Abstract
An infant developed severe right heart failure early after truncal repair with a pulmonary homograft. A mechanical obstruction by narrowing could not be identified at the homograft or pulmonary arteries. However, functional obstruction was caused by an extreme windkessel effect in a massively dilated homograft that absorbed rather than transmitted the pulse wave. Effective treatment consisted of replacing the dilated homograft by a rigid aortic homograft of equal size as the initial homograft. When confronted with circulatory failure after allograft placement, the clinician should not only look for obstruction by narrowing, but also consider the windkessel phenomenon.
Keywords: Allograft, Homograft, Heart failure, CHD truncus arteriosus, CHD valve
CASE PRESENTATION
A premature infant presented with an arterial trunk type III. Because of low birth weight (1.9 kg), she was considered too small for neonatal truncal repair. Bilateral pulmonary artery banding with 3 mm Gore-Tex bands (Gore & Associates Inc., Flagstaff, AZ, USA) was performed with good clinical result [1]. At the age of 9 months, she underwent truncal repair. The ostiae of the pulmonary arteries were prelevated and the Gore-Tex bands were removed. A 16 mm cryo-preserved pulmonary homograft (European Homograft Bank EHB, Brussels, Belgium) was used to construct the right ventricular outflow tract. Because of the small size of the pulmonary artery orifices, peroperative stenting of both pulmonary arteries was performed (6 mm diameter PG 1910 stent, Cordis Corporation, Warren, NJ, USA). The ventricular septum defect was closed. The patient was weaned from cardiopulmonary bypass without problems and had oxygen saturations >95%.
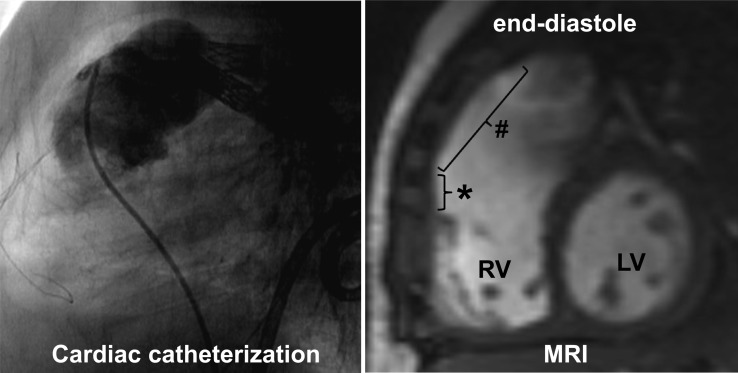
Twenty-four hours after the operation, the patient developed right heart failure. Transthoracic echocardiography demonstrated good RV function, no significant tricuspid regurgitation and good homograft valve function. Cardiac catheterization was performed 2 weeks postoperatively to exclude pulmonary artery problems: the pulmonary artery stents were patent (gradient 11 mmHg) with adequate run-off (Fig. 1, left panel). The catheterization was concise in order to limit contrast administration and radiation exposure.
Figure 1:
Left panel: sagittal view during cardiac catheterization depicting the pulmonary artery stents and the catheter in the pulmonary homograft. During contrast injection, the patency of the homograft and stents is confirmed. Right panel: sagittal view from cardiac MRI study. Dilated homograft (#) at end-diastole. *RV outflow patch, LV, left ventricle; RV, right ventricle.
During the following weeks, borderline haemodynamics persisted with inability to wean from mechanical ventilation despite optimal diuretic therapy. Echocardiography could only partially visualize the homograft due to its retrosternal position. A second catheterization was performed 4 weeks postoperatively and showed significant dilation of the homograft, moderately increased RV systolic pressure (48 mmHg, ∼2/3 of left ventricular systolic pressure) with neither homograft valve stenosis nor distal pulmonary artery stenosis. Additional cardiac MRI demonstrated severe dilation of the pulmonary homograft (from 16 mm diameter to oval shape 38 × 24 mm over a length of 21 mm, whereas pulmonary valve diameter 95th percentile for BSA is 12 mm), a mildly dilated RV with good contractility and competence of the homograft valve (Fig. 1, right panel). Since the aneurysmal dilation of the homograft acted as a reservoir absorbing rather than transmitting the pulse wave to the pulmonary arteries, replacement of the pulmonary homograft by a 16 mm aortic homograft (EHB, Brussels, Belgium) was performed 49 days after the initial pulmonary homograft placement. An aortic homograft was used to have a more rigid, less compliant graft. This procedure soon resulted in significant clinical improvement. Peripheral oedema visibly decreased and the child was weaned from respiratory support within 4 days to be discharged 2 weeks later. At 1-year follow-up, the child is doing well, RV and homograft function are good and the aortic homograft is not dilated.
DISCUSSION
Right heart failure is not exceptional after cardiac surgery in infants. When confronted with right heart failure, the clinician will focus on causes such as RV failure, severe tricuspid valve regurgitation and increased RV afterload by pulmonary hypertension or obstruction of pulmonary blood flow. If obstruction of flow is suspected, clinicians are trained to search for narrowing by mechanical obstruction. However, this case demonstrates we also should consider the possibility of a distended reservoir absorbing the pulse wave instead of transmitting it. This phenomenon can be explained using the windkessel model.
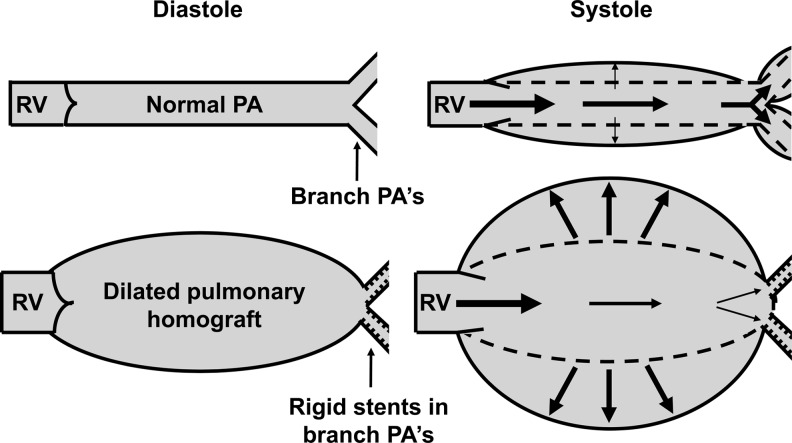
Windkessel means ‘air chamber’ in German, and refers to the concept of transforming pulsatile flow into laminar flow by use of an air chamber in the eighteenth century fire engines. The windkessel effect was introduced in medicine as a ‘compliant, elastic reservoir’ by Stephen Hales (1733) and later by Otto Frank (1899) [2]. The normal aorta, pulmonary artery and their large branches act as windkessel reservoirs: during systole, the pulse wave is attenuated by their compliance whereas during diastole, sufficient peripheral perfusion is ensured by elastic recoiling (Fig. 2). In this way, the windkessel properties allow conversion of ventricular pulsatile flow into continuous flow in the peripheral arteries [2, 3]. In our patient, two haemodynamic phenomena contributed to the extreme windkessel effect; first, the massive dilation of the homograft to 38 × 24 mm diameter and second, peripheral resistance was increased by the presence of rigid stents in the proximal pulmonary arteries. The extreme windkessel effect of this severely dilated homograft led to excessive attenuation of the pulse wave and insufficient pulmonary perfusion, thereby causing right heart failure.
Figure 2:
Schematic representation of normal physiological windkessel effect of the normal pulmonary artery (PA) and branch PAs (upper panel) resulting in adequate pulmonary perfusion. Pathological extreme windkessel effect of a dilated pulmonary homograft that absorbs rather than transmits the pulse wave (lower panel). In the lower panel, impedance is increased due to rigid stents in the proximal branch PAs and due to moderately increased PA pressure. RV, right ventricle.
We estimate that initial homograft dilation was caused by increased homograft wall stress due to the increased intraluminal hydrostatic pressure. Histological study to exclude intrinsic abnormalities of the pulmonary homograft tissue was not performed.
Our hypothesis that the windkessel effect by the massively dilated pulmonary homograft caused right heart failure is supported by the fact that effective treatment consisted solely of replacing the dilated homograft by a rigid one, leaving all other structural components of the circulation unaltered.
Both the pulmonary and aortic homografts of 16 mm were of relatively large size to anticipate further growth of the infant. Use of such rather large homografts in this patient population is ‘common practice’ in paediatric cardiology, and generally very well tolerated without pathological homograft dilation.
CONCLUSION
This complicated course after homograft placement illustrates that, when confronted with postoperative circulatory failure, clinicians should not only look for obstruction of blood flow by narrowing, but also consider functional obstruction by a windkessel reservoir. In this case, replacing the massively dilated homograft by a rigid homograft effectively treated right heart failure.
Funding
W.Y.V. is supported in part by a research grant from Rotary Tienen and by grant no. 2010T042 of the Dutch Heart Foundation.
Conflict of interest: none declared.
REFERENCES
- 1.Galantowicz M, Cheatham JP, Phillips A, Cua CL, Hoffman TM, Hill SL, et al. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg. 2008;85:2063–70. doi: 10.1016/j.athoracsur.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Westerhof N, Lankhaar JW, Westerhof BE. The arterial windkessel. Med Biol Eng Comput. 2009;47:131–41. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 3.Waragai T, Awa S, Akagi M, Andou Y, Watanabe N, Hosaki A, et al. Clinical implication of main pulmonary artery windkessel size after banding operation. Pediatr Int. 2009;51:84–90. doi: 10.1111/j.1442-200X.2008.02695.x. [DOI] [PubMed] [Google Scholar]