Abstract
The balance between systemic O2consumption (VO2) and O2delivery (DO2) is impaired in children after cardiopulmonary bypass surgery, with decreased DO2and increased VO2. The major goal, and the major challenge, of postoperative management has been to match DO2to VO2in order to sustain cellular metabolism, particularly in neonates after the Norwood procedure. While much effort has been put into augmenting cardiac output and DO2, VO2remains largely ignored. Respiratory mass spectrometry allows the precise and continuous measurement of VO2. Measured VO2, using the direct Fick principle, allows for the calculation of each element of systemic O2transport in the complex Norwood circulation. The actual measurements of O2transport have allowed us, in the past five years or so, to extensively investigate the Norwood physiology in terms of the VO2–DO2relationship and the factors affecting it in clinical treatments. Therefore, the first objective of this article is to introduce the technique of respiratory mass spectrometry and its adaption to measure VO2across paediatric ventilators with continuous flow. The second objective is to give an interim review of the main findings in our studies on systemic O2transport in 17 neonates in the first 72 h after the Norwood procedure. These findings include the profiles of systemic O2transport, the important contribution of VO2to the impaired balance of O2transport and the complex effects of some routine clinical treatments on the VO2–DO2relationship (including catecholamines, PaCO2, Mg2+and hyperglycaemia, as well as patient-specific anatomical variations). The influence of systemic O2transport on cerebral oxygenation is also introduced. This information may help us to refine postoperative management in neonates after the Norwood procedure. Our initial studies mark the end of the beginning, but much is yet explored. Ultimately, the resultant improved systemic and regional O2transport in the early postoperative period may have an important impact on long-term outcomes, thereby improving the quality of life for these vulnerable children.
Keywords: Respiratory mass spectrometry, Oxygen transport, The Norwood procedure
INTRODUCTION
The element of O2transport has been increasingly appreciated in the care of children with congenital heart defects after cardiopulmonary bypass (CPB) surgery [1–8]. The fundamental requirement of postoperative management is to match systemic O2delivery (DO2) with O2consumption (VO2) in order to sustain cellular metabolism and end-organ function. This is also a major challenge. In most children after CPB, cardiac function and DO2are depressed due to myocardial injury by surgery and ischaemia reperfusion [4, 5]. At the same time, VO2is increased and highly dynamic [1–4, 9, 10], due to systemic inflammatory response [9, 11], rewarming from hypothermic CPB and fever [1, 9], and pharmacological [2] and ventilatory manipulations [10, 12, 13]. VO2constitutes an important component of the imbalance of O2transport after CPB and of the effects, beneficial or adverse, of some routine clinical treatments [1–4, 10, 13]. However, in clinical practice, VO2remains largely ignored.
The imbalance of O2transport is particularly profound in neonates after the Norwood procedure for hypoplastic left heart syndrome and similar anatomical variants [2–4]. Due to this, the Norwood procedure continues to have a significant morbidity and a mortality rate that ranges from 6 to 25% [14–17]. This is inherent in the single ventricle supplying parallel pulmonary and systemic circulations and is compounded by the variable effects of CPB, ischaemia and reperfusion injury, and systemic inflammatory and metabolic response. In the Norwood circulation, the reserve of DO2is marginal. On the other hand, the increase in VO2is more substantial in neonates because of the greater systemic inflammatory and metabolic responses [18, 19] and the greater stimulation of VO2by inotropes [2, 20].
In this dynamic milieu, with significant alterations in the VO2–DO2relationship, indirect indicators of haemodynamics such as heart rate, arterial blood pressure and arterial and venous O2saturations are most commonly used to guide postoperative management to optimize the balance of O2transport. Their adequacy has been questioned [6]. Although central venous O2saturation accurately reflects the overall balance of O2transport, it does not discriminate between the contributions of DO2and VO2[3, 21]. In some clinical reports, derived values of systemic and pulmonary blood flows (Qsand Qp) and DO2have been obtained, but these are based on the false assumption of a fixed VO2of 160 or 180 ml/(min/m2) [7, 8, 22, 23].
This situation has changed with the adaptation of the highly sensitive and precise technique of respiratory mass spectrometry to continuously measure VO2across paediatric ventilators with continuous flow. Measured VO2, using the direct Fick principle, allows for the calculation of the actual values for each element of systemic O2transport in the Norwood circulation, including Qsand Qpand systemic and pulmonary resistances (SVR and PVR), DO2and O2extraction ratio (ERO2). In the past five years or so, we have extensively investigated systemic O2transport and factors affecting it in routine clinical treatments after the Norwood procedure [2–4, 6, 10, 24–27].
Therefore, the objectives of this article are two fold: first, to introduce the technique of respiratory mass spectrometry and its adaptation to paediatric ventilators to continuously measure VO2; second, to give an interim review of the main findings from our studies on O2transport to serve as ‘food for thought’ in further exploration of the Norwood physiology.
RESPIRATORY MASS SPECTROMETRY
The introduction of mass spectrometry into respiratory physiology in the 1940s was the basis for the development of the state-of-the-art methods for measuring VO2using highly accurate and rapid multiple gas analyses. The reported precision of the earlier methods using the mass spectrometer was as low as 5% in spontaneous breathing at rest or during exercise against a Douglas bag [28]. The precision may increase in paralysed and ventilated patients. We have adapted the method to continuously measure VO2with a variety of paediatric ventilators with continuous flow, using the AMIS 2000 Medical Respiratory Mass Spectrometer System (Innovision A/C, Odense, Denmark), making it a unique and powerful research tool in the paediatric intensive care unit (ICU) [1–4, 6, 9, 10, 13, 24–27, 29–33].
Principles of mass spectrometry
Mass spectrometers analyse substances in the gas phase by performing a sequence of five operations: (i) accepting and vaporizing a minute controlled quantity of sample; (ii) reducing the sample vapour to a very low pressure; (iii) ioning a representative part of the vapour; (iv) separating the ionize particles produced, according to their mass-to-charge ratio; and (v) reading the abundance of particles at specific values of the mass-to-charge ratio.
Set-up of the respiratory mass spectrometer with paediatric ventilators
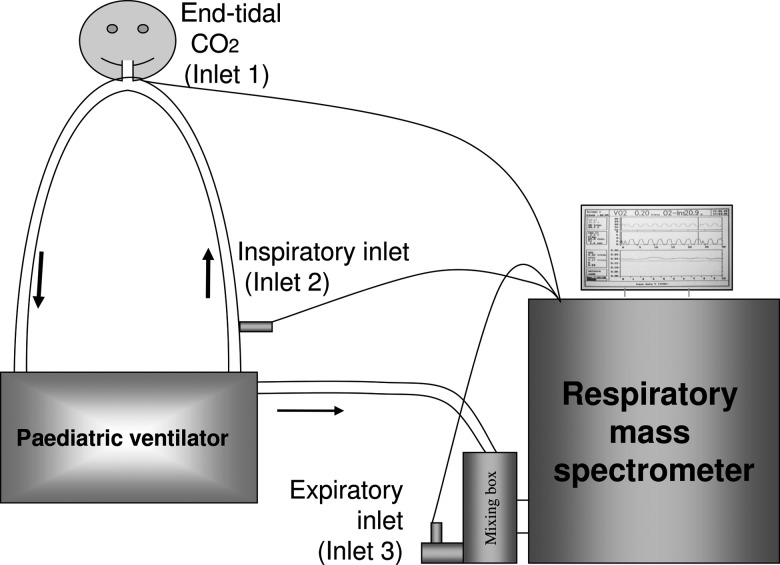
We have adapted the AMIS 2000 respiratory mass spectrometer in the ICU to various paediatric ventilators with continuous flow, such as the Servo 300 and Servo-i (Siemens AG, Munich, Germany) (Fig. 1). The mixing box is connected to the exit port of the paediatric ventilator to collect the expired gas and is also connected to an ‘expiratory’ inlet (Inlet 3). The expiratory inlet allows for the sampling of the ‘effluent’ mixed expirate from the distal end of the mixing box. Inlets 1 and 2 are placed at the mouth piece and in the inspiratory limb, respectively.
Figure 1:
The set-up of the AMIS 2000 respiratory mass spectrometer sampling inlets, the mixing box and the circuit of the paediatric ventilator in the ICU.
Accurate measurement of VO2relies on the complete collection of expired gas and thus a leak-free circuit. Patients are, therefore, intubated with a cuffed endotracheal tube (Microcuff-Heidelberg-Pediatric; Microcuff GmbH, Weinheim, Germany). Additionally, patients are usually sedated and paralysed in order to obviate the confounding effects of movement, agitation and pain on VO2and to ensure a steady state.
Calculation of O2transport parameters using VO2in the Norwood circulation
The Fick principle states that ‘The total uptake or release of any substance by an organ is the product of blood flow to the organ and the arteriovenous concentration difference of the substance’ [34]. The direct Fick principle using VO2is one of the oldest methods used for measuring Qpand Qs, but nonetheless remains the gold standard. The equations used for calculating O2transport in the Norwood circulation, i.e. single ventricular circulation, are as follows:
where CaO2CsvcO2and CpvO2are, respectively, the systemic arterial and superior vena caval O2contents and MaP, MsvcP and MpvP and pulmonary venous are the mean systemic arterial, superior vena caval and pulmonary venous pressures, respectively.
SYSTEMIC O2TRANSPORT AFTER THE NORWOOD PROCEDURE
The actual measurements of systemic O2transport parameters have enabled us to examine the alterations in the VO2–DO2relationship after the Norwood procedure and, more importantly, to explore the factors affecting the relationship, beneficial or adverse, in the routine treatments that are used to optimize O2transport. The rest of this paper summarizes the main findings from our studies in 17 neonates during the first 72 h after the classical Norwood procedure with the Blalock–Taussig shunt, between April 2004 and November 2006, at the Hospital for Sick Children in Toronto, Ont., Canada. These findings may also be largely applied to the modified Norwood procedure with a right ventricle to the pulmonary artery shunt.
Profiles of systemic O2transport after the Norwood procedure
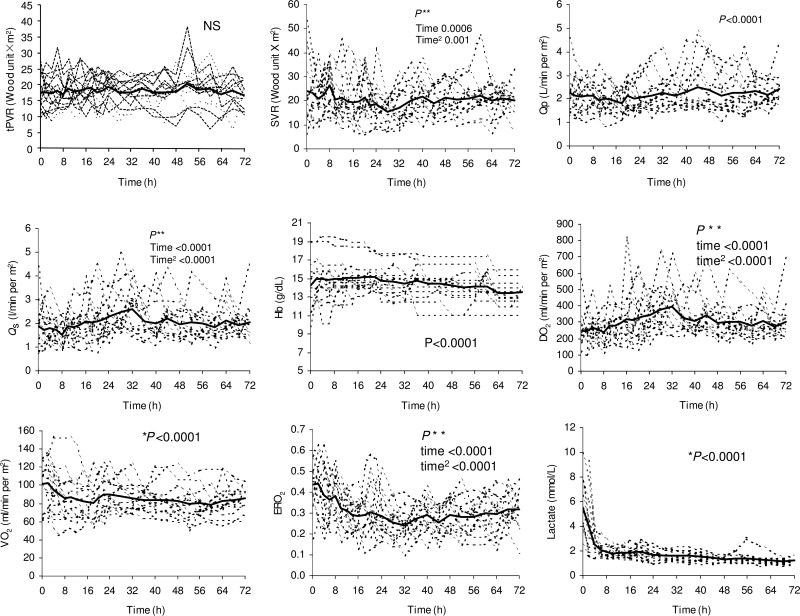
The wide, unstable inter- and intra-individual variations in all the elements of O2transport reflect the profound instability of the early postoperative course after the Norwood procedure (Fig. 2) [4]. The variability is particularly greater on the systemic side (including SVR and Qs). PVR and Qpare less variable due to the mechanical limitation of the Blalock–Taussig shunt in the classical Norwood procedure or to the right ventricle to the pulmonary artery shunt in the modified Norwood procedure. Over the first 72 h after the Norwood procedure, Qp, Qsand DO2gradually increase.
Figure 2:
Individual and mean values of systemic O2transport parameters. Total pulmonary vascular resistance with the BT shunt (tPVR) and SVR, Qp, Qs, haemoglobin (Hb), DO2, VO2, ERO2and arterial lactate levels during the first 72 h after patient arrival in the ICU.
VO2varies significantly both between and within patients, ranging from 45 to 152 ml/(min/m2). Note that these measured values are all below the previously assumed values of 160 or 180 ml/(min/m2) [7, 8, 22, 23]. Overall, VO2is high immediately after the Norwood procedure [3, 4]. It decreases rapidly in the first 24 h, followed by a slower decrease over the following 48 h, with a total decrease of about 20%. In the first 24 h, CO, Qsand DO2are the variables that decrease the most. However, during the critical first 24-h period, the balance of VO2–DO2improves significantly, as indicated by the rapid decrease in ERO2. The observed improvement in balance results primarily from a decrease in VO2, rather than from any significant improvement in DO2as reported previously [7, 23, 35]. This indicates the important contribution of VO2to the balance of O2transport in the early hours after the Norwood procedure.
Optimizing systemic O2delivery
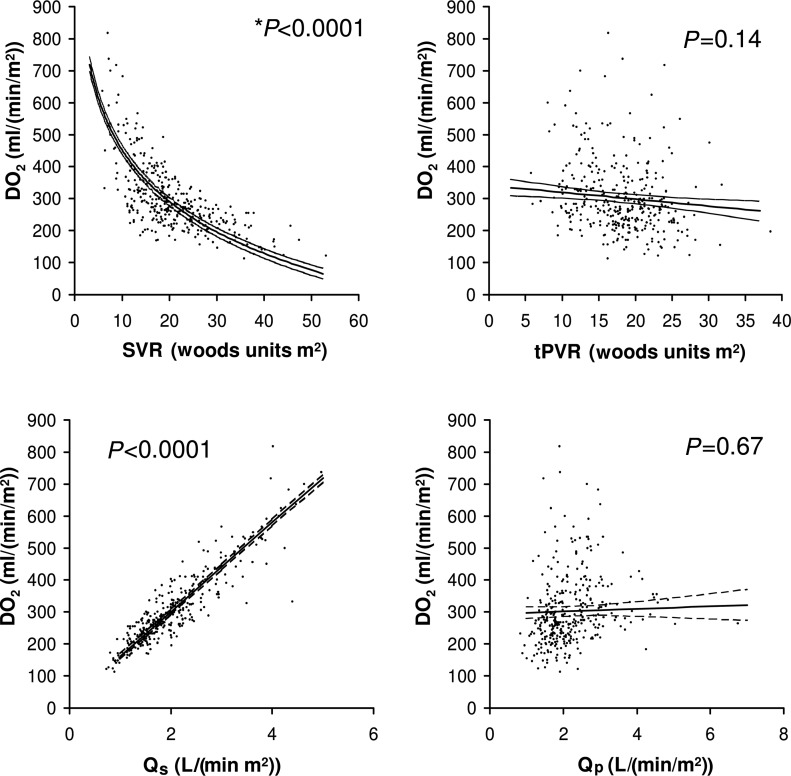
Correlation analyses of DO2with other systemic O2transport parameters reveal the important factors that contribute to DO2in the Norwood circulation [4]. There is a close positive correlation of DO2with Qsand a negative correlation with SVR, but not with Qpor tPVR (Fig. 3). Furthermore, among the variables of arterial O2content, DO2is not correlated with SaO2and only weakly correlated with PaO2. Haemoglobin value has a high positive correlation with DO2. Therefore, clinical management strategies aiming to increase DO2should target SVR and Qsas well as haemoglobin, but not tPVR, Qp, SaO2or PaO2. The routine use of systemic vasodilators such as phenoxybenzamine and nitroprusside to maintain a relatively low and stable SVR has been shown to improve O2transport and postoperative outcomes [8, 36]. In contrast, vasoconstrictors such as vasopressin may have adverse effects on almost all the elements of systemic O2transport, including decreases in Qs, CO and DO2as a result of an increase in SVR, leading to an increase in ERO2and lactate [27]. Additionally, more attention should be paid to the maintenance of a relatively high level of haemoglobin throughout the prolonged postoperative course [4].
Figure 3:
Correlations of systemic DO2with SVR, Qs, total pulmonary vascular resistance including the BT shunt (tPVR) and Qpin patients during the first 72 h after arrival in the ICU.
Factors affecting VO2and its balance with DO2
Direct and continuous measurements of VO2have allowed us to study the complex effects of clinical treatments on VO2itself and its relation with DO2. Some routine treatments used in an effort to improve the balance of O2transport have in fact complex or even adverse effects.
Catecholamines
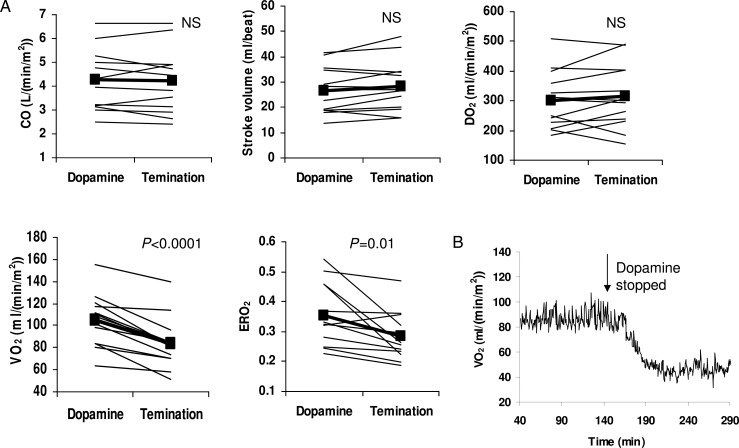
Catecholamines, such as dopamine, epinephrine and norepinephrine, are commonly used in children after CPB to augment cardiac contractility and DO2. Catecholamines also stimulate VO2through their effects on myocardial work and metabolic rate [37]. If the increase in DO2is greater than the increase in VO2, then catecholamines will improve the overall balance of O2transport and tissue oxygenation. In neonates, however, catecholamines have additional thermogenic actions through their effects on brown adipose tissue [38], resulting in an exaggerated increase in VO2[20]. Furthermore, neonatal hearts are known to have limited reserves to increase cardiac contractility. The reserves become marginal in a Norwood circulation. In these patients, efforts to improve DO2by catecholamines are more likely to be associated with predominately adverse effects. As we have reported [2], terminating a moderate dose of dopamine (5 μg/kg/min) was associated not with any significant changes in CO or DO2(Fig. 4A), but with a significant decrease in heart rate and rate pressure product, an indirect indicator of myocardial O2consumption. VO2also decreased by 16 ± 14 ml/min/m2, representing a change of 20 ± 11%. The termination of dopamine resulted in an overall improvement of the balance of O2transport, as indicated by the significant decrease in ERO2. In other words, a moderate dose of dopamine induces predominantly an increase in VO2, adversely affecting the VO2–DO2relationship. Fig. 4B shows an example of on-line VO2monitoring before and after dopamine termination.
Figure 4:
(A) The individual (thin line) and mean (bold line with closed squares) changes in systemic O2transport before and after termination of dopamine following the Norwood procedure. CO: cardiac output; DO2: O2delivery; ERO2: O2extraction ratio; VO2: O2consumption. (B) An example of the on-line measurement of VO2showing the rapid decrease in VO2after terminating dopamine (7.5 μg/kg/min).
CO2
CO2has been suggested as a factor increasing DO2in neonates both before and after the Norwood procedure [39]. Consequently, it is a common practice to maintain a relatively high arterial CO2tension (PaCO2), primarily by hypoventilation. The potent pulmonary vasoconstrictive effect of CO2was believed to decrease Qp, thereby increasing Qs. We studied the effect of stepwise increases in PaCO2from 40 to 50 to 60 mmHg and found complex effects of CO2on systemic and regional O2transport (Fig. 5) [10]. Moderate hypercapnia increases Qsas a result of its effect on SVR, rather than via PVR as proposed previously [39]. The increase in Qsis primarily a consequence of increased cerebral blood flow that compromises splanchnic circulation, as indicated by the increase in cerebral O2saturation and the decrease in splanchnic O2saturation measured by near infrared spectroscopy (Somanetics INVOS 5100A, Troy, MI, USA). Moderate hypercapnia also decreases VO2and stimulates the release of catecholamines. The decrease in VO2improves the balance of O2transport, but the increase in catecholamines may be undesirable. Clinically, CO2should be used with caution when the aim is to improve DO2.
Figure 5:
The changes (mean ± SD) in systemic O2transport including SVR, total pulmonary vascular resistance (tPVR), Qp, Qs, VO2, DO2, ERO2, lactate saturation, ScO2, SsO2and plasma epinephrine and norepinephrine concentration during the stepwise increases in PaCO2from 40 to 50 to 60 mmHg, and after termination of the additional inspired CO2at PaCO240 mmHg.
Plasma-ionized calcium and magnesium
The realization of Ca2+injury and Mg2+protection on myocardial function has indicated the use of a cardioplegic solution with low Ca2+and high Mg2+during CPB [40]. However, postoperative management strategies have not been clearly defined. While supplemental Ca2+is commonly used in current practice, little attention is paid to the supplementation of Mg2+. From our data [41], we found that Mg2+shows significant positive correlations with CO and DO2and negative correlations with heart rate, VO2, ERO2and lactate after the Norwood procedure. We further examined the effects of Mg2+and Ca2+on myocardial energetics, using cardiac power output and rate pressure product. Cardiac power output represents cardiac power, whereas rate pressure is an indirect measure of myocardial O2consumption [22, 23]. At any given unit of rate pressure product, Mg2+has a significant and positive correlation with cardiac power output. Ca2+shows the opposite trend, although without achieving statistical significance. As such, our data indicate the beneficial effects of Mg2+on myocardial energetics and systemic O2transport, whereas Ca2+may be potentially harmful. Therefore, maintaining a relatively high level of Mg2+and low level of Ca2+may promote the efficiency of myocardial work and improve the balance of systemic and myocardial O2transport.
Hyperglycaemia
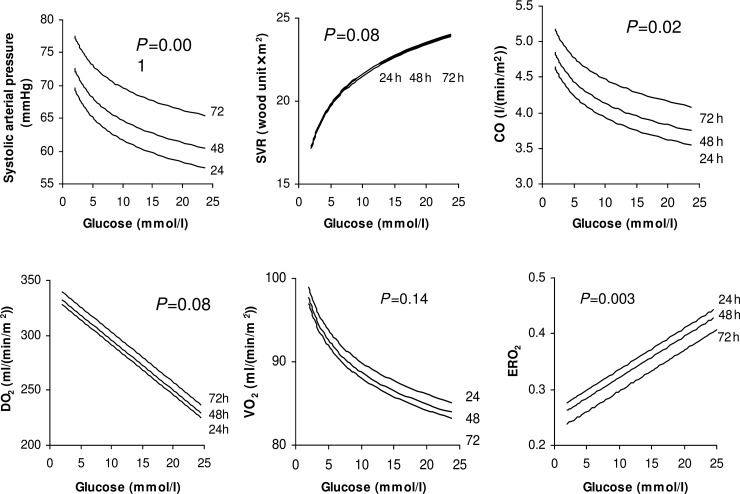
Hyperglycaemia has been identified as a risk factor for adverse outcomes in critically ill patients, including patients who have undergone CPB. Tight glucose control with insulin therapy has been shown to improve outcomes [42, 43], but is not a common practice followed for children after CPB. In our patients, blood glucose ranged from 2.8 to 24.6 mmol/l in the first 72 h after the Norwood procedure, with 60% of the measures being >6 mmol/l [33]. Correlation analysis of our data demonstrates a negative association between hyperglycaemia and systemic O2transport, with elevated glucose levels being closely and negatively correlated with CO and DO2and positively correlated with SVR and ERO2(Fig. 6). Our study was not designed to identify the cause-and-effect relationship. Randomized clinical trials of glucose control with insulin therapy are warranted to provide information regarding appropriate glucose management strategies in order to improve O2transport and clinical outcomes in neonates after the Norwood procedure and other cardiac surgeries.
Figure 6:
Representative regression lines for the predictive model of the correlations at 24, 48 and 72 h between blood glucose and the main variables of systemic O2transport, including systolic arterial pressure, SVR, cardiac output (CO), DO2, VO2and ERO2after the Norwood procedure.
Other factors
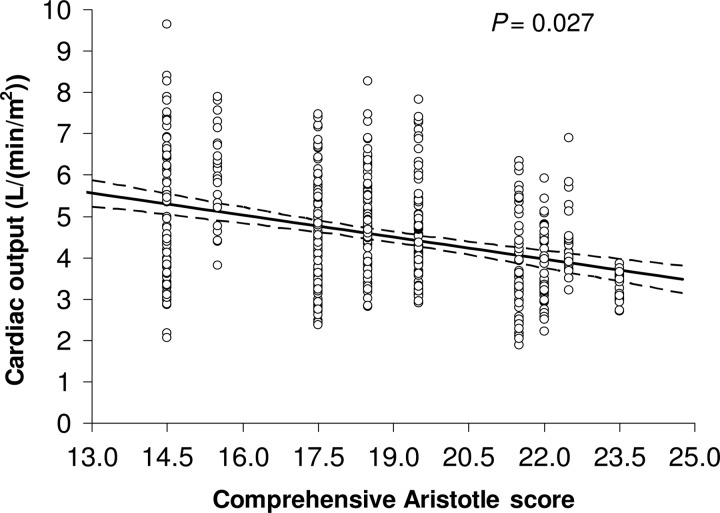
The Norwood procedure has a large dispersion of case complexity due to a wide spectrum of cardiac and non-cardiac lesions that certainly influence postoperative cardiac function and O2transport. The comprehensive Aristotle score, as an individualized measure of the complexity, summarizes the specific patient characteristics including the procedure-dependent factors (i.e. anatomical variations and associated procedures) and procedure-independent factors (i.e. clinical status of the patient) [44]. We have used the comprehensive Aristotle score with these specific factors and analysed the correlation with postoperative O2transport [25]. Not surprisingly, the comprehensive Aristotle score significantly correlates with CO following the Norwood procedure (Fig. 7). More importantly, our data analysis identified the specific risk factors contributing to a low postoperative CO level after the Norwood procedure, including preoperative myocardial dysfunction, mechanical ventilation to treat cardiorespiratory failure, atrioventricular valve regurgitation and aortic atresia. Therefore, a preoperative estimation of the comprehensive Aristotle score, particularly in association with these specific risk factors, may help to anticipate a high postoperative morbidity with low cardiac output syndrome and promote special prevention strategies.
Figure 7:
The correlation of the repeatedly measured total cardiac output and the comprehensive Aristotle score in neonates during the first 72 h after the Norwood procedure.
Influence of systemic O2transport on cerebral oxygenation
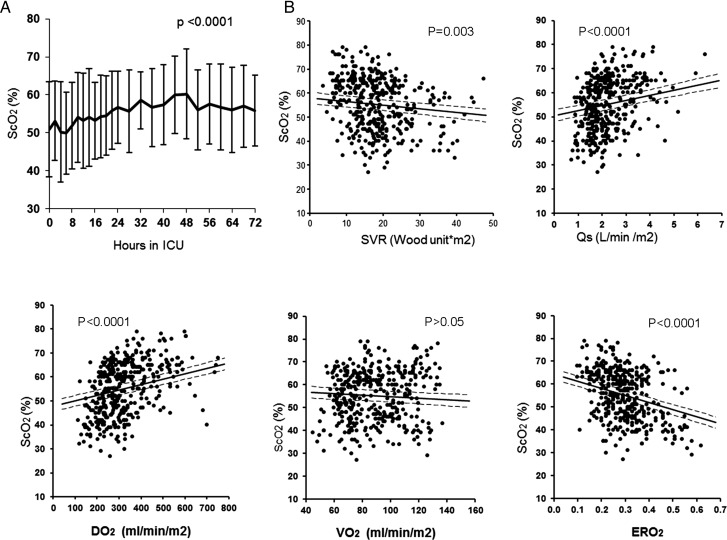
Ischaemic brain injury is a serious and frequent morbidity following the Norwood procedure in both the early postoperative period and the long-term follow-up [45]. Studies on neurological outcomes have largely focused on specific intraoperative risk factors such as pH imbalance and/or deep hypothermic circulatory arrest [46]. Preoperative reduction of cerebral blood flow and ischaemic injury are reported to be most severe in neonates with hypoplastic left heart syndrome [47]. The profound imbalance of systemic O2transport may place the cerebral oxygenation at further risk. We used near infrared spectroscopy to measure cerebral O2saturation after the Norwood procedure and found a significant reduction in the first 72 h that was particularly noticeable in the first 12 h (Fig. 8) [26]. Correlation analysis of systemic O2transport and cerebral O2saturation demonstrates that cerebral O2saturation is significantly influenced by all the parameters of systemic O2transport. As such, interventions to modify systemic O2transport may provide further opportunities to reduce the risk of cerebral ischaemia and improve neurodevelopmental outcomes.
Figure 8:
(A) Changes (mean ± SD) of ScO2during the first 72 h after the Norwood procedure. (B) Correlations between ScO2and systemic O2transport parameters of SVR, Qs, systemic VO2, DO2and ERO2during the first 72 h after the Norwood procedure.
CONCLUSION
Respiratory mass spectrometry is the state-of-the-art method for allowing the precise and continuous measurement of VO2. Measured VO2and the direct Fick principle allow for calculation of the actual values of each element of systemic O2transport in the Norwood circulation, which allows analysis of the complex physiology after the Norwood procedure. The important findings to date are summarized in this review. Based on these findings, a number of refined postoperative management strategies may be considered to optimize systemic O2transport. The lowering of VO2with careful use of inotropes and the maintenance of a high haemoglobin level and a low SVR with vasodilators appear to be rational approaches. Additionally, the maintenance of a moderate PaCO2level, normal blood glucose, relatively low plasma Ca2+and high Mg2+may promote the balance of systemic O2transport. The improvement in systemic O2transport may have beneficial effects on cerebral oxygenation. Finally, a preoperative estimation of the comprehensive Aristotle score with specific patient risk factors may help to anticipate a high postoperative morbidity with low cardiac output syndrome and promote special strategies to prevent it. Ultimately, the resultant improved systemic and regional O2transport in the early postoperative period may have important impacts on long-term outcomes, thereby improving the quality of life for these vulnerable children. Our initial studies mark the end of the beginning. Much is yet to be explored.
ACKNOWLEDGEMENT
My grateful appreciation goes to the clinicians at the Hospital for Sick Children in Toronto, Canada, for their help with conducting the study, including the surgical team for placing the intravenous lines, the anaesthesia team for inserting cuffed endotracheal tubes, the nurses for drawing blood samples during the study period and the respiratory therapists for the blood gas measurements.
Funding
Heart Stroke Foundation of Canada; Canadian Institute of Health Research.
Conflict of interest:none declared.
References
- 1.Li J, Schulze-Neick I, Lincoln C, Shore D, Scallan M, Bush A, et al. Oxygen consumption after cardiopulmonary bypass surgery in children: determinants and implications. J Thorac Cardiovasc Surg. 2000;119:525–33. doi: 10.1016/s0022-5223(00)70132-2. doi:10.1016/S0022-5223(00)70132-2. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Zhang G, Holtby H, Humpl T, Caldarone CA, Van Arsdell GS, et al. Adverse effects of dopamine on systemic hemodynamic status and oxygen transport in neonates after the Norwood procedure. J Am Coll Cardiol. 2006;48:1859–64. doi: 10.1016/j.jacc.2006.07.038. doi:10.1016/j.jacc.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Zhang G, Holtby HM, McCrindle BW, Cai S, Humpl T, et al. Inclusion of oxygen consumption improves the accuracy of arterial and venous oxygen saturation interpretation after the Norwood procedure. J Thorac Cardiovasc Surg. 2006;131:1099–107. doi: 10.1016/j.jtcvs.2005.10.057. doi:10.1016/j.jtcvs.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zhang G, McCrindle BW, Holtby H, Humpl T, Cai S, et al. Profiles of hemodynamics and oxygen transport derived by using continuous measured oxygen consumption after the Norwood procedure. J Thorac Cardiovasc Surg. 2007;133:441–8. doi: 10.1016/j.jtcvs.2006.09.033. doi:10.1016/j.jtcvs.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Wernovsky G, Wypij D, Jonas RA, Mayer JE, Jr, Hanley FL, Hickey PR, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants: a comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–35. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 6.Yu XY, Zhang G, Cai S, Li J. Clinical indirect indicators of hemodynamics do not accurately reflect systemic oxygen transport status in neonates after the Norwood procedure. Circulation. 2011;124:A14568. [Google Scholar]
- 7.Hoffman GM, Ghanayem NS, Kampine JM, Berger S, Mussatto KA, Litwin SB, et al. Venous saturation and the anaerobic threshold in neonates after the Norwood procedure for hypoplastic left heart syndrome. Ann Thorac Surg. 2000;70:1515–20. doi: 10.1016/s0003-4975(00)01772-0. discussion 1521 doi:10.1016/S0003-4975(00)01772-0. [DOI] [PubMed] [Google Scholar]
- 8.Tweddell JS, Hoffman GM, Fedderly RT, Berger S, Thomas JP, Jr, Ghanayem NS, et al. Phenoxybenzamine improves systemic oxygen delivery after the Norwood procedure. Ann Thorac Surg. 1999;67:1–167. doi: 10.1016/s0003-4975(98)01266-1. discussion 167–168. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Hoschtitzky A, Allen ML, Elliott MJ, Redington AN. An analysis of oxygen consumption and oxygen delivery in euthermic infants after cardiopulmonary bypass with modified ultrafiltration. Ann Thorac Surg. 2004;78:1389–96. doi: 10.1016/j.athoracsur.2004.02.032. doi:10.1016/j.athoracsur.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhang G, Holtby H, Bissonnette B, Wang G, Redington AN, et al. Carbon dioxide—a complex gas in a complex circulation: its effects on systemic hemodynamics and oxygen transport, cerebral, and splanchnic circulation in neonates after the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136:1207–14. doi: 10.1016/j.jtcvs.2008.02.096. doi:10.1016/j.jtcvs.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 11.Oudemans-van Straaten HM, Jansen PG, te Velthuis H, Beenakkers IC, Stoutenbeek CP, van Deventer SJ, et al. Increased oxygen consumption after cardiac surgery is associated with the inflammatory response to endotoxemia. Intensive Care Med. 1996;22:294–300. doi: 10.1007/BF01700449. doi:10.1007/BF01709521. [DOI] [PubMed] [Google Scholar]
- 12.Hoskote A, Li J, Hickey C, Erickson S, Van Arsdell G, Stephens D, et al. The effects of carbon dioxide on oxygenation and systemic, cerebral, and pulmonary vascular hemodynamics after the bidirectional superior cavopulmonary anastomosis. J Am Coll Cardiol. 2004;44:1501–9. doi: 10.1016/j.jacc.2004.06.061. doi:10.1016/j.jacc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Hoskote A, Hickey C, Stephens D, Bohn D, Holtby H, et al. Effect of carbon dioxide on systemic oxygenation, oxygen consumption, and blood lactate levels after bidirectional superior cavopulmonary anastomosis. Crit Care Med. 2005;33:984–9. doi: 10.1097/01.ccm.0000162665.08685.e2. doi:10.1097/01.CCM.0000162665.08685.E2. [DOI] [PubMed] [Google Scholar]
- 14.Azakie T, Merklinger SL, McCrindle BW, Van Arsdell GS, Lee KJ, Benson LN, et al. Evolving strategies and improving outcomes of the modified Norwood procedure: a 10-year single-institution experience. Ann Thorac Surg. 2001;72:1349–53. doi: 10.1016/s0003-4975(01)02795-3. doi:10.1016/S0003-4975(01)02795-3. [DOI] [PubMed] [Google Scholar]
- 15.Gaynor JW, Mahle WT, Cohen MI, Ittenbach RF, DeCampli WM, Steven JM, et al. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg. 2002;22:82–9. doi: 10.1016/s1010-7940(02)00198-7. doi:10.1016/S1010-7940(02)00198-7. [DOI] [PubMed] [Google Scholar]
- 16.Salim MA, Case CL, Sade RM, Watson DC, Alpert BS, DiSessa TG. Pulmonary/systemic flow ratio in children after cavopulmonary anastomosis. J Am Coll Cardiol. 1995;25:735–8. doi: 10.1016/0735-1097(94)00441-R. doi:10.1016/0735-1097(94)00441-R. [DOI] [PubMed] [Google Scholar]
- 17.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. doi:10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand KJ, Brown MJ, Bloom SR, Aynsley-Green A. Studies on the hormonal regulation of fuel metabolism in the human newborn infant undergoing anaesthesia and surgery. Horm Res. 1985;22:115–28. doi: 10.1159/000180083. doi:10.1159/000180083. [DOI] [PubMed] [Google Scholar]
- 19.Anand KJ, Brown MJ, Causon RC, Christofides ND, Bloom SR, Aynsley-Green A. Can the human neonate mount an endocrine and metabolic response to surgery? J Pediatr Surg. 1985;20:41–8. doi: 10.1016/s0022-3468(85)80390-0. doi:10.1016/S0022-3468(85)80390-0. [DOI] [PubMed] [Google Scholar]
- 20.Penny DJ, Sano T, Smolich JJ. Increased systemic oxygen consumption offsets improved oxygen delivery during dobutamine infusion in newborn lambs. Intensive Care Med. 2001;27:1518–25. doi: 10.1007/s001340101044. doi:10.1007/s001340101044. [DOI] [PubMed] [Google Scholar]
- 21.Yu X, Zhang G, Cai S, Li J. Clinical indirect indicators of hemodynamics do not accurately reflect systemic oxygen transport status in neonates after the Norwood procedure. Oral abstract presentation at the American Heart Association: Scientific Sessions 2011. Circulation. 124:A14568.. [Google Scholar]
- 22.Barnea O, Austin EH, Richman B, Santamore WP. Balancing the circulation: theoretic optimization of pulmonary/systemic flow ratio in hypoplastic left heart syndrome. J Am Coll Cardiol. 1994;24:1376–81. doi: 10.1016/0735-1097(94)90123-6. doi:10.1016/0735-1097(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 23.Maher KO, Pizarro C, Gidding SS, Januszewska K, Malec E, Norwood WI, Jr, et al. Hemodynamic profile after the Norwood procedure with right ventricle to pulmonary artery conduit. Circulation. 2003;108:782–4. doi: 10.1161/01.CIR.0000087338.09589.21. doi:10.1161/01.CIR.0000087338.09589.21. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Zhang G, Benson L, Holtby H, Cai S, Humpl T, et al. Comparison of the profiles of postoperative systemic hemodynamics and oxygen transport in neonates after the hybrid or the Norwood procedure: a pilot study. Circulation. 2007;116:I179–87. doi: 10.1161/CIRCULATIONAHA.106.679654. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Zhang G, Holtby H, Cai S, Walsh M, Caldarone CA, et al. Significant correlation of comprehensive Aristotle score with total cardiac output during the early postoperative period after the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136:123–8. doi: 10.1016/j.jtcvs.2007.12.056. doi:10.1016/j.jtcvs.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Zhang G, Holtby H, Guerguerian AM, Cai S, Humpl T, et al. The influence of systemic hemodynamics and oxygen transport on cerebral oxygen saturation in neonates after the Norwood procedure. J Thorac Cardiovasc Surg. 2008;135:83–90. doi: 10.1016/j.jtcvs.2007.07.036. e81–82 doi:10.1016/j.jtcvs.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 27.Zhang G, Holtby H, Cai S, Al Radi O, Li J. Aortic atresia is associated with an inferior systemic, cerebral, and splanchnic oxygen-transport status in neonates after the Norwood procedure. Eur J Cardiothorac Surg. 2011;39:e13–21. doi: 10.1016/j.ejcts.2010.10.009. doi:10.1016/j.ejcts.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Davies NJ, Denison DM. The measurement of metabolic gas exchange and minute volume by mass spectrometry alone. Respir Physiol. 1979;36:261–7. doi: 10.1016/0034-5687(79)90029-x. doi:10.1016/0034-5687(79)90029-X. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Van Arsdell GS, Zhang G, Cai S, Humpl T, Caldarone CA, et al. Assessment of the relationship between cerebral and splanchnic oxygen saturations measured by near-infrared spectroscopy and direct measurements of systemic haemodynamic variables and oxygen transport after the Norwood procedure. Heart. 2006;92:1678–85. doi: 10.1136/hrt.2005.087270. doi:10.1136/hrt.2005.087270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulze-Neick I, Li J, Penny DJ, Redington AN. Pulmonary vascular resistance after cardiopulmonary bypass in infants: effect on postoperative recovery. J Thorac Cardiovasc Surg. 2001;121:1033–9. doi: 10.1067/mtc.2001.113747. doi:10.1067/mtc.2001.113747. [DOI] [PubMed] [Google Scholar]
- 31.Schulze-Neick I, Li J, Reader JA, Shekerdemian L, Redington AN, Penny DJ. The endothelin antagonist bq123 reduces pulmonary vascular resistance after surgical intervention for congenital heart disease. J Thorac Cardiovasc Surg. 2002;124:435–41. doi: 10.1067/mtc.2002.121492. doi:10.1067/mtc.2002.121492. [DOI] [PubMed] [Google Scholar]
- 32.Shekerdemian LS, Bush A, Shore DF, Lincoln C, Redington AN. Cardiopulmonary interactions after Fontan operations: augmentation of cardiac output using negative pressure ventilation. Circulation. 1997;96:3934–42. doi: 10.1161/01.cir.96.11.3934. [DOI] [PubMed] [Google Scholar]
- 33.Zhang G, Cai S, Li J. Hyperglycaemia is negatively associated with systemic and cerebral oxygen transport in neonates after the Norwood procedure. Cardiol Young. 2011;22(1):1–8. doi: 10.1017/S1047951111000904. [DOI] [PubMed] [Google Scholar]
- 34.Fick A. Uber die messung des blutquantums in der herzventrikeln. Sitzungsberichte der physikalisch-medicinischen Gesellschaftzu Wurtzberg. 1870;8:XVI–VII. [Google Scholar]
- 35.Hoffman GM, Mussatto KA, Brosig CL, Ghanayem NS, Musa N, Fedderly RT, et al. Systemic venous oxygen saturation after the Norwood procedure and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2005;130:1094–100. doi: 10.1016/j.jtcvs.2005.06.029. doi:10.1016/j.jtcvs.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 36.De Oliveira NC, Ashburn DA, Khalid F, Burkhart HM, Adatia IT, Holtby HM, et al. Prevention of early sudden circulatory collapse after the Norwood operation. Circulation. 2004;110:II133–38. doi: 10.1161/01.CIR.0000138399.30587.8e. [DOI] [PubMed] [Google Scholar]
- 37.Ensinger H, Weichel T, Lindner KH, Grunert A, Ahnefeld FW. Effects of norepinephrine, epinephrine, and dopamine infusions on oxygen consumption in volunteers. Crit Care Med. 1993;21:1502–8. doi: 10.1097/00003246-199310000-00018. doi:10.1097/00003246-199310000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Sell H, Deshaies Y, Richard D. The brown adipocyte: update on its metabolic role. Int J Biochem Cell Biol. 2004;36:2098–104. doi: 10.1016/j.biocel.2004.04.003. doi:10.1016/j.biocel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Bradley SM, Simsic JM, Atz AM. Hemodynamic effects of inspired carbon dioxide after the Norwood procedure. Ann Thorac Surg. 2001;72:2088–93. doi: 10.1016/s0003-4975(01)03169-1. discussion 2093–2094 doi:10.1016/S0003-4975(01)03169-1. [DOI] [PubMed] [Google Scholar]
- 40.Ji B, Feng Z, Liu J, Long C. Myocardial protection related to magnesium content of cold blood hyperkalemic cardioplegic solutions in CABG. J Extra Corpor Technol. 2002;34:107–10. [PubMed] [Google Scholar]
- 41.Dhillon D, Yu X, Zhang G, Cai S, Li J. The relationship between plasma concentrations of ionized calcium and magnesium with cardiac energetics and systemic oxygen transport in neonates after the Norwood procedure. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2011.12.023. Jan 12. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. doi:10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 43.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. doi:10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 44.Lacour-Gayet F, Clarke D, Jacobs J, Comas J, Daebritz S, Daenen W, et al. The Aristotle score: a complexity-adjusted method to evaluate surgical results. Eur J Cardiothorac Surg. 2004;25:911–24. doi: 10.1016/j.ejcts.2004.03.027. doi:10.1016/j.ejcts.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16(Suppl 1):92–104. doi: 10.1017/S1047951105002398. doi:10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 46.Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KC, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–64. doi: 10.1056/NEJM199310073291501. doi:10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 47.Glauser TA, Rorke LB, Weinberg PM, Clancy RR. Acquired neuropathologic lesions associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85:991–1000. [PubMed] [Google Scholar]