Abstract.
A noninvasive approach to vasectomy may eliminate male fear of complications related to surgery and increase its acceptance. Noninvasive laser thermal occlusion of the canine vas deferens has recently been reported. Optical coherence tomography (OCT) and high-frequency ultrasound (HFUS) are compared for monitoring laser thermal coagulation of the vas in an acute canine model. Bilateral noninvasive laser coagulation of the vas was performed in six dogs ( vasa) using a Ytterbium fiber laser wavelength of 1075 nm, incident power of 9.0 W, pulse duration of 500 ms, pulse rate of 1 Hz, and 3-mm-diameter spot. Cryogen spray cooling was used to prevent skin burns during the procedure. An OCT system with endoscopic probe and a HFUS system with 20-MHz transducer were used to image the vas immediately before and after the procedure. Vasa were then excised and processed for gross and histologic analysis for comparison with OCT and HFUS images. OCT provided high-resolution, superficial imaging of the compressed vas within the vas ring clamp, while HFUS provided deeper imaging of the vas held manually in the scrotal fold. Both OCT and high HFUS are promising imaging modalities for real-time confirmation of vas occlusion during noninvasive laser vasectomy.
Keywords: coagulation, laser, male sterilization, noninvasive, optical coherence tomography, ultrasound, vasectomy
1. Introduction
Approximately 500,000 vasectomies are performed in the United States per year, making it the most common urological procedure in the U.S.1 Although vasectomy is more effective and less likely to have complications than tubal ligation, the number of men undergoing surgical sterilization is approximately three times less than women.2–4 Fear of complications related to surgical vasectomy (e.g. hematoma, infection, acute and chronic pain, and sterilization failure) is a major factor in a couples’ choice of surgical sterilization.5,6 A noninvasive technique for male sterilization may improve acceptance of vasectomy.
Our laboratory is currently developing a noninvasive vasectomy technique utilizing near-infrared laser irradiation in conjunction with cryogen spray cooling of the scrotal skin surface for thermal occlusion of the vas. During previous preliminary noninvasive laser vasectomy studies, we reported successful thermal occlusion and scarring of the vas in both ex vivo and in vivo canine models,7–9 with the thermally coagulated vas segment withstanding burst pressures over twice that of normal canine ejaculation pressures.10 However, this completely noninvasive therapeutic procedure may still benefit from the use of a compact and inexpensive imaging modality to confirm, in real time, accurate targeting, thermal coagulation, and occlusion of the vas.
Optical coherence tomography (OCT) and high-frequency diagnostic ultrasound (HFUS) have recently been used to measure the anatomy of the normal human vas deferens.11–13 Our laboratory has also used HFUS (with a 13-MHz probe) for imaging the canine vas before and after laser thermal coagulation.14 This study aims to improve on these previous studies by using a higher-frequency (20 MHz) probe capable of improved resolution and to compare HFUS with another high-resolution imaging modality, optical coherence tomography (OCT), for imaging the canine vas before and after laser thermal coagulation. Both imaging modalities are safe, compact, and inexpensive and are therefore evaluated as possible choices for potential use in laser vasectomy during this preclinical study. However, each imaging modality serves a slightly different purpose: HFUS provides deep imaging with intermediate scale resolution while OCT provides high resolution but is limited in its superficial imaging depth.
2. Methods
All procedures were conducted under an animal protocol approved by the Johns Hopkins Animal Review Committee. Noninvasive thermal occlusion of the vas was performed bilaterally in a total of six dogs ( vasa). After completion of the procedure, all vasa were harvested and processed for histology with H&E staining. The animals were neutered, monitored for a few days during recovery, and then adopted out to caring homes.
A compact, tabletop, 50-watt Ytterbium fiber laser (Model TLR1075-50, IPG Photonics, Oxford, MA) emitted near-infrared laser radiation with a wavelength of 1075 nm, which was focused with a lens into a 400-μm-core fiber optic patch-cord (Thorlabs, Newton, NJ). Another lens at the end of the patch-cord delivered a collimated laser beam to the tissue surface. The laser was externally triggered by a function generator (DS345, Stanford Research Systems, Sunnyvale, CA) to operate in long-pulse mode, with an average incident power of 9.0 W, 500-ms pulse duration, 1-Hz pulse rate, and 3-mm-diameter () spot at the scrotal skin surface.
A dynamic cooling device (DCD, Candela Laser Corporation, Wayland, MA) was used to deliver cryogen (1,1,1,2-tetrafluoroethane, ) to the scrotal skin surface through a solenoid valve to prevent overheating and formation of skin burns during the procedure. Three cryogen pulses were applied to pre-cool the skin prior to laser irradiation over a period of eight seconds. For all of the procedures, laser irradiation was performed for a period of 60 sec for each vas. Cryogen spray was delivered intermittently between laser pulses with a 60-ms pulse duration, pulse rate of 0.25 Hz, and a 2-cm-diameter spot concentric with the laser spot. A cryogen mask was used to thermally insulate surrounding scrotal skin from cryogen spray to avoid superficial freeze burns. Details of the experimental setup have been previously reported.9 A modified, 4-mm-OD, vasectomy ring clamp was used to manually isolate the vas beneath the scrotal skin surface for co-location with the cryogen and laser spots (Fig. 1). It should be noted that although a modified vasectomy ring clamp design was used for these procedures, the base of the clamp (hemostat) is essentially the same as the instrument currently used during a standard no scalpel vasectomy procedure.
Fig. 1.
A conventional no-scalpel vasectomy ring clamp was used to isolate the vas deferens beneath the scrotal skin surface, and then a detachable customized clamp extension was used to safely co-locate the cryogen spray spot and laser spot on the tissue surface inside the ring.
A compact, tabletop, inexpensive, FDA-approved, endoscopic, optical coherence tomography system (Niris II, Imalux, Cleveland, OH) with a handheld 8-Fr (2.7-mm-OD) probe was used [Fig. 2(a)]. The OCT system acquired real-time images at eight frames/s with axial resolution and lateral resolution in tissue with a lateral scan length of 2 mm and an imaging depth of 1.6 mm. The OCT system was used for superficial imaging of the canine vas once it was compressed inside the vas ring clamp, immediately before and after laser irradiation. This approach to OCT imaging of the vas is entirely feasible during a clinical procedure.
Fig. 2.
(a) Photograph of the optical coherence tomography system and probe used in these studies; (b) photograph of the high-frequency ultrasound system and probe used in these studies.
A compact, tabletop, inexpensive, FDA-approved high-frequency ultrasound system (Episcan-I-200, Longport International, Silchester, United Kingdom) with 20-MHz linear scanning transducer was used for comparison with OCT [Fig. 2(b)]. This transducer provided a scan length of 15 mm, an axial image resolution of , and an imaging depth of . The HFUS transducer was used to provide deeper imaging of the canine vas when it was held manually within a scrotal fold immediately before and after application of the vas ring clamp.
Several indicators were used to determine successful laser thermal coagulation and occlusion of the canine vas, including OCT, HFUS, gross analysis, and histology.
3. Results
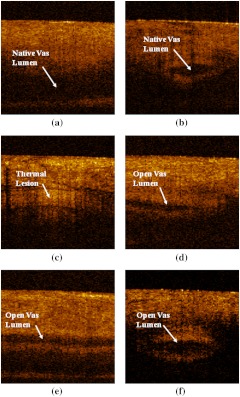
Longitudinal and cross-sectional OCT images of the vas were taken, in vivo, before and after the procedure to confirm successful thermal coagulation of the vas [Fig. 3(a), 3(b), and 3(c)]. OCT images of the excised vas were also taken, ex vivo, immediately after the procedure for comparison [Fig. 3(d), 3(e), and 3(f)]. The native vas appeared with a dark, fluid-filled lumen (absent of reflected signal intensity), while the thermal lesion was represented by a lighter region (indicative of enhanced reflected signal intensity). This is consistent with a significant increase in the scattering coefficient of soft tissue once it has been thermally denatured. It should also be noted that since the thermal lesion length along the vas () was longer than the OCT lateral scanning distance of 2 mm, it was impossible to capture the entire lesion in a single image. Furthermore, the vas was not a straight tube, but rather traveled in and out of the OCT (and HFUS) image plane.
Fig. 3.
(a) and (b) Longitudinal and cross-sectional OCT images of the native canine vas acquired while the vas and scrotal skin was compressed inside the vas ring clamp prior to the procedure. (c) Longitudinal OCT image of the thermally coagulated canine vas acquired while the vas and scrotal skin were compressed inside the vas ring clamp after the procedure. (d) Longitudinal OCT image of the thermally coagulated canine vas acquired ex vivo after the vas tissue was harvested; (e) and (f) longitudinal and cross-sectional OCT images of the native canine vas acquired ex vivo after the tissue was harvested. All OCT images measure ().
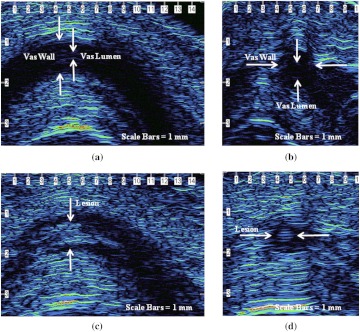
Longitudinal and cross-sectional HFUS images of the vas were also taken, in vivo, before and after the procedure to confirm successful thermal coagulation of the vas (Fig. 4). In the native vas images, the fluid-filled lumen also appears dark (with an absence of reflected signal intensity), while the thermally denatured vas appears lighter (again indicative of increased reflected signal intensity). Using HFUS, the average vas lumen diameter, vas wall thickness, and vas thermal lesion length were measured to be (), (), and (), respectively. These lumen diameter and wall dimensions are within a similar range to that previously reported for the native human vas deferens.12,13 The canine vas deferens in general is known to have similar dimensions to that of the human vas deferens.15 The thermal lesion length is strongly correlated with the 3-mm-diameter laser spot used in these studies and is also similar to measurements reported in previous studies.7–9,14
Fig. 4.
(a) and (b) Longitudinal and cross-sectional HFUS images of the native canine vas, acquired by manually isolating the vas within the scrotal skin fold prior to placement of the vas ring clamp. The vas wall and lumen can be identified. (c) and (d) Longitudinal and cross-sectional HFUS images of the thermally coagulated canine vas acquired by manually isolating the vas within the scrotal skin fold after removal of the vas ring clamp. A thermal lesion encompassing both the vas wall and lumen is observed.
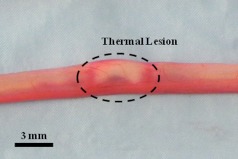
The thermally coagulated vas was excised for examination to verify the OCT and HFUS images. Figure 5 shows a representative image of the thermally coagulated vas segment, which can be identified by several indicators including blanching and shrinkage of the vas wall.
Fig. 5.
Gross image of the thermally coagulated vas segment showing significant blanching and shrinkage of the vas wall.
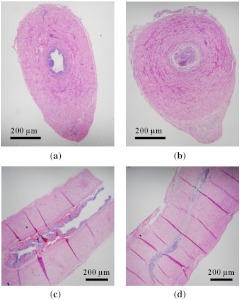
The vas samples were then processed for histology using standard techniques. For each dog, serial sectioning of the vas was performed in both the longitudinal and cross-sectional directions (one orientation for each vas—left and right sides) through the entire vas sample at 100 μm intervals. Figure 6 provides representative longitudinal and cross-sectional histologic sections of the canine vas with open lumen sections (taken beyond the thermally coagulated segment) also provided for comparison.
Fig. 6.
H&E-stained histologic sections of the vas: (a) cross-section of open lumen; (b) cross-section of thermally occluded lumen; (c) longitudinal section of open lumen; (d) longitudinal section of thermally occluded lumen.
4. Discussion
Vasectomy is a safe, simple, effective, and inexpensive surgical procedure for male sterilization. Despite the low morbidity of this procedure, societal pressures, psychological factors such as the perception of the loss of “manhood,” and male fear of surgery are reasons frequently cited by couples choosing other forms of contraception. A noninvasive laser vasectomy technique may eliminate many of these concerns and increase male acceptance of vasectomy. While some of our earlier noninvasive laser vasectomy studies have noted the formation of minor scrotal skin burns with the use of higher laser powers,8 there was no evidence of skin burns in this study. Only temporary skin irritation and reddening was observed due to the blunt trauma of the vasectomy ring clamp tips on the skin. Such trauma caused by the vas ring clamp is normal for the conventional vasectomy technique as well. This irritation disappeared approximately 15 min after the clamp was removed and the procedure completed.
The development of any completely noninvasive therapeutic procedure may benefit from the introduction of a diagnostic modality to confirm success since no tissue is removed for analysis during the noninvasive procedure. OCT and HFUS are obvious choices for use in vas imaging during noninvasive laser vasectomy because they are both relatively compact, inexpensive imaging modalities that provide sufficient image resolution and depth to view the vas deferens, which has a lumen and wall thickness of and , respectively.
OCT has an order of magnitude better resolution and an order of magnitude worse imaging depth than HFUS. Therefore, OCT was limited in these studies to imaging the vas once it was fixed and compressed beneath the scrotal skin inside the 4-mm-diameter vas ring clamp. However, while the native vas was easily visible during OCT imaging before the procedure, it was more difficult to identify the thermally coagulated vas segment after the procedure. Only longitudinal sections were obtained, and cross-sectional images of the thermally coagulated vas could not easily be confirmed. It may be that the need to perform OCT imaging of the compressed vas and scrotal skin within the clamp resulted in a distorted view of the anatomy, which in turn made interpretation of the OCT images more difficult. HFUS imaging of the vas was more successful, with longitudinal and cross-sectional images of the native and thermally coagulated vas obtained while the vas was held manually within a scrotal fold, both before and after removal of the vas from the ring clamp.
However, it should be noted that the limited imaging depth of the HFUS system required manual isolation of the vas prior to measurement. This made locating the region of interest during postoperative imaging of the thermal lesion more difficult and resulted in a slightly lower vas sample set for evaluation. In some vas, it was also difficult to differentiate the lumen from the wall before the procedure and provide two distinct measurements for these two structures. This may have been due to suboptimal probe placement or the method of manual isolation causing excessive pressure and flattening of the vas. It may be possible in future studies to design the imaging probe tip and vas clamp to minimize these limitations.
From a clinical perspective, it is also worth briefly mentioning the costs associated with noninvasive laser vasectomy, since surgical vasectomy is currently a low-cost procedure ( to $1,000). For noninvasive laser vasectomy, there are capital costs associated with purchase of the laser () and cryogen () systems. The compact, portable, tabletop, time-domain OCT system and probe used in this study is relatively inexpensive (), in comparison with other OCT systems. The compact, tabletop HFUS system and probe used in this study is also relative inexpensive () in comparison with conventional clinical HFUS consoles. However, a patient may be willing to pay a slightly higher fee for nonsurgical vasectomy.
Finally, more definitive and longer-term chronic canine studies are currently being performed to examine vas recanalization and azospermia rates, with comparison of surgical vasectomy and noninvasive laser vasectomy, prior to clinical studies. Both OCT and HFUS may be used in these chronic studies for imaging of the scarred vas.
5. Conclusions
High-frequency ultrasound may be used as a diagnostic tool to assist in determining successful laser thermal coagulation and scarring of the vas during noninvasive laser vasectomy. While optical coherence tomography also shows promise, further improvements are necessary before this imaging modality can be reliably used for evaluation of the thermally coagulated vas.
Acknowledgments
This project is supported by a research grant from the NIH, National Institute of Child Health and Human Development, and the United States Agency for International Development, through a subcontract from Family Health International (Durham, NC). The authors thank Dawn Ruben and Laurie Pipitone for assisting with the animal studies, James Hsia of the Candela Corporation (Wayland, MA) for providing the cryogen cooling system, Paul Wilson of Longport (Chadd’s Ford, PA) for providing the high-frequency ultrasound system, and Nancy Tresser of Imalux (Cleveland, OH) for providing the optical coherence tomography system used in these studies.
References
- 1.Barone M. A., et al. , “Vasectomy in the United States, 2002,” J. Urol. 176(1), 232–236 (2006). 10.1016/S0022-5347(06)00507-6 [DOI] [PubMed] [Google Scholar]
- 2.Mosher W. D., et al. , “Use of contraception and use of family planning services in the United States: 1982–2002,” Adv. Data 350, 1–36 (Dec. 10, 2004). [PubMed] [Google Scholar]
- 3.Chandra A., et al. , “Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth,” Vital Health Stat. 25, 1–160 (Dec. 2005). [PubMed] [Google Scholar]
- 4.Martinez G. M., et al. , “Fertility, contraception, and fatherhood: data on men and women from cycle 6 (2002) of the 2002 National Survey of Family Growth,” Vital Health Stat. 26, 1–142 (May2006). [PubMed] [Google Scholar]
- 5.Miller W. B., et al. , “Tubal sterilization or vasectomy: how do married couples make the choice,” Fertil. Steril. 56(2), 278–284 (1991). [DOI] [PubMed] [Google Scholar]
- 6.Shain R. N., et al. , “Factors associated with married women’s selection of tubal sterilization and vasectomy,” Fertil. Steril. 43(2), 234–244 (1985). [PubMed] [Google Scholar]
- 7.Cilip C. M., et al. , “Noninvasive laser vasectomy: preliminary ex vivo tissue studies,” Lasers Surg. Med. 41(3), 203–207 (2009). 10.1002/lsm.v41:3 [DOI] [PubMed] [Google Scholar]
- 8.Cilip C. M., et al. , “Noninvasive laser coagulation of the canine vas deferens, in vivo,” Proc. SPIE 7548, 75481D (2010). 10.1117/12.839601 [DOI] [Google Scholar]
- 9.Cilip C. M., et al. , “Application of an optical clearing agent during noninvasive laser coagulation of the canine vas deferens,” J. Biomed. Opt. 15(4), 048001 (2010). 10.1117/1.3463009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafik A., “Electrovasogram: a canine study of the electromechanical activity of the vas deferens,” Urology 46(5), 692–696 (1995). 10.1016/S0090-4295(99)80303-3 [DOI] [PubMed] [Google Scholar]
- 11.Davis C., Kuang W., “Optical coherence tomography: a novel modality for scrotal imaging,” Can. Urol. Assoc. J. 3(4), 319–322 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puttemans T., Delvigne A., Murillo D., “Normal and variant appearances of the adult epididymis and vas deferens on high-resolution sonography,” J. Clin. Ultrasound 34(8), 385–392 (2006). 10.1002/(ISSN)1097-0096 [DOI] [PubMed] [Google Scholar]
- 13.Middleton W. D., et al. , “High-resolution sonography of the normal extrapelvic vas deferens,” J. Ultrasound Med. 28(7), 839–846 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Cilip C. M., et al. , “High-frequency ultrasound imaging of noninvasive laser coagulation of the canine vas deferens,” Lasers Surg. Med. 43(8), 838–842 (2011). 10.1002/lsm.21098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leocadio D. E., et al. , “Anatomical and histological equivalence of the human, canine, and bull vas deferens,” Can. J. Urol. 18(3), 5699–5704 (2011). [PubMed] [Google Scholar]