(08)60811-9. [DOI] [PubMed] [Google Scholar]
- 10.McMichael AJ, Woodruff RE, Hales S. Climate change and human health: present and future risks. Lancet. 2006;367(9513):859–869. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- 11.Drayna P, McLellan SL, Simpson P. Association between rainfall and pediatric emergency department visits for acute gastrointestinal illness. Environ Health Perspect. 2010;118:1439–1443. doi: 10.1289/ehp.0901671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin J, Zhang J. The impacts of extreme events of weather and climate on infectious disease. Wei Sheng Yan Jiu. 2009;38(6):762–764. [PubMed] [Google Scholar]
- 13.Patz JA, Vavrus SJ, Uejio CK, McLellan SL. Climate change and waterborne disease risk in the Great Lakes region of the U.S. American J Prev Med. 2008;35(5):451–458. doi: 10.1016/j.amepre.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Greer A, Ng V, Fisman D. Climate change and infectious diseases in North America: the road ahead. CMAJ. 2008;178(6):715–722. doi: 10.1503/cmaj.081325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas KM, Charron DF, Waltner-Toews D, Schuster C, Maarouf AR, et al. A role of high impact weather events in waterborne disease outbreaks in Canada, 1975–2001. Int J Environ Health Res. 2006;16(3):167–180. doi: 10.1080/09603120600641326. [DOI] [PubMed] [Google Scholar]
- 16.Curriero FC, Patz JA, Rose JB, Lele S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am J Public Health. 2001;91(8):1194–1199. doi: 10.2105/ajph.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dura G, Pándics T, Kádár M, Krisztalovics K, Kiss Z, et al. Environmental health aspects of drinking water-borne outbreak due to karst flooding: case study. J Water Health. 2010;8(3):513–520. doi: 10.2166/wh.2010.099. [DOI] [PubMed] [Google Scholar]
- 18.Hunter PR. Climate change and waterborne and vector-borne disease. J Appl Microbiol. 2003;94 doi: 10.1046/j.1365-2672.94.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 19.Shuman EK. Global climate change and infectious diseases. N Engl J Med. 2010;362(12):1061–1063. doi: 10.1056/NEJMp0912931. [DOI] [PubMed] [Google Scholar]
- 20.Weniger BG, Blaser MJ, Gedrose J, Lippy EC, Juranek DD. An outbreak of waterborne giardiasis associated with heavy water runoff due to warm weather and volcanic ashfall. Am J Public Health. 1983;73(8):868–872. doi: 10.2105/ajph.73.8.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mac Kenzie WR, Hoxie NJ, Proctor ME, Gradus MS, Blair KA, et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331(3):161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 22.Nichols G, Lane C, Asgari N, Verlander NQ, Charlett A. Rainfall and outbreaks of drinking water related disease and in England and Wales. J Water Health. 2009;7(1):1–8. doi: 10.2166/wh.2009.143. [DOI] [PubMed] [Google Scholar]
- 23.Jean JS, Guo HR, Chen SH, Liu CC, Chang WT. The association between rainfall rate and occurrence of an enterovirus epidemic due to a contaminated well. J Appl Microbiology. 2006;101(6):1224–1231. doi: 10.1111/j.1365-2672.2006.03025.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee TC, Guo HR, Su HJ, Yang YC, Chang HL. Diseases Caused by Enterovirus 71 Infection. Pediatr Infect Dis J. 2009;28:904–910. doi: 10.1097/INF.0b013e3181a41d63. [DOI] [PubMed] [Google Scholar]
- 25.Islam MS, Sharker MA, Rheman S, Hossain S, Mahmud ZH, et al. Effects of local climate variability on transmission dynamics of cholera in Matlab, Bangladesh. Trans R Soc Trop Med Hyg 2009. 2009;103(11):1165–1170. doi: 10.1016/j.trstmh.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Bi P, Tong S, Donald K, Parton KA, Ni J. Climate variability and transmission of Japanese encephalitis in eastern China. Vector Borne Zoonotic Dis. 2003;3(3):111–115. doi: 10.1089/153036603768395807. [DOI] [PubMed] [Google Scholar]
- 27.Bi P, Wu X, Zhang F, Parton KA, Tong S. Seasonal rainfall variability, the incidence of hemorrhagic fever with renal syndrome, and prediction of the disease in low-lying areas of China. Am J Epidemiol. 1998;148(3):276–281. doi: 10.1093/oxfordjournals.aje.a009636. [DOI] [PubMed] [Google Scholar]
- 28.Semenza JC, Menne B. Climate change and infectious diseases in Europe. Lancet Infect Dis. 2009;9(6):365–375. doi: 10.1016/S1473-3099(09)70104-5. [DOI] [PubMed] [Google Scholar]
- 29.Akhtar R, McMichael AJ. Rainfall and malaria outbreaks in western Rajasthan. Lancet. 1996;348(9039):1457–1458. doi: 10.1016/s0140-6736(04)70109-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Bi P, Hiller JE, Sun Y, Ryan P. Climate variations and bacillary dysentery in northern and southern cities of China. J Infect. 2007;55(2):194–200. doi: 10.1016/j.jinf.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Echeverria P, Pitarangsi C, Eampokalap B, Vibulbandhitkit S, Boonthai P, et al. A longitudinal study of the prevalence of bacterial enteric pathogens among adults with diarrhea in Bangkok, Thailand. Diagn Microbiol Infect Dis. 1983;1(3):193–204. doi: 10.1016/0732-8893(83)90018-4. [DOI] [PubMed] [Google Scholar]
- 32.Huang D, Guan P, Guo J, Wang P, Zhou BS. Investigating the effects of climate variations on bacillary dysentery incidence in northeast China using ridge regression and hierarchical cluster analysis. BMC Infect Dis. 2008;8:130. doi: 10.1186/1471-2334-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benitez MA. Climate change could affect mosquito-borne diseases in Asia. Lancet. 2009;373(9669):1070. doi: 10.1016/s0140-6736(09)60634-6. [DOI] [PubMed] [Google Scholar]
- 34.Shaman J, Day JF. Reproductive Phase Locking of Mosquito Populations in Response to Rainfall Frequency. PLoS ONE. 2007;2(3):e331. doi: 10.1371/journal.pone.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soverow JE, Wellenius GA, Fisman DN, Mittleman MA. Infectious disease in a warming world: how weather influenced West Nile virus in the United States (2001–2005). Environ Health Perspect. 2009;117(7):1049–1052. doi: 10.1289/ehp.0800487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pappachan MJ, Sheela M, Aravindan KP. Relation of rainfall pattern and epidemic leptospirosis in the Indian state of Kerala. J Epidemiol Community Health. 2004;58(12):1054. doi: 10.1136/jech.2003.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis. 2003;9(12):1538–1542. doi: 10.3201/eid0912.020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sam IC, Puthucheary SD. Melioidosis and rainfall in Kuala Lumpur, Malaysia. J Infect. 2007;54(5):519–520. doi: 10.1016/j.jinf.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Suputtamongkol Y, Hall AJ, Dance DA, Chaowagul W, Rajchanuvong A, et al. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int J Epidemiol. 1994;23(5):1082–1090. doi: 10.1093/ije/23.5.1082. [DOI] [PubMed] [Google Scholar]
- 40.Corkeron ML, Norton R, Nelson PN. Spatial analysis of melioidosis distribution in a suburban area. Epidemiol Infect. 2010;139(9):1346–1352. doi: 10.1017/S0950268809991634. [DOI] [PubMed] [Google Scholar]
- 41.Robertson J, Levy A, Sagripanti JL, Inglis TJ. The survival of Burkholderia pseudomallei in liquid media. Am J Trop Med Hyg. 2010;82(1):88–94. doi: 10.4269/ajtmh.2010.09-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slack AT, Symonds ML, Dohnt MF, Smythe LD. The epidemiology of leptospirosis and the emergence of Leptospira borgpetersenii serovar Arborea in Queensland, Australia, 1998–2004. Epidemiol Infect. 2006;134(6):1217–1225. doi: 10.1017/S0950268806006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gubler DJ, Reiter P, Ebi KL, Yap W, Nasci R, et al. Climate variability and change in the United States: potential impacts on vector- and rodent-borne diseases. Environ Health Perspect. 2001;109:223–233. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brockmann S, Piechotowski I, Bock-Hensley O, Winter C, Oehme R, et al. Outbreak of leptospirosis among triathlon participants in Germany, 2006. BMC Infect Dis. 2010;10:91. doi: 10.1186/1471-2334-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su HP, Chou CY, Tzeng SC, Ferng TL, Chen YL, et al. Possible Typhoon-related melioidosis epidemic, Taiwan, 2005. Emerg Infect Dis. 2007;13(11):1795–1707. doi: 10.3201/eid1311.061461. [DOI] [PubMed] [Google Scholar]
- 46.Chou DW, Chung KM, Chen CH, Cheung BM. Bacteremic melioidosis in southern Taiwan: clinical characteristics and outcome. J Formos Med Assoc. 2007;106(12):1013–1022. doi: 10.1016/S0929-6646(08)60077-7. [DOI] [PubMed] [Google Scholar]
- 47.Ge X, Li T, Zhang S, Peng M. What causes the extremely heavy rainfall in Taiwan during Typhoon Morakot (2009)? Atmos Sci Lett. 2010;11(1):46–50. [Google Scholar]
- 48.Hong CC, Lee MY, Hsu HH, Kuo JL. Role of submonthly disturbance and 40–50 day ISO on the extreme rainfall event associated with Typhoon Morakot (2009) in Southern Taiwan. Geophysical Research Letters. 2010;37:L08805. [Google Scholar]
- 49.Hung MN, Tuan YC, Ke CM, Yen CH, Yang HW, et al. Investigation of Melioidosis Outbreak after Typhoon Morakot in Zuoying and Nanzih Districts, Kaohsiung City. Taiwan Epidemiol Bull. 2009;25(9):625. [Google Scholar]
- 50.Lai SK, Huang SY, Hsu YF, Sun CP, Lin CH, et al. Review of Significant Epidemics Occurred in Taiwan and International Community in 2009. Taiwan Epidemiol Bull 26. 2010;(10):178–201. [Google Scholar]
- 51.Shiu CJ, Liu SC, Chen JP. Diurnally asymmetric trends of temperature, humidity and precipitation in Taiwan. J Climate. 2009;22:5635–5649. [Google Scholar]
- 52.Liu SC, Fu C, Shiu CJ, Chen JP, Wu FT. Temperature dependence of global precipitation extremes. Geophys Res Lett. 2009;36:L17702. [Google Scholar]
- 53.Chen YJ, Wu PC, Yang TC, Su HJ. Examining non-stationary effects of social determinants on cardiovascular mortality after cold surges in Taiwan. Sci Total Environ. 2010;48:2042–4049. doi: 10.1016/j.scitotenv.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 54.Wu PC, Lin CY, SC Lung, Guo HR, Chou CH, et al. Cardiovascular mortality during heat and cold events: determinants of regional vulnerability in Taiwan. Occup Environ Med. 2011;68:525–530. doi: 10.1136/oem.2010.056168. [DOI] [PubMed] [Google Scholar]
- 55.Majani BS. Analysis of external drift kriging algorithm with application to precipitation estimation in complex orography: ITC. 2007.
- 56.Davis JC. Statistics and Data Analysis in Geology. New York: INC. 1986.
- 57.The Taiwan Central Weather Bureau. Precipitation Classification. Available: http://www.cwb.gov.tw/V7e/observe/rainfall/define.htm?. Accessed 2012 April 4.
- 58.Centers for Disease Control (CDC), Taiwan. Surveillance System. Available: http://www.cdc.gov.tw/english/list.aspx?treeid=00ed75d6c887bb27&nowtreeid=f0131176aa46d5db. Accessed 2012 April 4.
- 59.Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 2004;99:673–686. [Google Scholar]
- 60.Lin X, Zhang D. Inference in generalized additive mixed models by using smoothing splines. J R Statist Soc 61(part. 1999;2):381–400. [Google Scholar]





