Abstract
Remote ischaemic preconditioning (RIPC) gained attention as a possibility to reduce myocardial injury after a subsequent sustained episode of myocardial ischaemia. This prospective randomized study was carried out to assess whether RIPC reduces myocardial injury in coronary artery bypass grafting patients. Eighty patients were assigned to remote preconditioning or control treatment. Ischaemic preconditioning was induced by three 5-min cycles of upper limb ischaemia and reperfusion after anaesthesia induction. Haemodynamic and markers of myocardial damage were analysed preoperatively and over 48 h postoperatively. The cardiac index was higher immediately after remote preconditioning in the main group. There were no differences in other haemodynamic, troponin I and creatine kinase-MB concentrations at any time point between groups. Thus, short-term remote preconditioning improves haemodynamics and does not reduce myocardial injury after coronary artery bypass surgery. Further study of high-risk patients may be needed to fully evaluate the clinical effect of RIPC.
Keywords: Remote ischaemic preconditioning, Myocardial protection, Troponin I, Coronary artery bypass grafting
INTRODUCTION
One recognized approach for reduction in myocardial injury sustained during this surgery is ischaemic preconditioning, which describes the cardioprotection obtained from the application of cycles of non-lethal myocardial ischaemia and reperfusion before a potentially lethal heart ischaemic insult [1]. However, the clinical application of local preconditioning induced by aortic cross-clamping is limited by the need to induce ischaemia in the target organ, a process that itself may stimulate heart dysfunction and that is clearly inappropriate for global myocardial protection. More recently, it was established that a less invasive approach to same cardioprotection might be achieved by remote ischaemic preconditioning (RIPC). The concept of RIPC was first discovered in animal models by Przyklenk et al. [2], which showed that transient ischaemia of the left circumflex artery could reduce the effects of subsequent potentially lethal ischaemia in the left anterior descending artery. Further studies reported that brief ischaemia of non-cardiac tissue such as the kidney, the intestine [3] or skeletal muscle [4] could also protect the heart against a subsequent myocardial infarction. Although the exact mechanism for remote preconditioning is not yet known, the effects of RIPC are relatively benign, there being no myocardial dysfunction, arrhythmia or low cardiac output.
At the present, several clinical reports of RIPC in cardiac surgery have been published. In children undergoing congenital heart defect repairs, lower limb RIPC has been shown to reduce troponin I release and inotrope requirements [5]. In adults undergoing coronary artery surgery, RIPC using transient upper arm ischaemia has been followed by reductions in the postoperative release of lactate dehydrogenase, creatine kinase-MB (CK-MB) and troponin I [6]. Although these studies have shown reduced biomarker release by RIPC, until today it is not known whether the reduction in myocardial damage will translate into better outcome of cardiac patients. Moreover, in contrast to previous smaller studies, Rahman et al. [7] reported that in patients after on-pump coronary surgery, upper limb RIPC did not reduce troponin T release and improve haemodynamics. Therefore, we aimed to assess whether RIPC is effective in myocardial protection in patients with coronary heart disease (СHD) undergoing on-pump coronary artery bypass grafting (CABG).
MATERIALS AND METHODS
We performed a prospective randomized study with a cohort of patients scheduled for cardiac surgery between June 2010 and March 2011. Eighty adult patients with stable CHD referred for CABG under cardiopulmonary bypass (CPB) were recruited. The study protocol was approved by the local ethics committee and informed consent was obtained from all the patients before entering into the study. Patients were excluded from consideration for the following reasons: reduced left ventricular ejection fraction (<50%), renal failure, hepatic or pulmonary disease, diabetes and myocardial infarction within the past 4 weeks. Patients were randomly assigned to receive either RIPC (40 patients) or control (40 patients) before CABG. All patients had preoperative placement of Swan–Ganz for haemodynamic monitoring throughout the study.
The RIPC protocol comprised three 5 min cycles of right upper limb ischaemia, induced by a blood pressure cuff placed on the right upper arm and inflated to 200 mm Hg, with an intervening 5 min of reperfusion during which the cuff was deflated [8]. Control patients had a deflated cuff placed on the right upper arm for 30 min. The RIPC was applied after anaesthesia induction and baseline measurements. The time taken from the termination of the RIPC to the aortic cross-clamp was not more than 20 min in all patients.
Standard anaesthetic, surgical and CPB techniques were employed. Anaesthesia was induced with fentanyl (5–15 μg/kg), propofol (1–2.5 mg/kg) and pipecuronium bromide (0.1 mg/kg). Anaesthesia maintenance was done with isoflurane (0.6–1.2 vol%), and fentanyl, propofol and pipecuronium bromide were given as needed. All operations were performed under normothermic CPB (nasopharyngeal temperature >35°C). Antegrade cold-crystalloid cardioplegia (St Thomas solution) used and repeated every 20 min until completion. After construction of all the grafts, the CPB was discontinued and protamine was used to reverse the effect of heparin.
All patients had recordings of the cardiac index, stroke index, central venous pressure, pulmonary capillary wedge pressure, mean arterial pressure and heart rate before RIPC, after RIPC and 5 min, 30 min, 2, 4, 6 and 24 h after discontinuing CPB.
In order to assess the impact of RIPC to myocardial injury, we studied the dynamics of biochemical markers. Blood samples to assess troponin I (cTnI) and CK-MB isoenzyme were taken preoperatively and 6, 24 and 48 h after CPB. Plasma troponin I was determined quantitatively using the immunochemiluminescence method (Architect i2000sr; Abbot Diagnostics, USA) with an upper normal limit of 0.3 ng/ml. Plasma CK-MB activity was measured with an assay kit (ThermoFisher Scientific, USA), using a Konelab 20 analyser (Finland), which has an upper normal limit of 24 U/l.
The duration of ventilation postoperatively, intensive care unit stay, complications, blood loss and mortality was recorded.
The statistical analysis was performed with STATISTICA 7.0 for Windows (StatSoft Inc., Tulsa, OK, USA). The qualitative data are expressed in absolute numbers (percentage). The quantitative data are presented as the average (standard deviation), adding or substituting them with the median (25th and 75th percentiles) when the distribution of the values of this variable is markedly outside of the normal distribution; this last value was calculated using the Kolmogorov–Smirnoff test. For comparison of quantitative data, t-test for independent samples, Mann-Whitney U and Friedman tests were used depending on the case. Comparison of the qualitative data was performed using the χ2 (Chi-square) test, applying Yates’ correction to obtain the most conservative results. Values of P < 0.05 were considered statistically significant.
RESULTS
The major demographics and baseline characteristics were similar in the two groups (Table 1). There were no significant differences between the mean values of age, ejection fraction, cross-clamp time or CPB time between the two groups.
Table 1:
Demographics and baseline characteristics
| Variable | RIPC | Control | P-value |
|---|---|---|---|
| No. of patients | 40 | 40 | |
| Sex (male/female) | 36/4 | 37/3 | ns |
| Age (years) | 56.5 ± 8.7 | 58.1 ± 6.4 | ns |
| NYHA functional class | |||
| Class II | 12 (30%) | 10 (25%) | ns |
| Class III | 28 (70%) | 30 (75%) | ns |
| Class IV | 0 | 0 | ns |
| EuroSCORE | 2.2 ± 0.6 | 2.5 ± 0.8 | ns |
| Body mass index (kg/m2) | 28.4 ± 4.1 | 29.3 ± 3.2 | ns |
| LV ejection fraction (%) | 58.1 ± 6.2 | 59.8 ± 6.8 | ns |
| Cross-clamp time (min) | 36.6 ± 14.8 | 38.8 ± 13.9 | ns |
| Bypass time (min) | 62 ± 17.7 | 67.1 ± 20.3 | ns |
| Number of grafts | 2.6 ± 0.9 | 2.8 ± 0.7 | ns |
| Coronary endarterectomy | 5 (12.5%) | 5 (12.5%) | ns |
| Medication (% of group) | |||
| Ca2+ blockers | 8 (20%) | 9 (22.5%) | ns |
| β-Blockers | 32 (80%) | 34 (85%) | ns |
| Nitrates | 34 (85%) | 33 (82.5%) | ns |
| ACE-inhibitors | 22 (55%) | 21 (52.5%) | ns |
| Cholesterol-lowering drug | 31(77.5%) | 33 (82.5%) | ns |
RIPC: remote ischaemic preconditioning group; LV: left ventricle; ns: not significant; NYHA: New York Heart Association.
The assessment haemodynamic data indicated that mean arterial pressure decreased significantly in the RIPC group compared with the control group at 5 min after CPB [71.5 (69; 80) with RIPC versus 81 (72; 84) mmHg for the controls; P < 0.05]. The cardiac index increased significantly in the RIPC group immediately after RIPC [2.3 (2; 2.5) with RIPC versus 2.1 (1.9; 2.3) l/min/m2 for the controls; P < 0.05]. Statistically significant differences in the stroke index between groups were noted immediately after RIPC and 5 min after CPB. At this period in the RIPC group, the stroke index increased compared with the control group [41.4 (34.7; 51.3) and 40.9 (36.5; 42.2) with RIPC versus 36 (28.9; 41.3) and 35.1 (32.2; 37.2) ml/m2 for the controls; P < 0.05]. The systemic vascular resistance index in the RIPC group decreased significantly at 5 min after CPB [1774 (1485; 2044) with RIPC versus 2102 (1869; 2509) dyne s/cm5/m2 for the controls; P < 0.05]. There were no other differences in haemodynamic variables between the two groups in the perioperative period at any time point (Table 2).
Table 2:
Perioperative and postoperative haemodynamic data
| Parameter | Group | Before RIPC | After RIPC | 5 min after CPB | 30 min after CPB | 2 h after CPB | 4 h after CPB | 6 h after CPB | 24 h after CPB |
|---|---|---|---|---|---|---|---|---|---|
| Heart rate (bpm) | RIPC | 53 (48.6; 65.5) | 57 (50; 66) | 78 (76; 85) | 78 (72; 84) | 79 (72; 89) | 77 (68.5; 90) | 78.5 (66; 88) | 82.5(74; 90) |
| Control | 54 (50; 61) | 56 (50; 67) | 79 (74; 83) | 77.5(72.5; 83.5) | 88 (78; 92) | 81 (71; 93) | 81.5 (76; 88) | 83 (77; 90) | |
| MAP (mmHg) | RIPC | 83.5 (76; 95) | 83 (81; 92) | 71.5* (69; 80) | 77 (70; 81) | 78 (73; 87) | 80 (72.5; 85) | 78.5 (74; 82) | 82 (77; 91) |
| Control | 84 (75; 87) | 86(80; 93) | 81 (72; 84) | 75.5 (68.5; 84) | 86 (78; 91) | 82 (76; 84) | 84 (75; 93) | 85 (78; 89) | |
| PCWP (mmHg) | RIPC | 10 (8; 13) | 10 (9; 12) | 12 (10; 15) | 12 (9; 13) | 10 (7; 11) | 10.5 (8.5; 12) | 11.5 (9; 13) | 11 (10; 15) |
| Control | 9 (8; 13) | 10 (9; 13) | 11 (9; 13) | 10 (8.5; 12) | 8 (6.5; 9) | 9 (8; 12) | 10 (8; 14) | 13 (9; 15) | |
| CVP(mmHg) | RIPC | 7 (6; 9) | 7.5 (6; 9) | 8 (7; 11) | 9 (7.5; 10.5) | 8 (6.5; 10.5) | 9 (8; 11.5) | 9.5 (8; 11) | 10 (9; 11) |
| Control | 8 (6; 10) | 7 (6; 9) | 7 (5; 10) | 8 (5; 9) | 8 (6; 10) | 9 (5; 11) | 7 (6; 12) | 9 (7; 11) | |
| PAP (mmHg) | RIPC | 17.5 (15; 23.5) | 17 (16; 18) | 22.4 (15; 26) | 14.5 (14; 17.5) | 23 (16; 23) | 20 (14; 27) | 20 (16.5; 22.5) | 20 (15; 25) |
| Control | 21.5 (18.5; 24.5) | 16 (14; 22.5) | 25 (21; 28.3) | 17 (15; 18) | 22.5 (20; 25.5) | 23 (21; 24) | 21 (17; 25) | 23.8 (18.1; 25) | |
| Cardiac index (l/min/m2) | RIPC | 1.8 (1.7; 2) | 2.3* (2; 2.5) | 3.1 (2.7; 3.3) | 2.5 (2.3; 2.9) | 2.5 (2.3; 2.7) | 2.5 (2.3; 2.8) | 2.5 (2.3; 2.8) | 2.4 (2.3; 2.5) |
| Control | 1.6 (1.5; 2.2) | 2.1 (1.9; 2.3) | 2.8 (2.6; 3) | 2.3 (2.1; 2.8) | 2.4 (2.2; 2.7) | 2.7 (2; 2.9) | 2.7 (2.3; 3) | 2.5 (2.2; 2.7) | |
| Stroke index (ml/m2) | RIPC | 33 (27.2; 38.7) | 41.4* (34.7; 51.3) | 40.9* (36.5; 42.2) | 31.3 (29.2; 39.3) | 33.6 (27.1; 34.9) | 33.3 (29; 35.1) | 33.3 (29.8; 36.1) | 28.5 (26.2; 33) |
| Control | 32.6 (26; 34.6) | 36 (28.9; 41.3) | 35.1 (32.2; 37.2) | 29.9 (27.8; 33.7) | 29.7 (25; 32.6) | 30.8 (28.2; 35.6) | 32 (27.9; 37) | 28.3 (24.7; 31.1) | |
| SVRI (dyne·s/cm5/m2) | RIPC | 3503 (2923; 4145) | 2614 (2276; 3036) | 1774* (1485; 2044) | 2219 (1977; 2462) | 2370 (1931; 2677) | 2107 (1891; 2647) | 2091 (1971; 2305) | 2178 (2008; 2581) |
| Control | 3351 (2819; 4571) | 3068 (2467; 3272) | 2102 (1869; 2510) | 2206 (1953; 2607) | 2415 (2330; 2830) | 2440 (1916; 2703) | 2355 (1961; 2672) | 2449 (2379; 2752) |
CPB: cardiopulmonary bypass; CVP: central venous pressure; RIPC: remote ischaemic preconditioning group; MAP: mean arterial pressure; PAP: pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; SVRI: systemic vascular resistance index.
Difference confidence level: *P < 0.05 when compared RIPC patients with the control group.
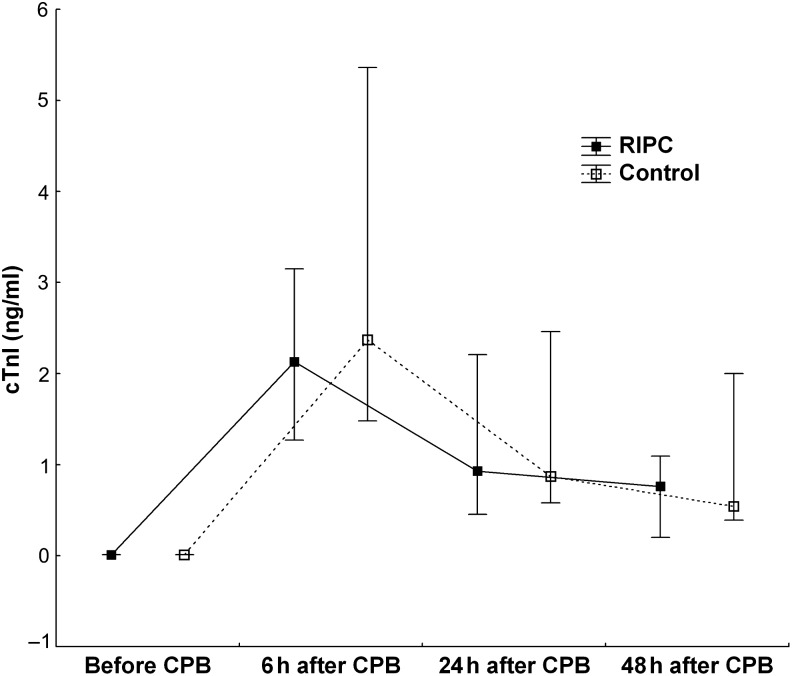
The evaluation biochemical markers of myocardial injury indicated that baseline troponin I concentrations were below the upper normal range of the assay (<0.3 ng/ml) in all patients. There was a rise in plasma cTnI in both groups postoperatively, indicating some myocardial injury during the operation (Table 3). Peak cTnI release occurred at 6 h after completion of CPB in all patients: the RIPC group 2.1 (1.3; 3.2) ng/ml; the control group 2.4 (1.5; 5.4) ng/ml. At 24 and 48 h after CPB, plasma cTnI in both the groups marked decreased (Table 3). There were no statistical differences in cTnI levels between RIPC and control groups at any time point. The total troponin I released in the RIPC group, expressed as the area under the curve of troponin I over the 48 h after surgery, did not differ significantly compared with the control group: the RIPC group, 54.4 (20; 97.3) ng/ml/48 h; the control group, 53.3 (34.7; 140) ng/ml/48 h (Fig. 1).
Table 3:
Markers of myocardial damage
| Marker | Group | Before RIPC | 6 h after CPB | 24 h after CPB | 48 h after CPB |
|---|---|---|---|---|---|
| cTnI (ng/ml) | RIPC | 0.01 (0.01; 0.01) | 2.13 (1.27; 3.15)^ | 0.93 (0.45; 2.21)^ | 0.76 (0.2; 1.09)^ |
| Control | 0.01 (0.01; 0.01) | 2.37 (1.48; 5.36)^ | 0.87 (0.58; 2.46)^ | 0.54 (0.39; 2)^ | |
| CK-MB (U/l) | RIPC | 20 (14; 22) | 45.5 (32; 53.5)^ | 48.5 (32; 66)^ | 49.5 (42; 70)^ |
| Control | 18 (14; 23) | 39 (33; 58)^ | 45 (33; 58)^ | 47.5 (38.5; 69.5)^ |
Difference confidence level: *P < 0.05 when RIPC patients are compared with the control group, ^P < 0.05 when compared with values before CPB.
Figure 1:
The area under the curve of troponin I over the 48 h after surgery in the RIPC group did not differ compared with the control group. Values presented are median (25th and 75th percentiles). RIPC: remote ischaemic preconditioning. cTnI: cardiac troponin I.
The baseline CK-MB activity did not exceed the upper normal limits (<24 U/l) in all patients. There was a marked elevation in plasma in both the groups at 6, 24 and 48 h after CPB compared with the preoperative values (Table 3). The peakСК-МВlevel occurred at 48 h after CPB in all patients: the RIPC group, 49.5 (42; 70); the control group, 47.5 (38.5; 69.5) U/l. However, there were no significant differences in activities between RIPC and control groups at any time point.
The data of the postoperative period are presented in Table 4. There were no operative deaths (to 30 days postoperatively) in the two groups. None of the patients in this study required any form of renal replacement therapy after surgery. The mean duration of postoperative ventilation, intensive care unit stay and the blood loss was similar in both the groups. One patient in the RIPC group required inotropic support. In this case at 5 h, infusion of dopamine (2–4 mcg/kg/min) was carried out which did not affect further clinical course of this patient.
Table 4:
Postoperative findings and outcome
| Variable | RIPC | Control | P-value |
|---|---|---|---|
| Ventilation time (h) | 4.5 ± 1.5 | 5 ± 1.5 | ns |
| ICU stay (days) | 1.9 ± 0.5 | 1.9 ± 0.5 | ns |
| Mortality | 0 | 0 | ns |
| Blood loss in POD 1 (ml/kg) | 4.9 ± 2.1 | 5.1 ± 2.2 | ns |
| Reoperation for bleeding | 0 | 0 | ns |
| Need for inotropic support | 1/40 (2.5%) | 0 | ns |
| Postoperative dialysis | 0 | 0 | ns |
| Mediastinitis | 0 | 0 | ns |
RIPC: remote ischaemic preconditioning group; ICU: intensive care unit; POD: postoperative day; ns: not significant.
There were no untoward consequences of the RIPC protocol.
DISCUSSION
Recently RIPC has gained attention as a way of reducing myocardial injury after a subsequent sustained episode of myocardial ischaemia. Due to the safe and non-invasive nature of the intervention, this cardioprotection might be applicable in numerous patients with CHD. However, published evidence from the cardiac surgical literature is conflicting and the independent influence of RIPC on the myocardial damage of patients following surgical coronary revascularization is unclear [9].
In this clinical study, we demonstrate that RIPC induced by brief ischaemia and reperfusion to the upper limb can, in the short-term, improve the contractile function of the heart. This was shown by the increase in the cardiac index immediately after RIPC compared with the control group. Both the decrease in peripheral vascular resistance and the increase in the stoke index may account for the increase in the cardiac index seen in the RIPC group. The haemodynamic differences between the groups were not revealed at any time point. The most possible mechanisms of the short-term increase in the cardiac pump function after RIPC have been described by some authors [10] and are closely related to the release of numerous substances, including adenosine, bradykinin, opioids, calcitonin and endocannabinoids into the blood stream.
It is proven that the cardiac enzyme release during cardiac surgery occurs as a result of combined ischaemia–reperfusion injury [11]. Cardiac-specific markers of myocardial injury such as troponin I and CK-MB have been used to quantify ischaemia–reperfusion form of myocardial injury and have been reported by several clinical studies to be associated with worse short-term and long-term outcomes after surgery [12]. In this study, we established that RIPC did not reduce myocardial injury in patients undergoing CABG surgery. There were no significant differences in plasma levels of biomarkers of myocardial injury and total troponin I release over the 48 h after surgery between the groups throughout the study period [13]. The mean duration of postoperative ventilation, intensive care unit stay and hospital stay did not differ between the groups.
There are several limitations of our study. First, we have demonstrated that remote preconditioning, induced by transient upper limb ischaemia, does not reduce myocardial injury in low-risk patients undergoing coronary artery surgery. High-risk patients tend to have greater myocardial injury and therefore may derive greater benefit from RIPC, but this remains to be determined by future clinical studies.
Furthermore, Kottenberg et al. [14] found that the effect of RIPC is inhibited by the use of propofol. In contrast, Lucchinetti et al. [15] found that RIPC applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on-pump CABG. The existence of a large number of conflicting studies emphasizes the need for further research. Hence, patients in this study were randomized to RIPC or control groups and all underwent the same anaesthetic, CPB and intensive care management protocols to minimize bias.
Funding
The study was supported only by institutional funding.
Conflict of interest: none declared.
REFERENCES
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whitaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–9. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 3.Gho BC, Shoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischaemia in non-cardiac tissue. Circulation. 1996;94:2193–200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 4.Addison PD, Neligan PC, Ashrafpour H, Khan A, Zhong A, Moses M, et al. Noninvasive remote ischemic preconditioning for global protection of skeletal muscle against infarction. Am J Physiol Heart Circ Physiol. 2003;285:H1435–43. doi: 10.1152/ajpheart.00106.2003. [DOI] [PubMed] [Google Scholar]
- 5.Cheung MMH, Kharbanda RK, Konstantinov IE, Shimizu M, Frnova H, Li J, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–82. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 6.Gunaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, Sancak B, et al. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000;41:493–6. doi: 10.1006/phrs.1999.0611. [DOI] [PubMed] [Google Scholar]
- 7.Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, et al. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation. 2010;122(11 suppl):S53–9. doi: 10.1161/CIRCULATIONAHA.109.926667. [DOI] [PubMed] [Google Scholar]
- 8.Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, et al. Remote ischemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567–71. doi: 10.1136/hrt.2008.155770. [DOI] [PubMed] [Google Scholar]
- 9.Kanoria S, Jalan R, Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote ischemic preconditioning: a novel method for reducing ischemia reperfusion injury. Transplantation. 2007;84:445–58. doi: 10.1097/01.tp.0000228235.55419.e8. [DOI] [PubMed] [Google Scholar]
- 10.Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol. 1998;275:H1542–7. doi: 10.1152/ajpheart.1998.275.5.H1542. [DOI] [PubMed] [Google Scholar]
- 11.Heusch G, Schulz R, Haude M, Erbel R. Coronary microembolization. J Mol Cell Cardiol. 2004;37:23–31. doi: 10.1016/j.yjmcc.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Lattouf OM, Thourani VH, Kilgo PD, Halkos ME, Baio KT, Myung R, et al. Influence of on-pump versus off-pump techniques and completeness of revascularization on long-term survival after coronary artery bypass. Ann Thorac Surg. 2008;86:797–805. doi: 10.1016/j.athoracsur.2008.04.065. [DOI] [PubMed] [Google Scholar]
- 13.Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–64. doi: 10.1007/s00395-010-0104-5. [DOI] [PubMed] [Google Scholar]
- 14.Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–8. doi: 10.1111/j.1399-6576.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 15.Lucchinetti E, Bestmann L, Feng J, Freidank H, Clanachan AS, Finegan BA, et al. Remote ischemic preconditioning applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on-pump coronary artery bypass graft surgery: lack of synergy or evidence of antagonism in cardioprotection? Anesthesiology. 2012;116:296–310. doi: 10.1097/ALN.0b013e318242349a. [DOI] [PubMed] [Google Scholar]