Abstract
The hypothesis driving this study was that photodynamic therapy (PDT) may limit abdominal aortic aneurysm growth due to matrix changes. The aortas of 12 rats were incubated with elastase using a newly modified experimental aneurysm model (3.5 mg/ml). Rats were allocated to an elastase-only group (n = 6) to study the elastase-induced aneurysm growth and an elastase ± PDT group to evaluate if PDT limited aneurysm growth (n = 6). PDT was performed with the photosensitizer methylene blue, and thermoneutral laser light (660 nm) was applied (120 J/cm2, 100 mW/cm2) using a diode laser. Four untreated rats served as controls. The arteries were analysed after 4 weeks based on histology, immunohistochemistry and morphometry. This modified rat elastase model led to reproducible aneurysm development with no elastase-induced mortality compared with control animals (circumference, controls: 2.9 ± 0.2 vs. elastase: 5.5 ± 0.9 mm; P < 0.01). PDT after elastase incubation did not inhibit inflammatory cell infiltration. No significant change in the circumference was observed between elastase incubation and PDT treatment after elastase incubation (circumference, elastase: 5.5 ± 0.9 vs. elastase and PDT: 6.1 ± 0.8 mm; P < 0.01). Despite a PDT-induced resistance to protease digestion, PDT did not reduce aortic dilatation in the elastase-treated rat aorta. These findings suggest that PDT may not be a useful modality to prevent aneurysm growth.
Keywords: Aortic aneurysm, Photodynamic therapy, Immunohistochemistry
INTRODUCTION
Abdominal aortic aneurysms are characterized by the segmental dilatation of the aortic wall and are the most common type of aneurysms of the human vascular system. Aneurysms are frequently identified when small and followed until they reach a size necessitating prophylactic therapy to prevent a rupture. There is still no commonly accepted prophylactic therapy to limit aneurysm growth and thus to avoid open surgery or endovascular therapy.
There is considerable evidence linking aortic aneurysms with extracellular matrix degradation and the loss of the structural integrity of the aortic wall [1, 2]. Vascular wall matrix proteins such as elastin and collagen provide the resilience and tensile strength of the aorta. The destruction of these proteins is considered necessary for aneurysm expansion and rupture of the aneurysm. Vascular wall matrix destruction in aortic aneurysms is mainly due to an inflammatory response within the media and adventitia [3]. The mode of action by which an inflammatory infiltrate destroys the connective tissue matrix is mainly by proteolytic digestion. The best studied groups of enzymes involved are metalloproteinases [4, 5]. Although pharmacological strategies aimed at preventing matrix degradation showed promising experimental results [6], there is currently no clinically accepted therapy to limit the growth of asymptomatic small aneurysms [7].
Photodynamic therapy (PDT), a technique that uses monochromatic and thermoneutral light to activate an otherwise non-toxic photosensitizer dye to produce local free-radical moieties, is clinically approved for the treatment of various proliferative diseases, mainly in oncology and dermatology [8]. In the vasculature, PDT was mainly studied to reduce vascular restenosis. In these studies, it has been shown that PDT led to cellular eradication in the arterial wall without an inflammatory response [9]. In addition, PDT has been demonstrated to stabilize the vascular wall and to render vessel resistance against cellular invasion, by inducing matrix protein cross-links [10, 11]. Although PDT led to a decellularization of the vascular wall, no aneurysmal dilatation was noted after PDT in short- and long-term experiments [12, 13].
Since PDT-inhibited inflammatory cell invasion and matrix degradation are main factors in the aneurysm development, the hypothesis for this project was that PDT would modulate the vascular wall matrix proteins in a way which would reduce abdominal aortic aneurysm growth. For this, a newly modified elastase-induced rat model was used, because elastase-induced mortality in earlier described experimental models was high [14].
MATERIALS AND METHODS
Animal model
Sixteen adult male Sprague–Dawley rats were used. All animals received human care in compliance with the European Convention on Animal care and the study was approved by the institutional ethics committee. A laparotomy was performed under sterile conditions, and the abdominal aorta and the common iliac arteries were exposed from the level of the left renal vein to the iliac bifurcation using the standard microsurgical technique. Four animals did not undergo further treatment (controls). In 12 animals, elastase (pancreatic elastase from porcine pancreas, enzyme commission number 3.4.21.36, 3.5 U/mg, E6883, Sigma-Aldrich Chemie, Germany) at a concentration of 1 mg/ml was applied. An intraluminal elastase application led to a high mortality in earlier described models [14] and, therefore, a different model using the external application at the site of the rat femoral artery was modified for use at the site of the aorta. The isolated aorta was externally incubated with elastase using a small paint brush for 15 min at 37°C, as described in a rat femoral artery model [15]. The animals were then divided into an elastase-only group (n = 6) and an elastase- and PDT-treated group (n = 6). For PDT, the photosensitizer dye methylene blue (250 µg/ml) was applied in an endovascular method for 3 min at 100 mm/Hg. Five minutes after the photosensitizer delivery, the aorta was externally irradiated with thermoneutral laser light at a wavelength of 660 nm, emitted by a diode laser (B&W Tek, Inc., Newark, USA), delivering a total fluence of 120 J/cm2 at an irradiance of 100 mW/cm2. The PDT dosimetry at the site of the rat aorta had been evaluated in a former study and was based on the rat carotid artery dosimetry. The diode laser was coupled to a 600-µm optical fibre through a microlens to obtain a uniform 1.5 cm spot. The light irradiation time was 20 min. The targeted artery was submerged in 0.9% saline and placed on a right-angled reflective mirror to achieve uniform irradiation. Animals were sacrificed 4 weeks after surgery. The iliac artery was cannulated, and the arterial system was flushed with saline and in situ perfusion fixed at 150 mmHg with 10% buffered formalin. The abdominal aortic segments were excised and placed in fresh 10% buffered formalin. After embedding in paraffin, multiple 4-μm-thick cross sections were obtained from the middle vessel segments.
Histomorphometry
The analyses of the vessels, stained with haematoxylin and eosin and van Gieson elastic stain using the standard techniques, comprised both a descriptive histological and a quantitative morphometric evaluation. The morphometric analyses of the specimens were performed using a digital system and the Axio Vision software (Axio Vision™ 6.0, Carl Zeiss AG, Germany). The vessel circumferences were measured and calculated for the identical segments in control animals, elastase-only animals and elastase- and PDT-treated animals in a blinded fashion.
Immunohistochemistry
To show an inflammatory cell infiltration in the aneurysm wall, immunohistochemistry, using the avidin–biotin complex method, was used. To identify macrophages, an antibody against CD68 (Dako; clone PG-M1; M0876; dilution 1:400) was used. T-lymphocytes were identified by an antibody against CD3 (NovoCastra, clone PS1; NCL-L-CD3PS1, dilution 1:100) and B-lymphocytes by antibodies against CD20 (Dako; L26; M0755; dilution 1:5000). The immunohistochemistry was carried out using standard protocols. Briefly, the specimens were deparaffinized with Xylol, then washed in a decreasing alcoholic row and again washed. The specimens were then incubated with the primary antibodies directed against CD3, CD20 and CD68 followed by the incubation with the biotinylated second antibody for 10 min and were washed with streptavidin (Zytomed, art. Nr. HRP 5000, biotinylated second antibody, polyvalent/streptavidin–HRP-conjugated). 3,3′-Diaminobenzidin (DAB) (Zytomed, art. Nr. DAB530, DAB substrate buffer + DAB chromogen) was used for visualization. Nuclei were counterstained with haematoxylin. Control slides with non-immune IgG as a primary antibody were included.
Statistical analyses
Results are expressed as mean ± SD. A one-way ANOVA and Tukey's post hoc test were applied using Statistica software (Statsoft, USA). A P-value <0.05 was considered significant.
RESULTS
Histology
The aortic incubation with elastase led to development of aortic aneurysms after 4 weeks, confirming a suitable experimental model to study the aneurysm development and therapeutic strategies to inhibit the aneurysm growth with no elastase-induced mortality. The significant dilatation after the elastase application (left side) compared with controls is shown in Fig. 1. PDT of elastase-incubated arteries did not limit the aneurysm growth but further damaged the vessel wall. The presence of a small thrombus and a thinned and fragile media after PDT can be observed in the left-hand side of Fig. 2.
Figure 1:
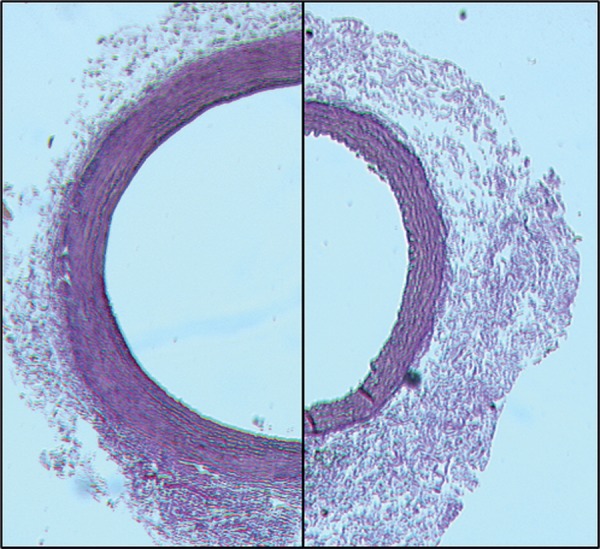
The representative light micrographs of elastase-only (left) compared with controls (right). Note the difference in the circumference.
Figure 2:
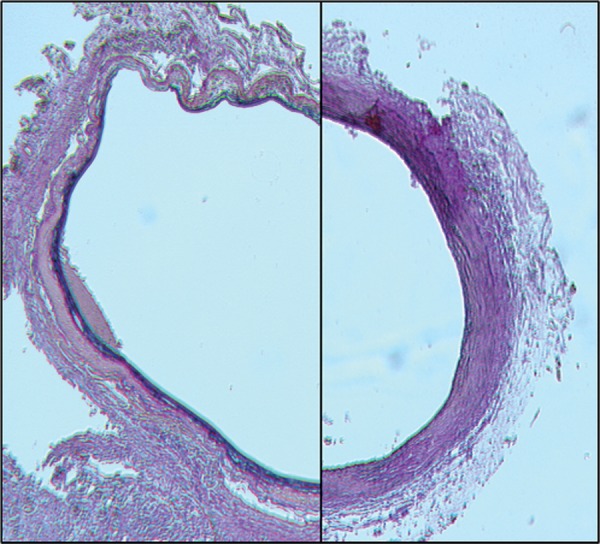
The representative light micrographs of elastase-only compared with elastase and PDT arteries. No significant difference in the circumference is observed. Note the cell free, but partially destroyed media and discrete thrombus in the PDT-treated specimen (left). Note the destruction of elastic fibers after incubation with elastase (right).
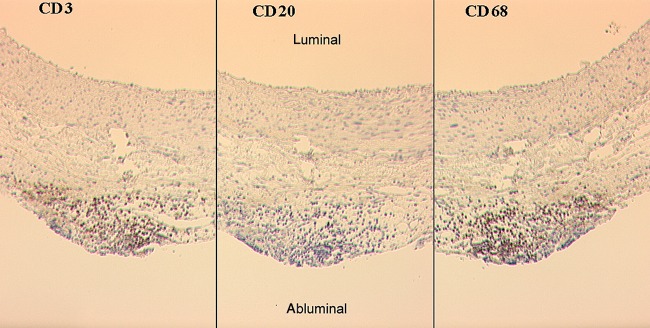
This elastase aneurysm model is based on an induction of an aortic wall inflammatory response (Fig. 3). The immunohistochemistry showed massive CD3- (Fig. 4) and CD68-positive cells in the vascular wall.
Figure 3:
The representative immunohistochemical staining of an elastase-treated animal. The same section of the vessel wall using the same magnification is shown. The specimens were stained with antibodies against CD3 (T-lymphocytes, left), CD20 (B-lymphocytes, middle) and CD68 (macrophages, right). Note the adventitial infiltration of inflammatory cells. Especially, T-lymphocyte and macrophage infiltration were found.
Figure 4:
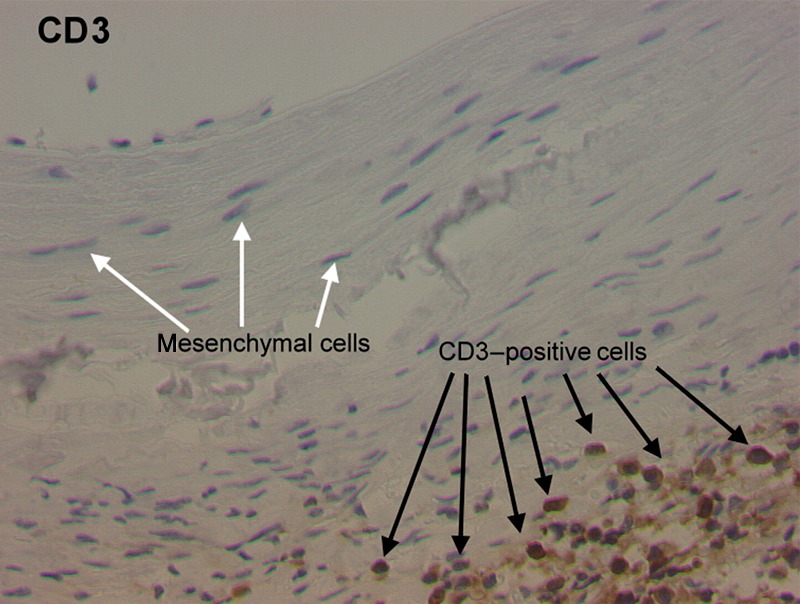
The representative immunohistochemical staining of an elastase-treated animal (CD3). Note the T-lymphocyte invasion into the adventitia of the vessel wall.
Vessel morphometry
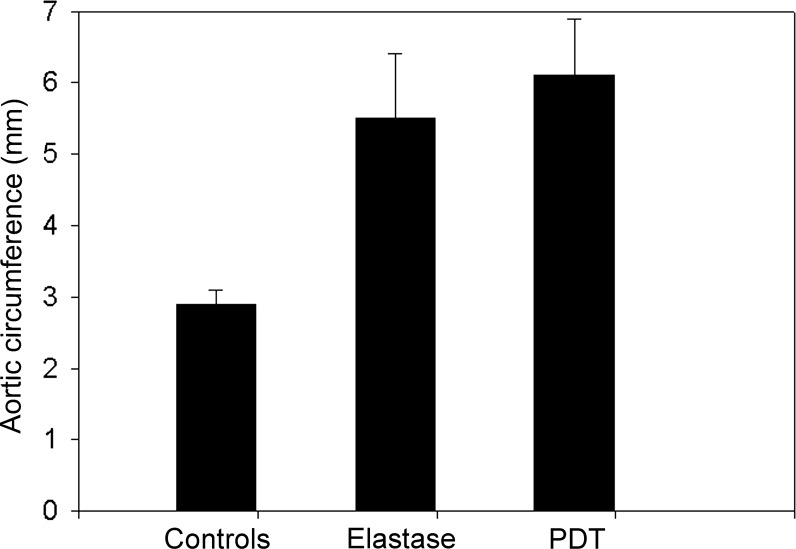
The circumferences were measured by digital morphometric analyses. There was no significant difference between arteries incubated with elastase-only and elastase-incubated and PDT-treated arteries (P = 0.21, Fig. 5). The control arteries showed a significantly smaller circumference, compared with the two experimental groups (P < 0.01; Fig. 5).
Figure 5:
The circumference of pressure-fixed abdominal aortic specimens. The data are expressed as mean ± SD. P < 0.01 in controls vs. elastase-only and elastase + PDT. No significant difference was noted between elastase-only and elastase + PDT confirming no positive effect on the arterial wall dilatation after PDT treatment in this model (P = 0.21).
DISCUSSION
PDT has been demonstrated to reduce intimal hyperplasia after vascular interventions in the experimental and clinical studies [9–11]. The underlying effects leading to a positive modulation of the vessel wall resulting in a reduction of intimal hyperplasia have been intensively studied. PDT leads to cellular eradication in the vascular wall by apoptosis without an inflammatory response [9]. In addition, PDT inhibits cellular migration into the vessel wall, modulates the vascular wall matrix proteins in a way that confers resistance against proteinase digestion and inactivates the growth factors and cytokines that play important roles in vascular disease [10].
These findings led to the hypothesis that PDT may stabilize the vascular wall and, therefore, may eventually be useful in the treatment of patients with small asymptomatic abdominal aortic aneurysms. This notion follows the previous studies emphasizing the critical role of vascular matrix degradation, mainly by proteinases, in aneurysm development and growth [4]. It has been shown that tetracycline derivates, which have efficacy as metalloproteinase inhibitors, have beneficial effects in the development of experimental aortic aneurysms. The finding that PDT modifies the vascular matrix in a way that renders the vessel wall resistant to protease digestion [10] suggests that PDT stabilizes collagens in the vascular wall. The earlier described rat elastase aneurysm models showed an insufficient aneurysm development and high mortality [14]. Therefore, a modified rat elastase aortic aneurysm model was developed. This was based on the described model to induce femoral artery aneurysms using an external elastase application [15]. It was investigated if the PDT-induced resistance to protease digestion might also lead to a limitation in the experimental aneurysm growth. Although the primary target of elastase is elastin, the basis for this aneurysm model is the induction of an aortic wall inflammatory response. After the initial elastin destruction, secondary connective tissue protein damage including collagen is caused by endogenous proteases released from attracted inflammatory cells as shown in these experiments. In this study, the aneurysm was induced before PDT treatment to mimic the clinical situation in humans. Transferring this model into the clinical situation would imply the treatment of existing small aneurysms to limit the further growth. In human vessels, PDT can be performed with an endovascular method using the systemic or endovascular photosensitizer application and endovascular laser irradiation. In this small animal study, laser light was applied externally due to the small size of the rat aorta. In this study, animals in the elastase-only group developed reproducible aneurysms in the investigated time frame. Although PDT has been shown to induce the matrix changes leading to a resistance to pepsin digestion in the balloon-injured carotid arteries [10], adjuvant PDT after incubating the aorta with elastase did not limit inflammatory cell ingrowth and aortic dilatation in this study. The regular matrix architecture and the absence of inflammatory cells, which is noted after PDT of a balloon-injured or untreated vessel [13], seem to be crucial for beneficial vascular PDT treatment.
In summary, although PDT induced a resistance to protease digestion in the balloon-injured arteries, this treatment modality did not reduce the aortic dilatation in this newly adapted experimental elastase model. Although PDT showed very promising results in preventing vascular restenosis, these findings suggest that PDT may not be a useful modality to prevent aneurysm growth.
Acknowledgements
The authors thank F. Adili for very helpful discussions, and M. Mueller, E. Haskamp and I. Karalus for their excellent technical assistance. The authors also express their gratitude to S. Tawadros for excellent animal care.
FUNDING
The present study was funded by the German Society of Vascular Surgery.
Conflict of interest: none declared.
REFERENCES
- 1.Daugherty A, Cassis LA. Mechanisms of abdominal aortic aneurysm formation. Curr Atheroscler Rep. 2002;4:222–7. doi: 10.1007/s11883-002-0023-5. [DOI] [PubMed] [Google Scholar]
- 2.Eskandari MK, Vijungo JD, Flores A, Boresztajn J, Shively V, Pearce WH. Enhanced abdominal aortic aneurysm in TIMP-1-deficient mice. J Surg Res. 2005;123:289–93. doi: 10.1016/j.jss.2004.07.247. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune response in abdominal aortic aneurysms. Arteriosler Thromb Vasc Biol. 2006;26:987–94. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 4.Lizarbe TR, Tarin C, Gomez M, Lavin B, Aracil E, Orte LM, et al. Nitric oxide induces the progression of abdominal aortic aneurysms through the matrix metalloproteinase inducer EMMPRIN. Am J Pathol. 2009;175:1421–30. doi: 10.2353/ajpath.2009.080845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies MJ. Aortic aneurysm formation. Lessons from human studies and experimental models. Circulation. 1998;98:193–5. doi: 10.1161/01.cir.98.3.193. [DOI] [PubMed] [Google Scholar]
- 6.Yang HH, Kim YL, Chum E, van Breemen C, Chung AW. Effectiveness of combination of losartan potassium and doxycycline versus single-drug treatments in the secondary prevention of thoracic aneurysms in Marfan syndrome. J Thorac Cardiovasc Surg. 2010;140:305–12. doi: 10.1016/j.jtcvs.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Liao S, Miralles M, Kelley BJ, Curci JA, Borhani M, Thompson RW. Suppression of experimental abdominal aortic aneurysms in the rat by treatment with angiotensin-converting enzyme inhibitors. J Vasc Surg. 2001;33:1057–64. doi: 10.1067/mva.2001.112810. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaMuraglia GM, Schiereck J, Heckenkamp J, Nigri G, Waterman P, Leszczynski D, et al. Photodynamic therapy induces apoptosis in intimal hyperplastic arteries [in process citation] Am J Pathol. 2000;157:867–75. doi: 10.1016/S0002-9440(10)64600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overhaus M, Heckenkamp J, Kossodo S, Leszczynski D, LaMuraglia GM. Photodynamic therapy generates a matrix barrier to invasive vascular cell migration. Circ Res. 2000;86:334–40. doi: 10.1161/01.res.86.3.334. [DOI] [PubMed] [Google Scholar]
- 11.Waterman PR, Overhaus M, Heckenkamp J, Nigri GR, Fungaloi PF, Landis ME, et al. Mechanisms of reduced human vascular cell migration after photodynamic therapy. Photochem Photobiol. 2002;75:46–50. doi: 10.1562/0031-8655(2002)075<0046:morhvc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Waksman R, McEwan PE, Moore TI, Pakala R, Kolodgie FD, Hellinga DG, et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J Am Coll Cardiol. 2008;52:1024–32. doi: 10.1016/j.jacc.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 13.LaMuraglia GM, ChandraSekar NR, Flotte TJ, Abbott WM, Michaud N, Hasan T. Photodynamic therapy inhibition of experimental intimal hyperplasia: acute and chronic effects. J Vasc Surg. 1994;19:321–31. doi: 10.1016/s0741-5214(94)70107-5. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Yokokawa M, Suzuki M, Higashide S, Katoh Y, Sugiyama S, et al. Factors influencing mortality in the rat elastase-induced-aneurysm model. J Surg Res. 2000;94:81–3. doi: 10.1006/jsre.2000.5995. [DOI] [PubMed] [Google Scholar]
- 15.Oskoui P, Stadler I, Lanzafame RJ. A preliminary study of laser tissue soldering as arterial reinforcement in an acute experimental aneurysm model. Lasers Surg Med. 2003;32:346–8. doi: 10.1002/lsm.10173. [DOI] [PubMed] [Google Scholar]