Abstract
OBJECTIVES
Risk assessment of patients with aortic stenosis (AS) undergoing aortic valve replacement (AVR) is challenging. We set out to determine the impact of myocardial late gadolinium enhancement (LGE), as detected by cardiovascular magnetic resonance (CMR), on postoperative outcomes following AVR.
METHODS
A prospective observational study was conducted on patients undergoing CMR using the LGE technique within 1 year of subsequent AVR. Patients were categorized into absent, mid-wall or infarct patterns of LGE by independent observers blinded to all clinical data, and data were collected with regard to 30-day mortality, major adverse cardiac and cerebrovascular events (MACCE) and postoperative complications.
RESULTS
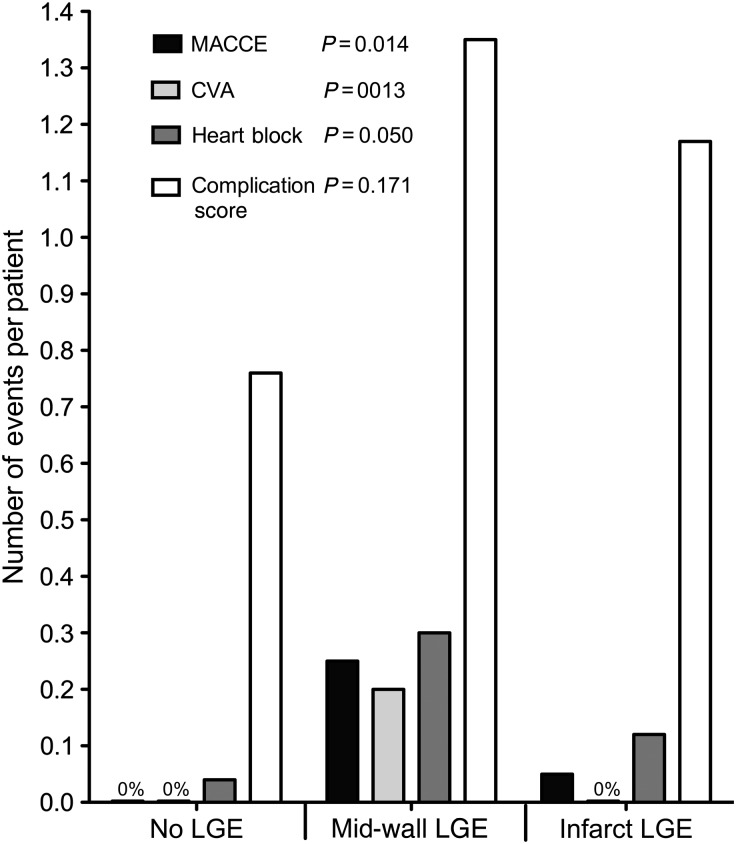
A total of 63 patients were studied. Twenty-five patients had no LGE; 20 had mid-wall LGE and 18 had an infarct pattern. The incidence of MACCE, cerebrovascular accident (CVA) and heart block were significantly higher in the mid-wall group compared with the other two groups (MACCE: 25 vs. 0 vs. 5%, P = 0.014; CVA: 20 vs. 0 vs. 0%, P = 0.013; heart block: 30 vs. 4 vs. 12%, P = 0.050). Patients with no LGE had no 30-day MACCE events and no deaths up to 2 years of follow-up.
CONCLUSIONS
The myocardial LGE holds promise as a means of predicting risk prior to AVR for AS.
Keywords: Aortic valve stenosis, Myocardial fibrosis, Magnetic resonance, Risk assessment
INTRODUCTION
Calcific aortic stenosis (AS) is the commonest form of valve disease in the western world. The only form of treatment is aortic valve replacement (AVR) once patients with severe disease develop symptoms [1]. The overall mortality for AVR in the UK is 2.4%; however, this figure rises to over 5% in patients >80 years and as high as 6.8% in elderly females who have excessive left ventricular (LV) hypertrophy [2, 3]. Indeed, the perioperative risk associated with AVR varies significantly from patient to patient, which can make the prediction of adverse events problematic. Clinical risk prediction tools have been developed, but these have been derived from clinical databases compiled predominantly of patients with coronary artery disease, and therefore may be of limited value to patients undergoing AVR.
In AS, the left ventricle hypertrophies in response to an increased afterload and while this initially restores wall stress, ultimately hypertrophy is believed to be maladaptive [4, 5]. Myocardial fibrosis occurs within the hypertrophied myocardium as part of this process and can be detected non-invasively using cardiovascular magnetic resonancing (CMR) and the late gadolinium enhancement (LGE) technique [6, 7]. The LGE also detects regions of prior myocardial infarction, with both patterns associated with an adverse prognosis in a range of conditions, including AS [8].
The aim of this study was to assess whether mid-wall and infarct patterns of LGE might also act as a marker of increased perioperative complications around the time of AVR.
MATERIALS AND METHODS
Patient population
We have previously reported a prospective, registry-based study of 143 patients with moderate/severe AS who underwent CMR with LGE [8]. All patients from this cohort who underwent AVR at the Royal Brompton Hospital between January 2003 and October 2009 and within 1 year of their scan were included in the present study. In our institution, we recommend CMR for all patients as a preoperative assessment prior to AVR. Exclusion criteria were disseminated by malignancy; moderate or severe aortic regurgitation, mitral regurgitation or mitral stenosis; contraindications to CMR, including pacemaker and defibrillator implantation and an estimated glomerular filtration rate of <30 ml/min. The study was conducted after obtaining approval from the local research ethics committee in 2003 and in accordance with the Declaration of Helsinki 1964.
Baseline data collection
Past medical history was documented from source patient record data. All patients underwent coronary angiography prior to surgery. The presence of coronary artery disease was defined by a significant coronary artery stenosis (>50% lumen diameter narrowing) and/or a previous history of myocardial infarction or revascularization.
CMR imaging
Cardiac imaging was performed with a 1.5-Tesla magnetic resonance scanner (Magnetom Sonato or Avanto, Siemens, Germany) using steady-state free precession sequences for aortic valve planimetry and for the assessment of LV volumes and mass. Ten to 15 min after the injection of 0.1 mmol/kg gadolinium contrast (Gd-DTPA; Schering, Germany), inversion-recovery-prepared spoiled gradient echo images were acquired in standard long and short-axis views to detect the areas of LGE as described previously [9].
Image analysis
The severity of AS was assessed using CMR-derived planimetry of aortic valve area according to the American Heart Association/American College of Cardiology criteria [1]. This technique has been validated against echocardiographic measures of AS severity [10]. The LV mass was calculated from the total end-diastolic myocardial volume multiplied by the specific gravity of the myocardium (1.05 g/ml). The presence and pattern of LGE was assessed by two independent observers, blinded to all clinical data. The LGE mass was calculated semi-automatically by a single operator using MRI-mass software as previously described (Medis, Netherlands) [8].
Clinical endpoints
The primary endpoint of the study was pre-specified as 30-day perioperative, major adverse, cardiac and cerebrovascular events (MACCE: death, cerebrovascular accident [CVA], myocardial infarction). Data were also collected with respect to other perioperative complications, including significant heart block (defined as new third-degree AV block or the need for ventricular pacing), supraventricular tachyarrhythmia, ventricular arrhythmia, pericardial tamponade, renal replacement therapy, requirement for tracheostomy and the need to return to theatre. Long-term mortality data were obtained from the National Strategic Tracing Service.
Statistical analysis
Continuous variables were assessed for normal distribution using the Shapiro–Wilk test. Parametric data were expressed as mean ± SD and compared using a one-way analysis of variance or an unpaired Student's t-test where appropriate. Non-parametric data were expressed as the median ± interquartile range (IQR) and compared using the Mann–Whitney or Krusak–Wallis tests. Categorical variables were expressed as percentages and analysed using Fisher's exact test or χ²-test depending on the frequencies of the variable in question. All analyses were performed using SPSS software (version 18; SPSS Inc., Chicago, IL, USA). A two-sided P < 0.05 was regarded as statistically significant. A combined complication score was derived for each pattern of LGE by addition of each of the complications and subsequent division by the number of subjects in that group.
RESULTS
Of the original 143 patients, 63 underwent AVR at the Royal Brompton Hospital within 1 year of their CMR scan and were enrolled into this study. Nine patients underwent AVR at other institutions, for whom perioperative data were not available.
Patterns of LGE
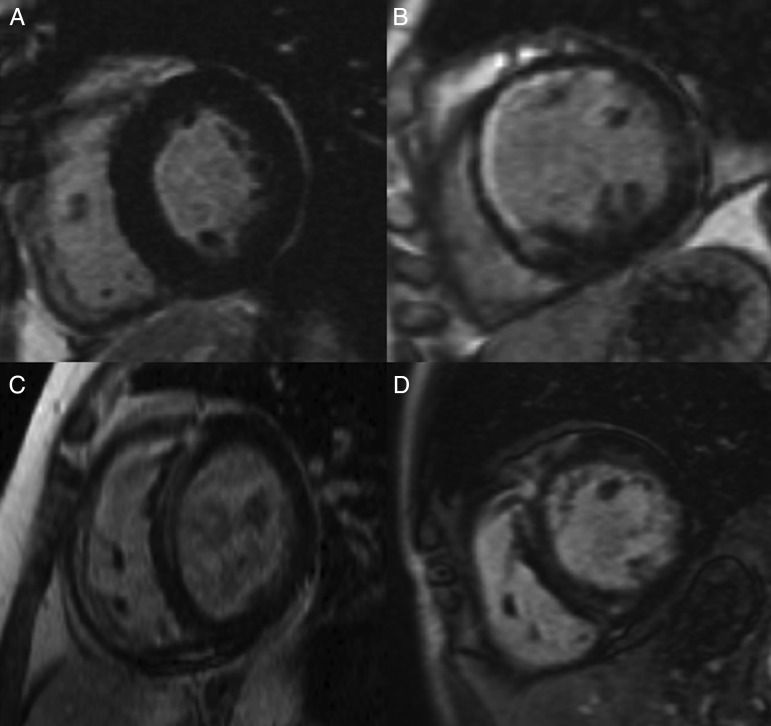
Three patterns of LGE were observed: no gadolinium enhancement (no LGE group); localized enhancement consistent with prior myocardial infarction (infarct LGE group); a mid-wall pattern of enhancement (mid-wall LGE group) (Fig. 1). Inter-observer agreement in determining the pattern of LGE in this cohort has previously been reported with a κ value of 0.89 [8]. Twenty-five (40%) patients had no LGE; 20 (32%) patients had mid-wall LGE and 18 (28%) had an infarct pattern (Table 1).
Figure 1:
Examples of the different patterns of LGE on steady-state free procession images of the left ventricle in end-diastole. (A) Short axis view of the left ventricle in a patient with no LGE. (B) Infarct pattern of LGE with subendocardial enhancement of the antero-septum and anterior wall. (C and D) Linear mid-wall fibrosis predominantly affecting the LV septum (mid-wall LGE). The patient in (C) went on to develop heart block perioperatively.
Table 1:
Baseline characteristics of the different patterns of LGE in 63 patients who underwent aortic vale replacement
| No LGE | Mid-wall LGE | Infarct pattern LGE | P-value | |
|---|---|---|---|---|
| Number | 25 | 20 | 18 | |
| Age | 73 (60–77) | 71 (59–80) | 73 (67–77) | 0.83 |
| Male gender % | 60 | 80 | 89 | 0.080 |
| Comorbidity [n (%)] | ||||
| Coronary artery disease | 10 (40) | 9 (45) | 18 (100) | <0.001* |
| Hypertension | 13 (52) | 15 (75) | 11 (61) | 0.29 |
| Diabetes mellitus | 8 (32) | 6 (30) | 6 (33) | 0.985 |
| Hypercholesterolaemia | 13 (52) | 7 (35) | 10 (56) | 0.38 |
| Active smoking | 1 (4) | 3 (15) | 5 (29) | 0.10 |
| Chronic kidney disease ≥3 | 1 (4) | 4 (20) | 5 (28) | 0.081 |
| Previous CVA | 3 (12) | 0 (0) | 7 (39) | 0.003* |
| AF | 7 (28) | 3 (15) | 2 (11) | 0.37 |
| Left bundle branch block | 4 (16) | 0 (0) | 2 (11) | 0.20 |
| Right bundle branch block | 1 (4) | 2 (10) | 4 (22) | 0.21 |
| Medication [n (%)] | ||||
| ACEi/ARB | 13 (52) | 9 (45) | 10 (56) | 0.80 |
| Beta-blocker | 15 (60) | 7 (35) | 7 (39) | 0.19 |
| Statin | 15 (60) | 14 (70) | 16 (89) | 0.12 |
| Diuretic therapy | 11 (44) | 11 (55) | 8 (44) | 0.73 |
| CMR scan | ||||
| Indexed LV volume | 76 (67–100) | 91 (66–109) | 91 (78–113) | 0.22 |
| Indexed LV mass | 99 ± 27 | 113 ± 33 | 104 ± 25 | 0.26 |
| Ejection fraction | 64 ± 16 | 55 ± 20 | 52 ± 15 | 0.046* |
| Aortic valve area | 0.87 ± 0.30 | 0.95 ± 0.29 | 0.86 ± 0.19 | 0.55 |
| Bicuspid valve | 8 (32%) | 6 (30%) | 3 (17%) | 0.52 |
| % LGE mass | 0 (0–0) | 3.5 (1.9–4.6) | 5.8 (3.1–10.2) | <0.001* |
Parametric data presented as mean ± SD; non-parametric data as median (25th–75th IQR).
AF: atrial fibrillation; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; CABG: coronary artery bypass graft operation; CVA: cerebrovascular accident; LGE: late gadolinium enhancement; LV: left ventricular.
*Denotes statistical significance (P < 0.05).
Patients were well matched across the patterns of LGE in terms of age, sex, aortic valve severity and comorbidity with the following exceptions. Coronary artery disease was assessed in all patients by invasive coronary angiography, and its prevalence was highest in those in the infarct LGE group (P < 0.001) and as was the proportion of subjects with previous CVA (P = 0.003). Patients in the no LGE group had higher ejection fractions than those in other groups (P = 0.046, Table 1).
Operative details
Twenty-three patients required concomitant coronary artery bypass graft (CABG) at the time of AVR. For 13 subjects, the operation represented a repeat sternotomy (Table 2). The infarct group had the highest incidence of concomitant CABG (61% P = 0.024) and of prior sternotomy (39% P = 0.038).
Table 2:
Operative data, 30-day perioperative complications, and 1 and 2 year mortality for patients undergoing AVR according to their pattern of LGE
| No LGE (n=25) | Mid-wall LGE (n=20) | Infarct LGE (n=18) | P-value | |
|---|---|---|---|---|
| Operative details [n (%)] | ||||
| AVR + CABG | 5 (20) | 7 (35) | 11 (61) | 0.024* |
| Previous sternotomy | 5 (20) | 1 (5) | 7 (39) | 0.038* |
| 30-day perioperative complications [n (%)] | ||||
| Back to theatre | 2 (8) | 3 (15) | 1 (6) | 0.65 |
| Tamponade | 1 (4) | 2 (10) | 1 (6) | 0.82 |
| Need for IABP | 2 (8) | 2 (10) | 1 (6) | 1.00 |
| Tracheostomy | 1 (4) | 2 (10) | 3 (17) | 0.36 |
| Renal replacement therapy | 3 (12) | 2 (10) | 1 (6) | 0.87 |
| Heart block | 1 (4) | 6 (30) | 2 (12) | 0.050* |
| Ventricular tachycardia | 3 (12) | 0 (0) | 5 (28) | 0.059 |
| Atrial fibrillation | 7 (28) | 5 (25) | 7 (39) | 0.61 |
| Total complication score (mean number of complications per patient) | 0.76 | 1.35 | 1.17 | 0.171 |
| 30-day MACCE | 0 (0%) | 5 (25%) | 1 (5%) | 0.014* |
| Death | 0 (0%) | 1 (5%) | 1 (5%) | 0.51 |
| Cerebrovascular accident | 0 (0%) | 4 (20%) | 0 (0%) | 0.013* |
| Myocardial infarction | 0 (0%) | 0 (0%) | 0 (0%) | |
| Long-term follow-up | ||||
| 1 year mortality | 0 | 2 (10%) | 2 (11%) | 0.23 |
| 2 year mortality | 0 | 3 (15%) | 2 (11%) | 0.11 |
Parametric data presented as mean ± SD; non-parametric data as median (25th–75th IQR).
AVR: aortic valve replacement; CABG: coronary artery bypass graft operation; CVA: cerebrovascular accident; IABP: intra-aortic balloon pump; TIA: transient ischaemic attack; MACCE: major adverse cardiac and cerebrovascular events; LGE: late gadolinium enhancement.
*Denotes statistical significance (P < 0.05).
While numerically more patients in the mid-wall group had concomitant CABG compared with the no LGE group (35 vs. 20%; P = 0.32), this was balanced by an increased number of repeat sternotomies in the latter (20 vs. 5%; P = 0.20).
30-day MACCE
Thirty-day MACCE was higher in the mid-wall LGE groups (P = 0.014) compared with the other groups. This occurred despite this group including the lowest number of patients undergoing repeat sternotomy (P = 0.038) and was predominantly driven an increased incidence of CVA, which also reached statistical significance (P = 0.013). Two perioperative deaths occurred, with one in the infarct group and one in the mid-wall group. No patient in the no LGE group suffered a MACCE event. No perioperative myocardial infarctions were observed in our cohort (Table 2).
Other perioperative complications
The incidence of heart block was three times higher in the mid-wall group (30%) than in the other two groups (P = 0.050) and required pacing in half of these patients. There was also a trend to an increased incidence of ventricular tachycardia in the infarct group (P = 0.059, Table 2). Otherwise, there were no significant differences in the perioperative outcomes between the different patterns of LGE. While the perioperative complication score in the mid-wall group was double that in the no LGE group and numerically higher than that in the infarct group, these differences did not reach statistical significance (P = 0.171, Table 2, Fig. 2).
Figure 2:
Comparison of 30-day perioperative complications following AVR for AS according to the pattern of LGE. The incidences of MACCE, cerebrovascular events, heart block and the perioperative complication score are expressed as the number of events per patient for each pattern of LGE. The incidences of MACCE, CVA and heart block were significantly higher in the mid-wall group compared with the other patterns of LGE.
Long-term mortality
Patients were followed up for an average of 2.0 ± 1.4 years (median 1.7 years). Five deaths occurred after 2 years of follow-up: three in the mid-wall group and two in the infarct group, with no deaths observed in the no LGE group.
DISCUSSION
This is the first study to examine the impact of mid-wall LGE on perioperative outcomes following AVR. We have demonstrated that the incidence of 30-day MACCE, CVA and heart block were all significantly higher in patients with this pattern of LGE compared with those with no enhancement or indeed an infarct pattern. By contrast, patients with no LGE appear to have particularly good postoperative outcomes with no 30-day MACCE events and no patient deaths at 2 years. These preliminary data therefore suggest that the pattern of myocardial LGE may be a useful clinical tool in predicting risk prior to AVR.
The LGE has been associated with an adverse prognosis in a number of conditions, including dilated cardiomyopathy, myocardial infarction and hypertrophic cardiomyopathy [9, 11, 12]. We have previously demonstrated a 7-fold increase in mortality in AS patients with mid-wall LGE compared with those with no enhancement [8]. In the present study, we have examined a subset of patients from this original cohort who went on to have AVR. We have demonstrated that mid-wall LGE is associated with increased 30-day perioperative MACCE, despite this group including the least number of patients undergoing repeat sternotomy and less patients undergoing concomitant CABG than in the infarct group. The increased MAACE rates appear to be predominantly driven by an increased incidence of cerebrovascular events; however, a signal to an increased death rate could also be observed in those with mid-wall and infarct patterns of LGE compared with the no LGE group at 30 days, 1 and 2 years. This is likely to reflect a combination of impaired LV function and increased arrhythmogenicity as has been previously hypothesized [8, 13]. The explanation for the increased incidence of CVA in this study is less clear but may be related to diastolic impairment and atrial arrhythmogenicity increasing the propensity to atrial thrombus formation and consequent perioperative stroke.
The incidence of the other perioperative complications examined in this study was similar across the patterns of LGE, with the exception of heart block, which was again highest in those with mid-wall fibrosis. Heart block is a common and important complication associated with AS and aortic valve surgery. It is caused by fibrosis of the AV node and His-Purkinje network, and it is therefore unsurprising that it should be associated with myocardial fibrosis detected by LGE. While this study is the first to establish an association between mid-wall LGE and heart block, it might also apply to other patient populations in which myocardial and conductive system fibrosis are also observed.
It has been postulated that the presence of mycardial fibrosis might act as an early marker of decompensated LV hypertrophy [14]. Our data indicate that AVR may be better performed before the onset of this decompensation, as detected by the mid-wall LGE. If our preliminary findings are confirmed, this could potentially have important implications for the timing of AVR in AS. However, we would not suggest that surgery should be avoided in those with mid-wall fibrosis. Indeed in the parent study of 143 patients, those with mid-wall LGE who subsequently underwent AVR were four times less likely to die than those who did not despite being well matched in terms of AS severity and comorbidity [8].
Limitations
While we have observed numerical trends, our study was not powered to detect statistical differences in mortality between the different patterns of LGE. Furthermore, despite achieving statistical significance, the MACCE rate was low, such that we have not been able to assess the impact of ejection fraction, concomitant CABG or other predictors of perioperative complications. Our data are therefore preliminary and can only be considered as hypothesis-generating. Nevertheless, they provide a strong rationale for large-scale multicentre studies aimed at providing definitive evidence for the role of LGE in predicting perioperative complications.
Conclusions
The mid-wall LGE was associated with increased rates of MACCE, CVA and heart block 30 days following AVR. By contrast, patients with no LGE appear to have particularly good postoperative outcomes with no MACCE events at 30 days and no patient deaths at 2 years. These preliminary data suggest that the pattern of myocardial LGE may be an important factor in predicting risk prior to AVR, and this now needs to be confirmed in large-scale multicentre trials.
FUNDING
This research was supported by the National Institutes of Health Research Cardiovascular Biomedical Research Unit, a collaboration between Royal Brompton Hospital and Imperial College. Support was also received from the British Heart Foundation and CORDA. Mark Dweck is supported by a British Heart Foundation Clinical PhD Training Fellowship (FS/10/026).
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr M. Kolowca (Rzeszow, Poland): You presented probably the biggest cohort of patients with significant aortic stenosis assessed by cardiac magnetic resonance with gadolinium. First question: did you include or exclude the papillary muscle in the assessment of LGE mass and did you find any mid-wall fibrosis in the papillary muscles? It is important, because mid-wall fibrosis could be one of the mechanisms of mitral regurgitation worsening survival in this group of patients. Did you find any mitral regurgitation due to this process?
And my second question is, did you try to correlate the value of the LGE mass with the end points? If you do this kind of correlation, you can find exactly the cutoff point above which you could say there is a significant higher odds ratio for MACE, and this cutoff point could be significant in the decision-making process.
Dr Quarto: About the first question, yes, obviously we studied myocardial fibrosis also in papillary muscle but, as I said, in this group only patients with aortic stenosis plus or minus coronary artery disease have been included. Therefore if there was any mitral regurgitation, the patient was excluded by the study, and therefore I can't be very helpful.
Dr Kolowca: Did you include the papillary muscle in the LGE mass analysis?
Dr Quarto: Yes, we did, but in these patients there was no mitral regurgitation at all, even mild.
Dr Kolowca: And what about fibrosis in the papillary muscle, that was present or absent?
Dr Quarto: We didn't find a high incidence of fibrosis of the papillary muscle. Obviously if there was a strong fibrosis of the papillary muscle, definitely we would find mitral regurgitation. Regarding the second point, we found out that the incidence of a worse prognosis for myocardial fibrosis increased after 10% of LGE mass inside the left ventricle.
Dr M. Cikirikcioglu (Geneva, Switzerland): It is a very interesting study and it is a very active field to add some additional risk assessments for aortic valve surgery. But if I understand well, your take-home message is if we perform that kind of imaging before the operation, we can calculate better the high-risk patients. But on the other hand, it is a little bit of a time-consuming and expensive method for all-comers.
The first question: do you advise the use of that technique for all patients before aortic valve surgery? And the second question is related to the image which you have shown us. The infarcted pattern patients had a dilated left ventricle. Is there any correlation between the infarcted pattern and left ventricle dimensions, which we can measure with the echocardiographic studies easily? If there is a correlation and the infarcted pattern patients had dilated ventricles, we can easily measure the diameter and therefore we can exclude this proposed new method.
Dr Quarto: I will try to answer your questions as one. Definitely do these tests on all the patients as I believe it will be better for the assessment of these patients. But I understand that there are three major issues. The first thing, it is time-consuming and cost-consuming; the second thing is the availability of the cardiac magnetic resonance in all the centres - it is not always available; and the third point is the potential unsuitability of the patients having these tests, because obviously patients with a pacemaker or patients who are claustrophobic, for example, cannot undergo this test. Therefore at the moment, in the literature I couldn't find any better noninvasive test for diagnosis of the mid-wall fibrosis. The 3D echo is the second test that we can use to identify these kinds of patients, but it is not as specific as MRI.
For the cohort of ischaemic patients, as I said, it is not the main point of the study. These patients we know go badly because they are infarcted patients, they have low ejection fraction, big end-diastolic volume. The interesting thing about this paper is more that the patients with quite normal left ventricular function, good ejection fraction in the mid-wall, have a worse outcome than the infarct group.
Dr Cikirikcioglu: And normal left ventricle volume also?
Dr Quarto: Volume but not index. If I can show the picture, this is the volume of the mid-wall LGE. There is no difference. There is a difference between the infarcted group and no LGE but there is no statistically significant difference between the no LGE and the mid-wall group.
Dr M. Glauber (Massa, Italy): Just a technical aspect. You evaluated the angiograms before or after the MRI?
Dr Quarto: All the patients were investigated with angiogram just after the MRI.
REFERENCES
- 1.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. Acc/aha 2006 guidelines for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the society of cardiovascular anesthesiologists endorsed by the society for cardiovascular angiography and interventions and the society of thoracic surgeons. J Am Coll Cardiol. 2006;48:e1–148. doi: 10.1016/j.jacc.2006.05.021. doi:10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Bridgewater B, Keogh B, Kinsman R, Walton P. The society for cardio-thoracic surgery in great britain and ireland, sixth national adult cardiac surgical data base report 2008. 2009. pp. 164–73. Dendrite clinical system ltd.
- 3.Morris JJ, Schaff HV, Mullany CJ, Morris PB, Frye RL, Orszulak TA. Gender differences in left ventricular functional response to aortic valve replacement. Circulation. 1994;90:II183–9. [PubMed] [Google Scholar]
- 4.Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97:301–7. doi: 10.1136/hrt.2010.192997. doi:10.1136/hrt.2010.192997. [DOI] [PubMed] [Google Scholar]
- 5.Gosse P. Left ventricular hypertrophy as a predictor of cardiovascular risk. J Hypertens Suppl. 2005;23:S27–33. doi: 10.1097/01.hjh.0000165625.79933.9a. doi:10.1097/01.hjh.0000165625.79933.9a. [DOI] [PubMed] [Google Scholar]
- 6.Debl K, Djavidani B, Buchner S, Lipke C, Nitz W, Feuerbach S, et al. Delayed hyperenhancement in magnetic resonance imaging of left ventricular hypertrophy caused by aortic stenosis and hypertrophic cardiomyopathy: visualisation of focal fibrosis. Heart. 2006;92:1447–51. doi: 10.1136/hrt.2005.079392. doi:10.1136/hrt.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudolph A, Abdel-Aty H, Bohl S, Boye P, Zagrosek A, Dietz R, et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284–91. doi: 10.1016/j.jacc.2008.08.064. doi:10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 8.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–9. doi: 10.1016/j.jacc.2011.03.064. doi:10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 9.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. doi:10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich M, Schulz-Menger J, Dietz R. Magnetic resonance to assess the aortic valve area in aortic stenosis. J Am Coll Cardiol. 2004;43:2148. doi: 10.1016/j.jacc.2004.03.010. author reply 2148–9 doi:10.1016/j.jacc.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 11.O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–74. doi: 10.1016/j.jacc.2010.05.010. doi:10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Kwong RY, Chan A, Brown KA, Chan CW, Reynolds HG, Tsang S, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–43. doi: 10.1161/CIRCULATIONAHA.105.570648. doi:10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 13.Nazarian S. Is ventricular arrhythmia a possible mediator of the association between aortic stenosis-related midwall fibrosis and mortality? J Am Coll Cardiol. 2011;58:1280–2. doi: 10.1016/j.jacc.2011.04.045. doi:10.1016/j.jacc.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 14.Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–91. doi: 10.1161/01.cir.0000051865.66123.b7. doi:10.1161/01.CIR.0000051865.66123.B7. [DOI] [PubMed] [Google Scholar]