Abstract
The anomalous origin of one pulmonary artery branch from the aorta (AOPA) is rare. We report our single-institution surgical experience with this condition. Between January 1994 and February 2011, 17 patients (age: 1 month–25 years) with AOPA underwent surgery at our institute. Thirteen patients had an anomalous origin of the right pulmonary artery (RPA) while four had an anomalous origin of the left pulmonary artery (LPA) from the aorta. In patients with anomalous RPA, 11 patients had the proximal type and two patients had the distal type of AOPA. Four patients had associated Tetralogy of Fallot (TOF). In 14 patients, direct implantation into the main pulmonary artery was performed, while three patients required interpositon of a graft. There was one operative death due to persistent hypoxia in a 7-month old child with TOF and an anomalous LPA from the aorta. At a median follow-up of 36.5 months (range: 2–192 months), all 16 survivors were asymptomatic. On echocardiography, two patients showed a gradient of 25 and 30 mmHg across the anastomosis and are being followed up. In our experience, early repair of AOPA results in acceptable haemodynamic and anatomic results. Long-term survival can be expected with a low incidence of re-operation or re-intervention.
Keywords: Congenital heart disease, Anomalous pulmonary artery, Hemitruncus
INTRODUCTION
The anomalous origin of a branch pulmonary artery from the aorta (AOPA), or hemitruncus, is a rare congenital cardiac anomaly characterized by the anomalous origin of one of the branch pulmonary arteries (PA) from the ascending aorta and a normal origin of the other PA from the right ventricular outflow tract (RVOT) in the presence of two semilunar valves. The lung connected to the normally arising PA receives the entire cardiac output from the right ventricle, while the other lung is exposed to both pressure and volume overload due to unrestricted shunting from the aorta. Untreated, pulmonary vascular obstructive disease develops rapidly and 1-year survival may be as low as 30%. Surgical management early in life has improved the outcomes in these patients [1]. We summarize our experience at a tertiary level institution with this condition over a 17-year period.
PATIENTS AND METHODS
Between January 1994 and February 2011, 17 patients with AOPA underwent surgery at the All India Institute of Medical Sciences, New Delhi, India. The study protocol was approved by the ethics committee. Clinical history and preoperative investigations were reviewed. Echocardiographic data were obtained regarding the site of the origin of the anomalous PA, additional defects, biventricular function and estimated pulmonary artery pressures. Intraoperative and intensive care unit (ICU) charts were reviewed for the surgical procedure and details of the cardiopulmonary bypass (CPB). Postoperative data included survival, ventilatory and inotropic support, and the duration of the hospital stay. After discharge from the hospital, the patients were seen in the outpatient department at 1 week, 1 month, 3 months, 6 months and then yearly intervals. Follow-up records were specifically reviewed for surgical complications and any re-interventions.
RESULTS
Of the 17 patients, 11 were male. The median age was 7 months (1 month– 25 years), and the median weight was 5.5 kg (2.9–40 kg). We classified this condition as follows (Tables 1 and 2).
Table 1:
Classification of the patients undergoing repair of the anomalous origin of the pulmonary artery from the aorta
| Group | Definition |
|---|---|
| Group I | AOPA without RVOT obstruction |
| Type 1 | Anomalous origin of RPA |
| 1a | Anomalous origin of RPA from the proximal ascending aorta |
| 1b | Anomalous origin of RPA from the distal ascending aorta near the origin of the innominate artery or origin from the right innominate artery as trifurcation |
| Type 2 | Anomalous origin of LPA from the ascending aorta |
| Group II | AOPA with RVOT obstruction |
| Type 1 | Anomalous origin of RPA |
| 1a | Anomalous origin of RPA from the proximal ascending aorta |
| 1b | Anomalous origin of RPA from the distal ascending aorta near the origin of the innominate artery or origin from the right innominate artery as trifurcation |
| Type 2 | Anomalous origin of LPA from the ascending aorta |
AOPA: anomalous origin of branch pulmonary artery; RPA: right pulmonary artery; LPA: left pulmonary artery.
Table 2:
Preoperative demographic and clinical characteristics, surgical management, early and late postoperative outcome
| Patient | Age (years) | Sex | Weight (kg) | PAH | CHF | Preoperative saturation (%) | PA pressure in AOPA (mmHg) | PA pressure in normal PA (mmHg) | Arch | Anomalous branch | Associated anomaly | Surgical procedure | Additional surgical procedure | CPB | Post-op complication | Outcome | Follow-up (months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group I | ||||||||||||||||||
| Type 1a | 1 | 4/12 | Male | 3.5 | + | + | 95 | 55/30 | 30 (FU no PAH) | Left | RPA | PDA | Direct implantation | PDA ligation | No | No | Survived | 30 |
| 2 | 1/12 | Male | 2.9 | + | + | 96 | NA | 85 (FU no PAH) | Left | RPA | PDA | Direct implantation | PDA ligation | Yes | No | Survived | 25 | |
| 3 | 3/12 | Male | 3.0 | + | + | 95 | NA | 85 (FU no PAH) | Left | RPA | PDA | Direct implantation | PDA ligation | Yes | No | Survived | 25 | |
| 4 | 11/12 | Female | 6.0 | + | + | 100 | 95/55 | 90/35 | Left | RPA | ASD severe TR | Direct implantation | ASD closure and tricuspid repair | Yes | No | Survived | 22 | |
| 5 | 5/12 | Male | 4.7 | + | + | 98 | 110/60 | 110 | Left | RPA | PDA | Direct implantation | PDA ligation | Yes | No | Survived | 156 | |
| 6 | 8/12 | Male | 5.0 | + | + | 97 | NA | 80 (post-op 2 mth severe PAH) | Left | RPA | – | Direct implantation | – | Yes | No | Survived | 120 | |
| 7 | 7/12 | Female | 5.5 | + | + | 95 | 95/40 | 100/40 (PVRI 6.23) | Left | RPA | – | Homograft | – | No | No | Survived | 71 | |
| 8 | 4/12 | Male | 5.5 | + | + | 100 | 100/60 | 130/70 (PVRI 22) | Left | RPA | PDA, TR | Direct implantation | PDA ligation and tricuspid repair | Yes | No | Survived | 73 | |
| 9 | 8 | Female | 16 | + | – | 94 | 130/70 | 110/70 (PVRI 7) | Left | RPA | PDA, small VSD | Direct implantation | PDA ligation | Yes | No | Survived | 174 | |
| Type 1b | 10 | 25 | Female | 40 | – | – | 55 | Could not enter | 100 | Left | RPA | ASD | Dacron graft | – | No | No | Survived | 10 |
| Type 2 | 11 | 1 | Male | 3.5 | + | + | 100 | 95/58 | 90/50 | Left | LPA | VSD, ASD | Direct implantation | VSD closure and ASD closure | Yes | PAH crisis, long ventilation | Survived | 2 |
| Group II | ||||||||||||||||||
| Type 1a | 12 | 18/12 | Male | 7 | – | – | 89 | 86/40 | Left | RPA | TOF | Direct implantation | VSD closure+ pericardial patch augmentation | Yes | No | Survived | 46 | |
| 13 | 7 | Male | 20 | – | – | 91 | 130/80 | 44/22 | Right | RPA | TOF | Direct implantation | VSD closure+ transannular pericardial patch augmentation | Yes | No | Survived | 166 | |
| Type 1b | 14 | 3 | Male | 12 | – | – | 66 | Could not enter | 29 | Left | RPA | TOF | Saphenous vein graft | VSD closure+ transannular monocusp pulmonary homograft | Yes | No | Survived | 1 |
| Type 2 | 15 | 7/12 | Male | 5.8 | – | – | 63 | 100/30 | Small size | Left | LPA | TOF | Direct implantation | Innominate- RPA shunt | No | No | Died | – |
| 16 | 6 | Female | 19.5 | – | – | 84 | 110/56 | 66/34 | Left | LPA | TOF | Direct implantation | VSD closure+ pericardial patch augmentation of MPA | Yes | No | Survived | 2 | |
| 17 | 13 | Female | 40 | – | – | 90 | 104/60 | 30/17 | Right | LPA | TOF | Direct implantation | VSD closure+ transannular pericardial patch augmentation | Yes | No | Survived | 40 | |
PAH: pulmonary arterial hypertension; CHF: congestive heart failure; CPB: cardiopulmonary bypass; RPA: right pulmonary artery; LPA: left pulmonary artery; PDA: patent ductus arteriosus; ASD: atrial septal defect; VSD: ventricular septal defect; TOF: Tetralogy of Fallot; TR: tricuspid regurgitation; MPA: main pulmonary artery; PAH: pulmonary artery hypertension; NA: not available.
Group I comprised of patients without RVOT obstruction (n = 11) and Group II comprised of those with RVOT obstruction (n = 6). In Group I, two patients had isolated AOPA and nine had additional left-to-right shunt. Nine patients in Group I with type 1a and type 2 origins presented with recurrent respiratory infection, congestive cardiac failure and failure to thrive within the first year of life. Patient No. 9 initially presented at 2 months of age with failure to thrive and was then lost to follow-up. She again presented at 8 years of age with dyspnoea on exertion NYHA class II. Echocardiography at this visit revealed AOPA with patent ductus arteriosus (PDA), small ventricular septal defect (VSD) and severe pulmonary arterial hypertension (PAH). One patient had an anomalous right coronary artery from the non-coronary sinus with a hypoplastic left lung. An anomalous right pulmonary artery (RPA) from the aorta was compressing the left bronchus of this patient. One patient with type 1b origin was found to have a cardiac murmur on routine physical examination and on echocardiography, and was diagnosed to have AOPA with an atrial septal defect. In Group II, all six patients had cyanosis but no congestive cardiac failure. Except one patient, all patients in this group presented beyond 1 year of age. Patient No. 15 presented at 7 months of age with cyanosis and recurrent cyanotic spells.
Radiographic findings
These differed in the two groups. In Group II, there was pulmonary oligaemia on the side normally connected to the RVOT, whereas the lung receiving the PA from the aorta showed pulmonary plethora (Fig. 1). In Group I, there was bilateral pulmonary plethora that was more marked on the side of the AOPA (Fig. 2) in all except one patient who had an anomalous RPA from the aorta with an ASD (Patient 10). In this patient, the left lung was Eisenmengerized and hence oligaemic, while the right lung also appeared oligaemic because of tight stenosis at the RPA origin.
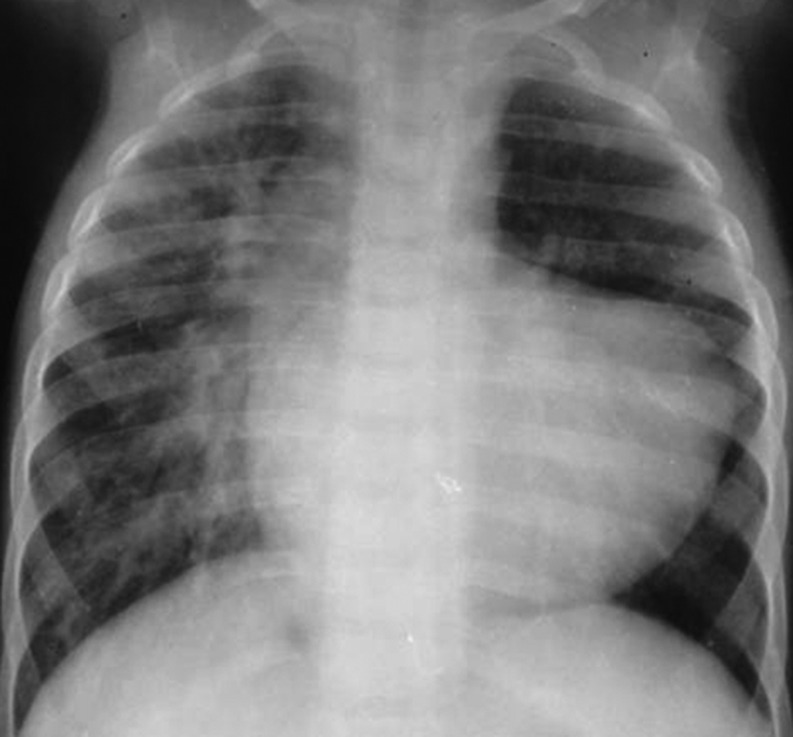
Figure 1:
Preoperative chest X-ray of Patient No. 12 shows cardiomegaly with hypervascularity of the right lung with features of pulmonary arterial hypertension. The left lung is hypovascular.
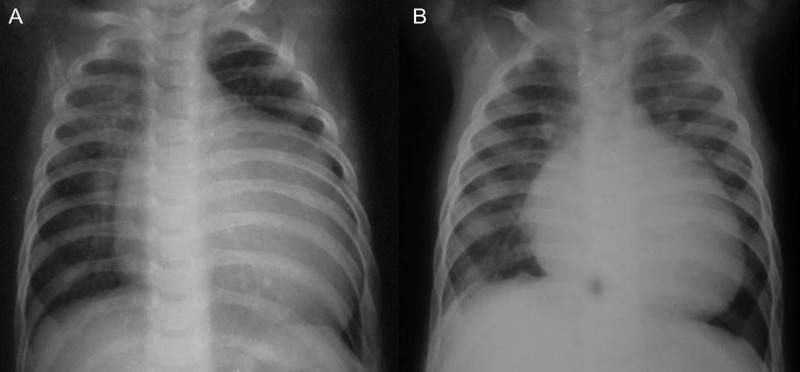
Figure 2:
Preoperative and postoperative chest X-rays of Patient No. 6. (A) The preoperative chest X-ray shows an increased vascularity of the right lung compared with the left lung with cardiomegaly. (B) The postoperative chest X-ray shows a decrease in heart size with normal vascularity of both the lungs.
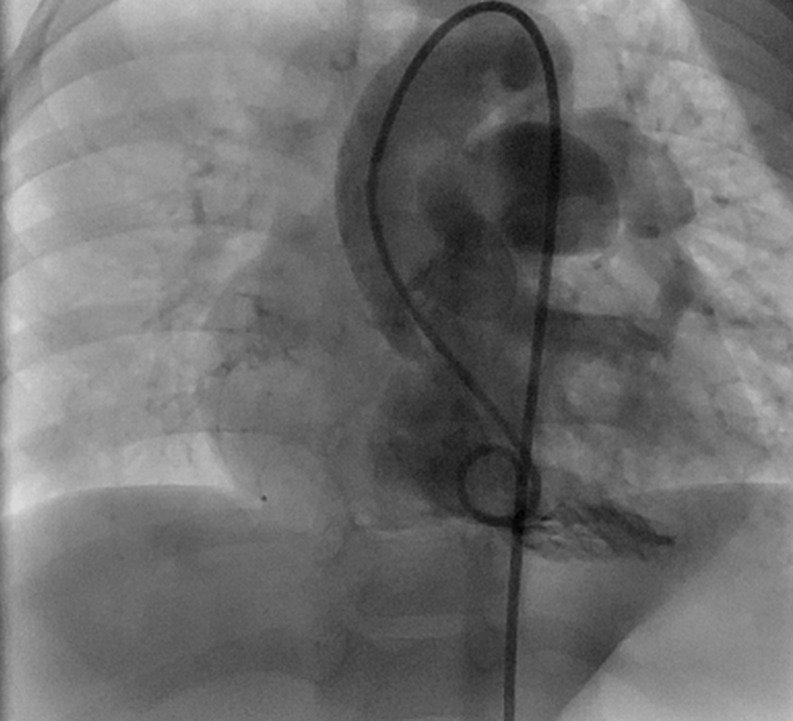
Echocardiography was confirmatory in 10 patients. In six patients, additional cardiac catheterization (Figs 3–5) and/or CT angiography (Fig. 6) was required for accurate delineation. Patient No. 14 was operated on with a preoperative diagnosis of Tetralogy of Fallot (TOF) with RPA atresia. At operation, a small stump of RPA was seen arising from the ascending aorta near the origin of the innominate artery with ∼2 cm intervening atretic segment with reformation as a 4-mm RPA at the hilum.
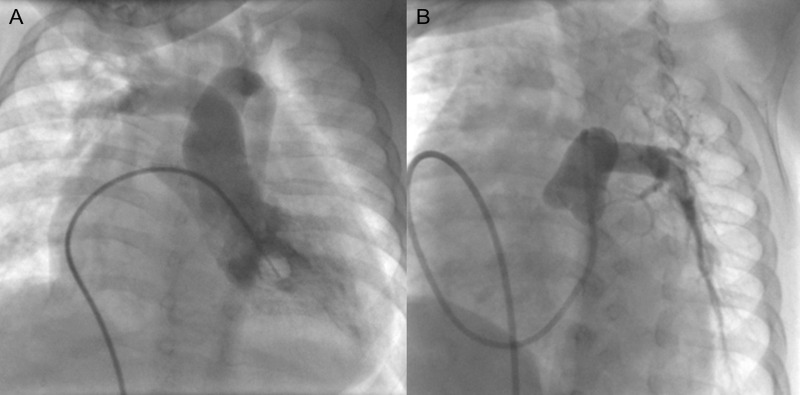
Figure 3:
Cine-angiograms of Patient No. 9 show (A) the right pulmonary artery arising from the ascending aorta and (B) the left pulmonary artery arising normally from the right ventricle.
Figure 4:
Cine-angiograms of Patient No. 15 with hemitruncus and Tetralogy of Fallot showing (A) left pulmonary artey arising from the aorta and (B) a hypoplastic right pulmonary artery arising from the right ventricle.
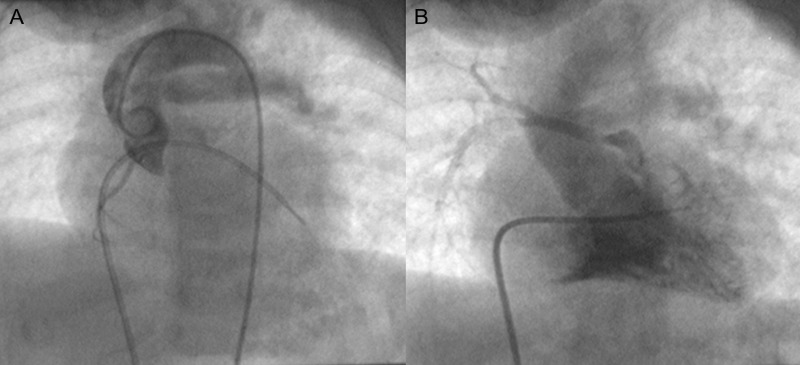
Figure 5:
A cine-angiogram of Patient No. 14 with hemitruncus and Tetralogy of Fallot showing the left pulmonary artery arising from the right ventricle. The right pulmonary artery could not be entered and was visualized only on CT angiogram.
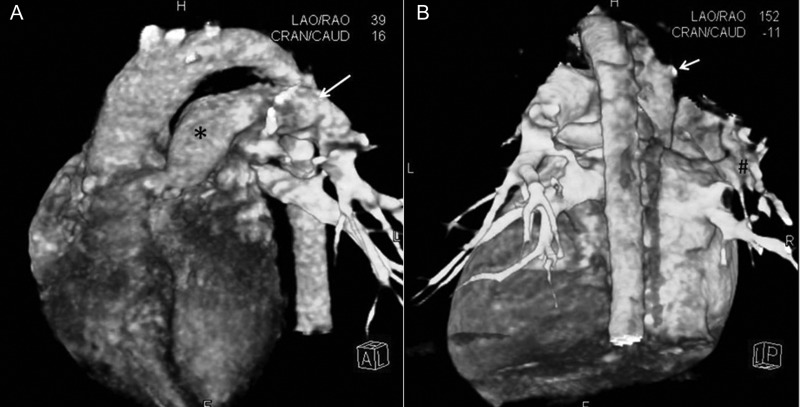
Figure 6:
CT angiograms of Patient No. 14. (A) Volume rendered (VR) reconstruction of the CT angiogram (CTA) in a left anterior oblique and cranial angulation, showing the main (*) and the left pulmonary artery (arrow). (B) VR reconstruction of CTA from a posterior vantage, showing the stump (arrow) of the occluded right pulmonary artery (RPA) arising from the ascending aorta (AA). # indicates the reformed distal RPA.
Morphology
Thirteen patients had an anomalous RPA origin, while four patients had an anomalous left pulmonary artery (LPA) origin. Eleven patients had type 1a AOPA with or without RVOT obstruction. In 10 of these, an anomalous RPA originated from the posterior surface of the ascending aorta, while in one patient it originated from the left postero-lateral surface. Two patients had type 1b AOPA, one each in Groups I and II. In both patients, the RPA originated from the distal ascending aorta at the base of the origin of the innominate artery. In both, there was a small patent stump of the RPA from the aorta with an atretic intervening segment with reformation at the hilum of the lung. Four patients had an anomalous origin of LPA from the left lateral surface of the ascending aorta. In one patient, anomalous LPA originated from the left lateral surface of the ascending aorta with an apparently inseparable plane/common intervening wall between the MPA and the LPA.
Surgical approach
Repair was performed through a median sternotomy. Surgical procedures were the division of AOPA and direct implantation into the MPA (n = 14), implantation with Dacron interposition graft (n = 1) and implantation with homograft interposition (n = 2). In patients with isolated AOPA, repair was performed without CPB in four patients; in the remaining five patients, normothermic CPB without cardioplegic arrest was used due to haemodynamic instability. If associated cardiac anomalies required simultaneous repair (n = 8), standard CPB with aorto-bicaval cannulation at 28°C was used. In patients operated on without CPB, the MPA, branch PA and aorta were mobilized. The anomalous PA was looped. The PDA was divided. The anomalous artery was clamped and divided at its origin from the aorta, and the aortic end was repaired directly. The divided end of the PA was passed under the aorta. The MPA was opened after applying the side-biting clamp followed by an end-to-side anastomosis between the anomalous PA and the MPA using a continuous 6-0 or 7-0 polypropylene suture. A total of 13 patients were operated on CPB (five with isolated AOPA and eight with associated anomalies). Associated procedures included ASD closure ASD and tricuspid valve repair (n = 1), tricuspid valve repair (n = 1), VSD closure (n = 1) and TOF repair (n = 5).
In Patient No. 15, the innominate artery to the RPA shunt was performed additionally. In Patient No. 10 (reported earlier) [2], an 8-mm Dacron tube graft was interposed between the MPA and hilar RPA. This patient also had an associated ASD, which was left open with a plan to perform cardiac catheterization later with the device closure of the ASD if the pulmonary vascular resistance (PVRI) was to decrease. In Patient No. 14, continuity between the MPA and RPA was established with a homograft saphenous vein graft with ligation of the aortic end of the RPA.
Postoperative complications, intensive care unit and hospital stay
The median duration of the ICU stay was 2 days (1–45 days). All patients received elective inotropic support as needed. Patient No. 11 had pulmonary hypertensive crisis in the early postoperative period requiring prolonged ventilation, tracheostomy and pulmonary vasodilators including nitric oxide. He was discharged from the hospital after 75 days.
There was one hospital death of a 7-month old male with an anomalous LPA from the aorta with TOF with the small MPA and RPA. Direct implantation of LPA to MPA and the innominate artery to the RPA shunt were performed using the 4-mm polytetrafluoroethylene graft. Postoperatively, this patient had persistent hypoxia and hypercarbia despite a patent shunt. There was unexplained bradycardia and cardiac arrest 6 h later. The parents did not consent to an autopsy. In the remaining 15 patients, the postoperative course was uneventful. The median hospital stay was 9 days (8–12 days).
Post-discharge haemodynamic function and re-intervention
The median follow-up was 36.5 months (2–192 months) and was 100% complete. Seven patients had a follow-up of more than 5 years, six had a follow-up of more than 2 years and three had a follow-up of less than 2 years. Presently, 14 patients are in NYHA class I and two are in NYHA class II. None has required re-intervention. Patient No. 9 was eight-year old with type 1a hemitruncus with a large PDA and small VSD with severe PAH at the time of surgery. PDA ligation with RPA implantation on the MPA was performed. The VSD was not closed because of the doubtful reversibility of PAH. Follow-up echocardiography showed small VSD with moderate PAH. Follow-up cardiac catheterization of Patient No. 10 six months later showed good flow to both the lungs. Her systemic saturation was 95%, although right ventricular and PA pressure and PVRI continued to be elevated. The remaining patients had good biventricular function and no significant tricuspid regurgitation. Two patients (Nos. 2 and 4) have a gradient of 30 and 25 mmHg, respectively, across the anastomosis, but because they are asymptomatic, they are being followed up.
DISCUSSION
AOPA accounts for 0.1% [3] of all congental heart diseases. Kutsche and Van Mierop [4], while reviewing 108 cases, proposed that abnormal migration of pleuripotent cells is important in its development. On the left side, this occurs due to failure of development of the left sixth arch leading to the failure of fusion of LPA to MPA and the persistence of the aortic sac from which LPA arises. On the right side, a proximal form of AOPA occurs due to the incomplete migration of the right sixth aortic arch to the left side [4]. Although embryogenesis of the anomalous origin of distal RPA is unclear, a plausible explanation may be the development of the right fifth arch and the disappearance of proximal PA and distal sixth arch. This explains that the narrowing present in anomalous distal RPA does not involve the first part but is located further distally. The anomalous origin of RPA is four to eight times more common than the left [4]. AOPA on the left side is more commonly associated with the right aortic arch. In our series, 13 patients (77%) had an anomalous origin of RPA. The right-sided aortic arch was present in two patients, one with anomalous RPA and another with anomalous LPA.
AOPA may occur in isolation or may be associated with PDA, TOF, aortopulmonary window, ASD, isthmic hypoplasia and interrupted aortic arch [5]. It has also been shown to be associated with DiGeorge syndrome. However, none of our patients had DiGeorge syndrome.
On the basis of the amount of the pulmonary blood flow and clinical presentation, we divided all our patients into two groups. In Group I, patients had increased pulmonary blood flow. These patients usually present within the first 6 months of life with lower respiratory tract infection and congestive heart failure and are prone to develop early PAH [6] due to increased pulmonary blood flow, left ventricular failure, high amount of circulating vasoconstrictors and neurogenic crossover from the unprotected lung [7]. Therefore, early surgery is needed to prevent irreversible PAH. Patients in Group II present relatively late due to RVOT obstruction. Peng et al. [8] have shown relatively poorer long-term outcomes in this group that may be related to the use of a conduit for the repair of RVOT or PA implantation predisposing the patient to re-interventions. If the patients in Group II can be repaired completely without using a prosthetic material, they may have as good long-term outcomes as in Group I. Our patients in both groups are doing equally well.
In both groups, based on the different embryological origin of all three types of AOPA, we classified our patients as mentioned above. In both type 1a and type 2, the anomalous branch PA is large with the dilated ascending aorta. In type 1b, on the contrary, at birth an anomalous distal RPA is dilated along with the dilated ascending aorta. The right lung is normally developed. But as ductal tissue in the anomalous RPA contracts, its size decreases with the severe stenosis or atresia of RPA distal from its origin and patent small RPA at both ends. Our hypothesis is based on observation of two of our patients with type 1b anomaly and a study of eight patients published by Kajihara et al. [9]. In that series, one patient had distal type 1b AOPA. During surgery at 8 days of age, this patient did not have any stenosis of RPA. Division of the RPA from the aorta and end-to-side anastomosis with MPA was performed. However, this patient developed stenosis at the anastomotic site after 1 month. During re-operation, exclusion of the stenotic segment with interposition grafting was performed. A histopathological examination of the stenotic segment revealed the presence of ductal tissue in the stenotic segment. In our series, in Patient Nos. 10 and 14, the right lung was well developed despite the small size of the RPA at the pulmonary hilum and the absence of pulmonary collaterals.
Since the first correction of AOPA by Armer et al. [10], various surgical techniques have been described. These include implantation using a synthetic graft [11–13], autologous pericardial patch, homograft interposition [11–13] and direct implantation [12–14]. Direct implantation is the procedure of choice [13–15] whenever feasible. This obviates the need for repeated re-interventions. In our series, 14 out of 15 patients (11 with type 1a and 4 with type 2) had direct implantation. Only one patient required homograft interposition. In patients with type 1b AOPA, exclusion of the mid segment with graft interposition is better. In our series, out of two patients with type 1b AOPA, a Dacron interposition graft was used in one patient, while a saphenous vein homograft was used in the other. None of these patients has required any re-intervention.
Patients with AOPA treated early in life have been shown to have good short- and long-term outcome [1, 8]. Patients with associated complex cardiac lesions require multiple reinterventions with the poor outcome [8]. Postoperative scintiscans to assess differential perfusion in both the lungs would be ideal to assess these patients. Financial constraints prevented us from performing them for the present study, but we expect to be able to perform these in the future.
CONCLUSION
AOPA is a rare but potentially treatable lesion with an acceptable long-term outcome if operated early in life. Direct pulmonary implantation is the preferred method for type 1a and type 2 AOPA. Repair without CPB in a simple lesion is the treatment of choice.
Conflict of interest: none declared.
References
- 1.Nathan M, Rimmer D, Piercey G, Del Nido PJ, Mayer JE, Bacha EA, et al. Early repair of hemitruncus: excellent early and late outcomes. J Thorac Cardiovasc Surg. 2007;133:1329–35. doi: 10.1016/j.jtcvs.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Talwar S, Kothari SS, Sharma P, Chauhan S, Gulati GS, Choudhary SK, et al. Successful surgical correction of anomalous origin of the right pulmonary artery from the aorta in an adult. J Card Surg. 2011;26:201–16. doi: 10.1111/j.1540-8191.2010.01185.x. [DOI] [PubMed] [Google Scholar]
- 3.Patel RJ, Zakir RM, Sethi V, Patel JN, Apovian J, Alexander JC, et al. Unrepaired Tetralogy of Fallot with right hemitruncus in an adult. Tex Heart Inst J. 2007;34:250–1. [PMC free article] [PubMed] [Google Scholar]
- 4.Kutsche LM, Van Mierop LH. Anomalous origin of a pulmonary artery from the ascending aorta: associated anomalies and pathogenesis. Am J Cardiol. 1988;61:850–6. doi: 10.1016/0002-9149(88)91078-8. [DOI] [PubMed] [Google Scholar]
- 5.Dodo H, Alejos JC, Perloff JK, Laks H, Drinkwater DC, Williams RG. Anomalous origin of the left main pulmonary artery from the ascending aorta associated with DiGeorge syndrome. Am J Cardiol. 1995;75:1294–5. doi: 10.1016/s0002-9149(99)80788-7. [DOI] [PubMed] [Google Scholar]
- 6.Fong LV, Anderson RH, Siewers RD, Trento A, Park SC. Anomalous origin of one pulmonary artery from the ascending aorta: a review of echocardiographic, catheter, and morphological features. Br Heart J. 1989;62:389–95. doi: 10.1136/hrt.62.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prifti E, Bonacchi M, Murzi B, Crucean A, Leacche M, Bernabei M, et al. Anomalous origin of the right pulmonary artery from the ascending aorta. Anomalous origin of the right pulmonary artery from the ascending aorta. J Card Surg. 2004;19:103–12. doi: 10.1111/j.0886-0440.2004.04023.x. [DOI] [PubMed] [Google Scholar]
- 8.Peng EW, Shanmugam G, Macarthur KJ, Pollock JC. Ascending aortic origin of a branch pulmonary artery—surgical management and longterm outcome. Eur J Cardiothorac Surg. 2004;26:762–6. doi: 10.1016/j.ejcts.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Kajihara N, Imoto Y, Sakamoto M, Ochiai Y, Kan-o M, Joo K, et al. Surgical results of anomalous origin of the right pulmonary artery from the ascending aorta including reoperation for infrequent complications. Ann Thorac Surg. 2008;85:1407–11. doi: 10.1016/j.athoracsur.2007.11.081. [DOI] [PubMed] [Google Scholar]
- 10.Armer RM, Shumacker HB, Klatte EC. Origin of the right pulmonary artery from the ascending aorta. Report of a surgically corrected case. Sogo Rinsho. 1961;24:662–8. doi: 10.1161/01.cir.24.3.662. [DOI] [PubMed] [Google Scholar]
- 11.Fucci C, di Carlo DC, Di Donato R, Marino B, Calcaterra G, Martelletti C. Anomalous origin of the right pulmonary artery from the ascending aorta: repair without cardiopulmonary bypass. Int J Cardiol. 1989;23:309–13. doi: 10.1016/0167-5273(89)90189-7. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Sulaiman RM, Hashmi A, McCrindle BW, Williams WG, Freedom RM. Anomalous origin of one pulmonary artery from the ascending aorta: 36 years’ experience from one centre. Cardiol Young. 1998;84:449–54. doi: 10.1017/s1047951100007101. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Yasui H, Kado H, Yonenaga K, Shiokawa Y, Tokunaga S. Anomalous origin of the right pulmonary artery from the ascending aorta. Ann Thorac Surg. 1991;52:1285–91. doi: 10.1016/0003-4975(91)90014-h. [DOI] [PubMed] [Google Scholar]
- 14.Salaymeh KJ, Kimball TR, Manning PB. Anomalous pulmonary artery from the aorta via a patent ductus arteriosus: repair in a premature infant. Ann Thorac Surg. 2000;69:1259–61. doi: 10.1016/s0003-4975(99)01428-9. [DOI] [PubMed] [Google Scholar]
- 15.Penkoske PA, Castaneda AR, Fyler DC, Van Praagh R. Origin of pulmonary artery branch from ascending aorta. Primary surgical repair in infancy. J Thorac Cardiovasc Surg. 1983;85:537–45. [PubMed] [Google Scholar]