Abstract
We investigate age and sex differences in acute myocardial infarction (AMI) after cardiac surgery in a prospective study of 2038 consecutive patients undergoing cardiac surgery with cardiopulmonary bypass. An age of ≥70 years implied changes in the type of AMI from the ST-segment elevation myocardial infarction (STEMI) to non-ST-segment elevation myocardial infarction (non-STEMI). Men were more likely than women to suffer from AMI after cardiac surgery (11.8% vs. 5.6%), as a result of the higher frequency of STEMI (6% of men vs. 1.8% of women; P < 0.001) in both age groups. A troponin-I (Tn-I) peak was significantly higher in patients ≥70 years old. In-hospital mortality was higher in patients ≥70 (7.3%) than in those <70 years old (3.3%), because of the increased mortality observed in men with non-AMI (2.1% vs. 6.3%) and women with STEMI (0% vs. 28.6%) and non-STEMI (0% vs. 36.8%, P < 0.05). Old age was associated with a higher frequency of non-STEMI, Tn-I peak, mortality and length of stay in the intensive care unit (ICU). Regardless of age, men more often suffer from AMI (particularly STEMI). AMI in women had a notable impact on excess mortality and ICU stay observed in patients ≥70 years of age. Clinical and Tn-I peak differences are expected in relation to age and gender after AMI post-cardiac surgery.
Keywords: Age differences, Sex differences, Cardiac surgery, Acute myocardial infarction, STEMI/non-STEMI, Outcome
INTRODUCTION
In acute coronary syndromes, although women suffer myocardial infarctions at older ages than men, mortality and morbidity rates are persistently higher in women [1].
A recent histological study of coronary arteries from men and women who died of acute coronary disease found similarities between the sexes, although active, inflammatory atherosclerosis developed earlier in men than in women. This difference gradually faded with age, and disappeared altogether around age 70 [2]. In women, some studies have found better outcomes in coronary artery bypass grafting (CABG) after adjusting for different risk factors [3]. Perioperative myocardial infarction is often silent, with transient ECG changes, and may occur as a result of an acute coronary thrombosis involving an unstable or vulnerable plaque (Type 1) or myocardial oxygen supply/demand imbalance (Type 2), which appears to be the most common cause of this complication after cardiac surgery [4]. Our group previously reported no clinically relevant sex-based differences in troponin-I (Tn-I) levels after cardiac surgery, without perioperative STEMI, in patients undergoing CABG or valve surgery [5]. This prompted us to investigate whether a patient's age and sex may impact the incidence and outcome of acute myocardial infarction (AMI) after cardiac surgery. The increasingly necessary consensus to define perioperative myocardial infarction is still far away, but establishing the incidence and characteristics of AMI after cardiac surgery is mandatory in order to improve the accuracy of its definition, if necessary adjusting it for gender and age.
MATERIALS AND METHODS
This prospective observational study included 2434 consecutive patients undergoing elective cardiac surgery with cardiopulmonary bypass (CPB; specifically, either valve surgery, CABG or both) between February 2004 and April 2009 in a tertiary level university hospital. General characteristics are shown in Table 1. Throughout the period there were no changes in the surgical procedures, anaesthetic or intensive care unit (ICU) management. Written informed consent was collected prior to surgery from all patients. The study was approved by the Ethics Committee.
Table 1:
General characteristics of the patients
| Age group (years) | Sex group | Mean | Statistical differences | |
|---|---|---|---|---|
| Age (years) | <70 | Women | 59.6 ± 8.8 | a |
| Men | 58.1 ± 9.5 | |||
| ≥70 | Women | 74.7 ± 3.3 | ||
| Men | 74.7 ± 3.4 | |||
| Body mass index | <70 | Women | 28.1 ± 5.1 | |
| Men | 27.9 ± 4.1 | |||
| ≥70 | Women | 28.5 ± 4.2 | ||
| Men | 27.3 ± 3.5 | |||
| Parsonnet score | <70 | Women | 12.6 ± 7.3 | |
| Men | 9.4 ± 7.1 | |||
| ≥70 | Women | 13.6 ± 6.4 | ||
| Men | 12.1 ± 7.1 | |||
| APACHE-II | <70 | Women | 11.3 ± 3.8 | |
| Men | 10.6 ± 4.3 | |||
| ≥70 | Women | 13.9 ± 4.2 | ||
| Men | 13.7 ± 4.2 | |||
| APACHE-III | <70 | Women | 45.6 ± 16.1 | |
| Men | 43.0 ± 16.3 | |||
| ≥70 | Women | 56.3 ± 18.1 | ||
| Men | 56.4 ± 16.5 | |||
| Cross-clamp time (min) | <70 | Women | 69.4 ± 28.3 | |
| Men | 69.7 ± 26.1 | |||
| ≥70 | Women | 72.8 ± 27.8 | ||
| Men | 72.3 ± 24.9 | |||
| Cardiopulmonary bypass time (min) | <70 | Women | 103.6 ± 37.6 | |
| Men | 108.9 ± 35.0 | |||
| ≥70 | Women | 111.8 ± 41.4 | ||
| Men | 113.6 ± 34.3 | |||
| Incidence (%) | ||||
| Hypertension | <70 | Men | 51.90 | a |
| Women | 56 | |||
| ≥70 | Men | 76.3 | ||
| Women | 70.8 | |||
| Diabetes | <70 | Men | 10.3 | b |
| Women | 5.6 | |||
| ≥70 | Men | 10.1 | ||
| Women | 8.3 | |||
| Dyslipaemia | <70 | Men | 44.9 | b |
| Women | 50.1 | |||
| ≥70 | Men | 48.4 | b | |
| Women | 56 | |||
| Chronic renal failure | <70 | Men | 3 | |
| Women | 3.8 | |||
| ≥70 | Men | 4.8 | b | |
| Women | 8.3 |
aStatistically significant differences between age groups (P < 0.05).
bStatistically significant differences between sex groups (P < 0.05).
Gender, age, the Parsonnet score, APACHE-II and -III scores, incidence of AMI (STEMI and non-STEMI), Tn-I curve and peak of this curve, in-hospital mortality and length of ICU stay were all recorded. Patients were classified into three groups: non-AMI, STEMI according to classical ECG criteria [6] and non-STEMI, defined as a Tn-I peak >20 ng/ml [7, 8] and a late peak in Tn-I [9].
All blood samples were obtained using a central venous catheter. Serum Tn-I samples were obtained immediately after surgery upon ICU admission, and again 6, 12, 24 and 48 h later. The samples were measured with a Dimension RxL analyser (Dade Behring, Newark, DE, USA).
Statistical methods
ANOVA with post hoc Bonferroni correction was applied in order to evaluate any differences in sex and/or age and/or clinical groups (non-AMI, STEMI and non-STEMI) for the quantitative samples with a normal distribution. For non-parametric data, the χ2 test was used to analyse differences in sex, age and/or clinical groups. Data are reported as means ± SD, with P-values <0.05 considered significant.
RESULTS
Acute myocardial infarction incidence
After applying the exclusion criteria to the initial sample of 2434 consecutive patients, the study population included 2038 patients: 1276 men and 762 women. Of these, 1271 (649 men and 622 women) underwent valve surgery, 614 (496 men and 118 women) CABG and 153 underwent (114 men and 39 women) both types of surgery. Age was <70 years in 1164 patients [798 (68.6%) men and 366 (31.4%) women] and ≥70 years in 874 [478 (54.7%) men and 396 (45.3%)]. Sex differences between the two age groups were statistically significant (P < 0.001).
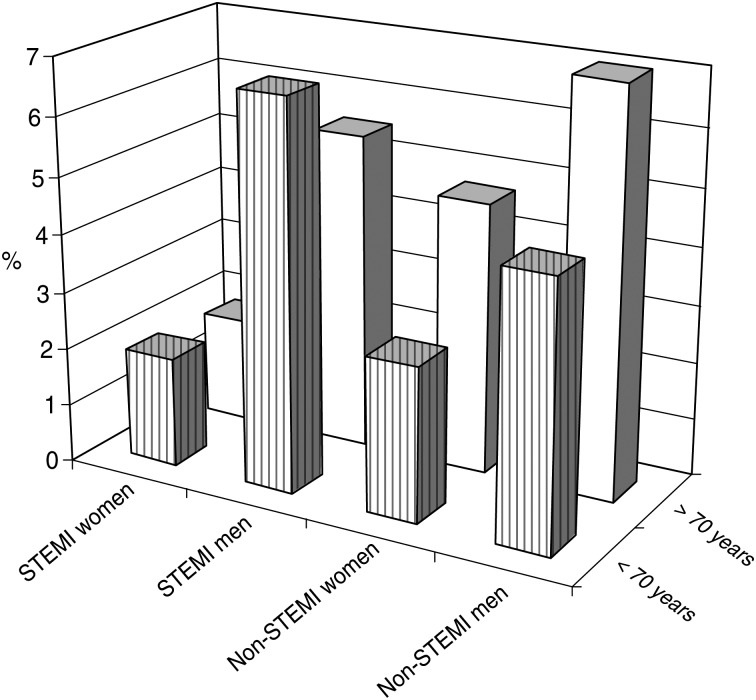
AMI incidence was significantly lower in the valve surgery group (valve group = 5.4%; CABG group = 14.6%, CABG and valve group = 19.8%; P < 0.001), although the distribution of STEMI and non-STEMI was similar for the three types of surgery (valve surgery: STEMI 2.3% and non-STEMI 3.1%; CABG: STEMI 7.1% and non-STEMI 7.6%; both types of surgery: STEMI 10.5% and non-STEMI 9.3%). Age ≥70 years old was not associated with a higher incidence of AMI after cardiac surgery when compared with <70 years, but age did imply a change in the predominant type of AMI. Patients ≥70 years old were more frequently non-STEMI (6.3%) than STEMI (3.5%), in contrast to patients <70 years who demonstrated more STEMI (5.1%) than non-STEMI (4.0%) (P < 0.05; Fig. 1). Overall, men were more likely than women to suffer from AMI after cardiac surgery (11.8% vs. 5.6%), as a result of the higher frequency of STEMI (6% of men vs. 1.8% of women; P < 0.0001). This difference was found in both age groups: <70 (6.7% of men vs. 1.9% of women; P < 0.001) and ≥70 years old (5.5% of men vs. 1.8% of women; P < 0.005; Table 2).
Figure 1:
Patients’ distribution between AMI groups. Percentage of patients in the STEMI group and non-STEMI group with respect to age and sex shows significantly higher values in STEMI (P < 0.001) and non-STEMI groups (P < 0.001) in men. In the group ≥70 years old, both sexes were more likely to suffer from non-STEMI (P < 0.05.)
Table 2:
Patients’ distribution between groups
| Age (years) | Sex | AMI group | n (%) |
|---|---|---|---|
| <70 | Women (n = 366) | Non-AMI | 349 (95.4) |
| STEMI | 7 (1.9) | ||
| Non-STEMI | 10 (2.7) | ||
| Men (n = 798) | Non-AMI | 708 (88.7) | |
| STEMI | 53 (6.7)a | ||
| Non-STEMI | 37 (4.6)a,b | ||
| Total (n = 1164) | Non-AMI | 1057 (90.8) | |
| STEMI | 60 (5.1) | ||
| Non-STEMI | 47 (4.0) | ||
| ≥70 | Women (n = 396) | Non-AMI | 370 (93.5) |
| STEMI | 7 (1.8) | ||
| Non-STEMI | 19 (4.7) | ||
| Men (n = 478) | Non-AMI | 418 (87.5) | |
| STEMI | 27 (5.5) | ||
| Non-STEMI | 33 (7.0)b | ||
| Total (n = 874) | Non-AMI | 788 (90.2) | |
| STEMI | 34 (3.5) | ||
| Non-STEMI | 52 (6.3) |
Number and percentage of patients in the non-AMI group, STEMI group and non-STEMI group with respect to age and sex.
aSignificantly higher values in STEMI (P < 0.001) and non-STEMI groups (P < 0.001) in men.
bIn the group ≥70 years old, both sexes were more likely to suffer from non-STEMI (P < 0.05).
Troponin-I peak
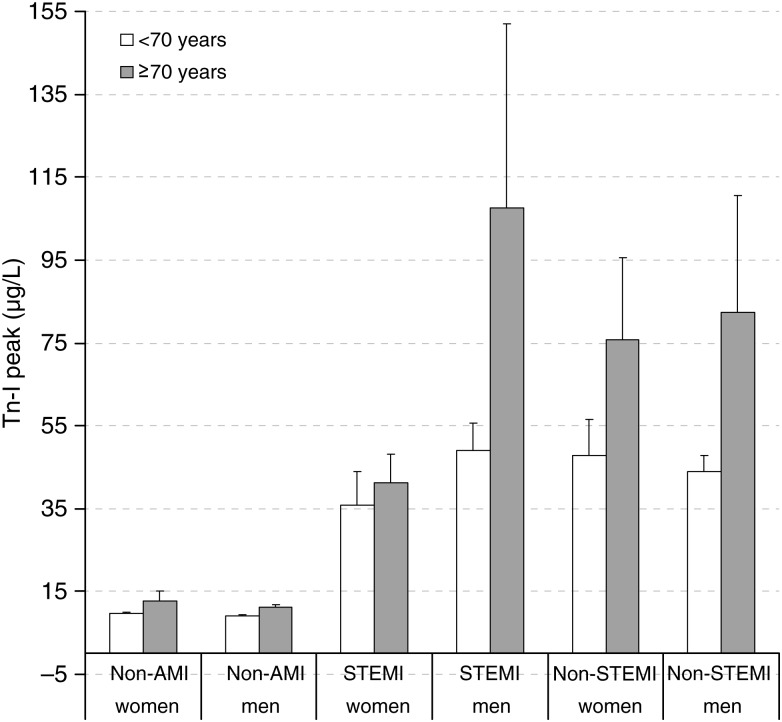
The Tn-I peak was significantly lower in the non-AMI group (10.4 ± 23.7 µg/l) than in the STEMI (62.8 ± 117.5 µg/l) and non-STEMI groups (63.8 ± 107.1 µg/l; P < 0.001). At 48 h post-surgery, Tn-I was 4.4 ± 5.6 µg/l in the non-AMI group, 24.6 ± 28.6 µg/l in the STEMI group and 25.3 ± 34.6 µg/l in the non-STEMI group (P < 0.001). The Tn-I peak also occurred significantly earlier after surgery in the non-AMI group (10.9 ± 9.0 h) than in the STEMI (17.0 ± 9.6 h) and non-STEMI groups (16.5 ± 8.7 h; P < 0.001). Tn-I peak was significantly higher in patients ≥70 than in those aged <70 years old (F = 19.95, P < 0.001). These differences resulted from the increase observed in men with STEMI and non-STEMI (F = 5.67, P < 0.005; Fig. 2).
Figure 2:
Tn-I peak. Non-AMI group, STEMI group and non-STEMI group. Mean values and standard deviation for the Tn-I peak of the three groups. Lower in the non-AMI group when compared with the STEMI and non-STEMI groups (P < 0.001). Higher in patients ≥70 when compared with those aged <70 years (P < 0.05) due to the increase in men with STEMI and non-STEMI.
Mortality, length of stay in intensive care unit and other data
The in-hospital mortality rate was significantly higher in patients ≥70 years (7.3%) than in those <70 years old (3.3%) (P < 0.001) because of the increase in mortality observed for men with non-AMI (2.1% vs. 6.3%, P < 0.001) and women with STEMI (0% vs. 28.6%, P < 0.05) and non-STEMI (0% vs. 36.8%, P < 0.05; Table 3). ICU stay was also significantly longer in patients ≥70 years old than in those aged <70 (F = 10.0, P < 0.001). Prolonged ICU stay was observed for both sexes in patients with non-AMI (F = 4.7, P < 0.05) and in women with STEMI and non-STEMI (F = 3.8, P < 0.05; Table 4).
Table 3:
In-hospital mortality
| In-hospital mortality | Exitus (%) | Exitus (%) | ||
|---|---|---|---|---|
| <70 years | ||||
| Women | 12/366 | 3.3 | Non-AMI | 12/349 (3.5) |
| STEMI | 0/7 (0)a | |||
| Non-STEMI | 0/10 (0)b | |||
| Men | 27/796 | 3.4 | Non-AMI | 15/708 (2.1) |
| STEMI | 8/53 (15.4) | |||
| Non-STEMI | 4/37/11.4) | |||
| Total | 39/1164 | 3.3 | Non-AMI | 27/1057 (3.2) |
| STEMI | 8/60 (13.6) | |||
| Non-STEMI | 4/47 (8.9) | |||
| ≥70 years | ||||
| Women | 30/396 | 7.6 | Non-AMI | 21/370 (5.9) |
| STEMI | 2/7 (28.6)a | |||
| Non-STEMI | 7/19 (36.8)b | |||
| Men | 34/478 | 7.1 | Non-AMI | 26/418 (6.3) |
| STEMI | 4/27 (16.7) | |||
| Non-STEMI | 4/33 (11.4) | |||
| Total | 64/874 | 7.3 | Non-AMI | 47/788 (6.0) |
| STEMI | 7/34 (20.6) | |||
| Non-STEMI | 10/52 (19.2) | |||
In-hospital mortality in the different groups with respect to age and sex.
aStatistically significant increase in women ≥70 years old in the STEMI group (P < 0.05).
bStatistically significant increase in women ≥70 years old in the non-STEMI group (P < 0.05).
Table 4:
ICU stay
| Age (years) | Sex | Group | ICU stay (h) | |
|---|---|---|---|---|
| <70 | Women | Non-AMI | (110.1 ± 126.8) | 106.8 ± 122.2 |
| STEMI | 221.7 ± 277.2 | |||
| Non-STEMI | 147.2 ± 103.9 | |||
| Men | Non-AMI | (107.8 ± 126.2) | 100.6 ± 117.6 | |
| STEMI | 164.0 ± 160.4 | |||
| Non-STEMI | 165.6 ± 187.3 | |||
| Total | Non-AMI | (108.5 ± 126.3) | 102.7 ± 119.1a | |
| STEMI | 170.8 ± 175.9 | |||
| Non-STEMI | 161.6 ± 171.8 | |||
| ≥70 | Women | Non-AMI | (150.9 ± 182.5) | 140.7 ± 165.1 |
| STEMI | 356.7 ± 309.7 | |||
| Non-STEMI | 269.3 ± 322.9 | |||
| Men | Non-AMI | (137.7 ± 157.9) | 132.0 ± 158.6 | |
| STEMI | 169.0 ± 106.4 | |||
| Non-STEMI | 183.1 ± 172.3 | |||
| Total | Non-AMI | (143.6 ± 169.5) | 136.0 ± 161.6a | |
| STEMI | 211.4 ± 185.0 | |||
| Non-STEMI | 213.4 ± 237.0 | |||
Non-AMI group, STEMI group and non-STEMI group. Mean values and SD for the ICU stay of the three groups.
aSignificantly lower values in the non-AMI group with respect to the STEMI (P < 0.001) and non-STEMI (P < 0.001) groups.
As regards the Parsonnet score, there were no significant sex differences in the whole sample or in patients with AMI. However, a significant difference was observed between patients aged <70 years (the Parsonnet score 10.4 ± 7.4) and those ≥70 years old (12.7 ± 6.8; P < 0.001).
DISCUSSION
In this study, we showed that both age and sex may influence the incidence, mortality and morbidity of AMI after cardiac surgery. Thus (i) men are more likely to suffer from AMI after cardiac surgery, especially STEMI; (ii) both men and women are more likely to suffer from non-STEMI when aged ≥70 years; (iii) the Tn-I peak increases in the AMI group when patients are ≥70 years old; (iv) women with STEMI have a lower Tn-I peak than men; (v) in-hospital mortality in patients with AMI increases only in women aged ≥70 years old; and (vi) in the group ≥70 years old, ICU stay increases in the non-AMI group in both men and women, while the STEMI and non-STEMI groups show only a longer stay in women.
We observed sex differences between patients <70 and ≥70 years old receiving cardiac surgery. This is because men suffer coronary syndromes younger than women, and therefore undergo surgery at an earlier age [10, 11]. The differences in the present study are mostly owing to CABG, performed in 496 men and 118 women.
There were no differences in the frequency of AMI after cardiac surgery between patients <70 and ≥70 years old. However, the predominant type of AMI switched from STEMI in patients aged <70 to non-STEMI in those ≥70 years old. The relatively frequent occurrence of perioperative non-STEMI, which is very often subendocardial [9], in both men and women, suggests that subendocardial perfusion deteriorates with age.
Despite the increase in non-STEMI observed in women ≥70 years old, the incidence of AMI was lower in women than men, as is the case in non-surgery-related AMI [12]. In addition, women did not have any substantial age-related differences in the STEMI incidence, in contrast to the findings for non-surgery acute coronary syndrome. This suggests that in the case of AMI after cardiac surgery, the surgical procedures, or the reactions to them, are an important cause of STEMI. The lower prevalence of AMI in women is suggestive of a greater resistance to this condition, as is generally the case with acute coronary syndromes [10, 11].
We did not use the universal definition of myocardial infarction [13], because it did not exist when we begun the protocol (2004) and also because it would be difficult to individualize the ‘arbitrary convention’ of increases in the biomarker of more than five times the 99th percentile of the normal reference range, as this reference will vary depending on the surgery characteristics (CABG, valve or valves, CABG plus valve, with or without CPB, and so on). The similarity in the time of the Tn-I peaks between the STEMI and non-STEMI groups, and their difference with regard to the non-AMI group stresses the accuracy of the late Tn-I peak for detecting perioperative AMI.
The Tn-I concentration and its evolution can indicate AMI in both STEMI and non-STEMI groups. The Tn-I elevation criteria obtained from magnetic resonance imaging [9] seems to be an important step forward in terms of identifying a condition that is often difficult to diagnose. New imaging studies have reported that in the event of a perioperative AMI, the Tn-I peak occurs later and the Tn-I concentration at 48 h remains high. These two data are the most reliable signs of myocardial necrosis [9].
The increase in Tn-I peak with age observed in the present study could be owing to a greater sensitivity to aggressions. The lower Tn-I peak concentrations in women with STEMI may have some mid- and long-term advantages that merit further investigation.
In general, it seems that women are less likely to suffer AMI after cardiac surgery, but that they tolerate this condition worse than men; furthermore, the potentially negative repercussions of age affects only some of the variables measured. At all events, women with non-perioperative STEMI also had higher adjusted mortality rates than men [1]. Compared with men, women present some physiological differences in relation to cardiac ischaemia, such as higher myocardial oxygen consumption and lower myocardial glucose extraction fraction and utilization; they also show greater sympathetic activation that lasts until resolution at 9 months following uncomplicated AMI [14]. These differences may explain some of the sex-based differences in AMI observed in this study.
The Parsonnet scores' similarity between the sexes suggests that the differences found are not due to any pre-surgery characteristics. This Parsonnet scores’ similarity between patients with and without non-STEMI and the lower Parsonnet scores in patients who go on to develop AMI illustrate the lack of sensitivity of this score for detecting a propensity to perioperative AMI. By contrast, the finding of higher APACHE-II and -III scores in patients with AMI shows that these scores are sensitive enough to detect important physiological changes resulting from AMI. The longer ICU stay in women may be a sex-related difference that is not correlated with the variables studied here. As regards the longer stay and mortality among patients with AMI, we do not believe that this merits further comment.
In summary, patients with age ≥70 years old do not have a higher incidence of AMI after cardiac surgery, though advanced age is associated with a higher frequency of non-STEMI, Tn-I peak, mortality and length of ICU stay when compared with patients <70 years. Interestingly, after cardiac surgery, men suffer from AMI (particularly STEMI) more often than women, regardless of age. However, AMI (STEMI and non-STEMI) in women had a notable impact on excess mortality and ICU stay observed in patients ≥70 years. Older men are more likely to suffer from non-STEMI and when they suffer STEMI or non-STEMI, they present a higher increase in the Tn-I peak.
CONCLUSION
Clinical and Tn-I peak differences are expected in relation with age and gender after AMI post-cardiac surgery.
Acknowledgements
We thank J. Valero for his analyses and the nursing team at our ICU.
Conflict of interest: none declared.
References
- 1.Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118:2803–10. doi: 10.1161/CIRCULATIONAHA.108.789800. doi:10.1161/CIRCULATIONAHA.108.789800. [DOI] [PubMed] [Google Scholar]
- 2.Frink RJ. Gender gap, inflammation and acute coronary disease: are women resistant to atheroma growth? Observations at autopsy. J Invasive Cardiol. 2009;21:270–7. [PubMed] [Google Scholar]
- 3.Jacobs AK, Kelsey SF, Brooks MM, Faxon DP, Chaitman BR, Bittner V, et al. Better outcome for women compared with men undergoing coronary revascularization: a report from the bypass angioplasty revascularization investigation (BARI) Circulation. 1998;98:1279–85. doi: 10.1161/01.cir.98.13.1279. [DOI] [PubMed] [Google Scholar]
- 4.Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation. 2009;119:2936–44. doi: 10.1161/CIRCULATIONAHA.108.828228. doi:10.1161/CIRCULATIONAHA.108.828228. [DOI] [PubMed] [Google Scholar]
- 5.Ricart A, Farrero E, Ventura JL, Javierre C, Carrio L, Rodríguez D, et al. Are there sex-based differences in serum troponin I after cardiac surgery? Crit Care Med. 2009;37:2210–15. doi: 10.1097/CCM.0b013e3181a0321b. doi:10.1097/CCM.0b013e3181a0321b. [DOI] [PubMed] [Google Scholar]
- 6.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. doi:10.1016/S0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 7.Benoit MO, Paris M, Silleran J, Fiemeyer A, Moatti N. Cardiac troponin I: its contribution to the diagnosis of perioperative myocardial infarction and various complications of cardiac surgery. Crit Care Med. 2001;29:1880–6. doi: 10.1097/00003246-200110000-00005. doi:10.1097/00003246-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bojar RM. Manual of perioperative care in adult cardiac surgery. 4th ed. Oxford: Blackwell; 2005. [Google Scholar]
- 9.Selvanayagam JB, Pigott D, Balacumaraswami L, Petersen SE, Neubauer S, Taggart DP. Relationship of irreversible myocardial injury to troponin I and creatine kinase-MB elevation after coronary artery bypass surgery: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;45:629–31. doi: 10.1016/j.jacc.2004.11.030. doi:10.1016/j.jacc.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel keys lecture. Circulation. 1997;95:252–64. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 11.Wexler LF. Studies of acute coronary syndromes in women: lessons for everyone. N Engl J Med. 1999;341:275–6. doi: 10.1056/NEJM199907223410409. doi:10.1056/NEJM199907223410409. [DOI] [PubMed] [Google Scholar]
- 12.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of myocardial infarction to participants. N Engl J Med. 1999;341:217–25. doi: 10.1056/NEJM199907223410401. doi:10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. doi:10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 14.Hogarth AJ, Graham LN, Mary DA, Greenwood JP. Gender differences in sympathetic neural activation following uncomplicated acute myocardial infarction. Eur Heart J. 2009;30:1764–70. doi: 10.1093/eurheartj/ehp188. doi:10.1093/eurheartj/ehp188. [DOI] [PubMed] [Google Scholar]