Abstract.
Glaucoma is the second-leading cause of blindness worldwide and is often associated with elevated intraocular pressure (IOP). Partial thickness intrascleral channels can be created with a femtosecond laser operating at a wavelength of 1700 nm. Such channels have the potential to increase outflow facility and reduce elevated IOP. Analysis of the dimensions and location of these channels is important in understanding their effects. We describe the application of two-photon microscopy and confocal microscopy for noninvasive imaging of the femtosecond laser created partial-thickness scleral channels in human cadaver eyes. High-resolution images, hundreds of microns deep in the sclera, were obtained to allow determination of the shape and dimension of such channels. This demonstrates that concept of integrating femtosecond laser surgery, and two-photon and confocal imaging has the future potential for image-guided high-precision surgery in transparent and translucent tissue.
Keywords: confocal microscopy, femtosecond laser, glucoma, sclera, two photon microscopy
1. Introduction
Glaucoma is the second leading cause of blindness worldwide. It is a common ophthalmic disease, often associated with elevated intraocular pressure (IOP). More than two million people in the United States are affected by glaucoma.1 Current medical and surgical therapies are directed at reducing IOP.2 Drug treatments have several associated side effects.3 Traditional and laser surgical interventions also have unsatisfying disadvantages such as perioperative complications and limited longevity of achieved IOP reductions. In addition, surgical treatments may be associated with increased risk of intra-ocular infection.3
Over the last decade, the field of femtosecond eye surgery has expanded rapidly, supporting the advantage of combined high ablation precision and minimized collateral tissue effects.4–9 The tissue can be removed cleanly and without thermal or mechanical damage by this effect. It was investigated and discussed by Teng et al., Stern et al., and Niemz et al.10–14 By employing femtosecond laser with appropriate wavelength, photodisruption can be used to create subsurface incisions in the translucent sclera without damaging the overlying tissues. Femtosecond laser with a wavelength of 1700 nm has the ability to create partial-thickness intrascleral channels.15,16 Femtosecond laser creates partial thickness fistulas that increased the rate of aqueous humor (AH) outflow in the cadaver eye and is mentioned in this report as AH drainage channel in the sclera.17
Perfusion experiment on the human cadaver eye was performed to explore the effect of femtosecond laser ablation drainage channels in the sclera on the outflow rate of aqueous humor (AH). Although perfusion on cadaver eye will increase the IOP, measurement results showed the decrease of IOP after the partial thickness intrascleral channels were created. The positive result indicates that IOP can be reduced by increasing the outflow facility with femtosecond laser created channels and that minimally invasive femtosecond laser treatments may have a future for surgical treatments of glaucoma.18
Dimensions and location of femtosecond-laser-induced partial-thickness intrascleral channels will affect the outflow rate resulting in IOP reduction. Analysis of the dimensions and location of such channels is important in understanding their effects. Observation of such channels will be important for optimization of laser treatment parameters and helpful for the future of femtosecond laser surgical treatments of glaucoma.
The two photon and confocal microscope integrates two functions: confocal microscopy and two-photon microscopy. A confocal microscope has the ability to optically section through a relatively thick, light-scattering tissue specimen to provide a three-dimensional (3-D) image, thereby eliminating the need for processing and sectioning procedures.19,20 Two-photon microscopy forms an image from the nonlinear optical process induced by ultrahigh photon flux/multiphoton absorption. The second harmonic generation (SHG) signals from the sample are collected to form a microscopic image. This technology has a series of advantages, such as diffraction-limited resolution and deep depth discrimination; reduced photo-damage or bleaching effect outside the laser focus and without the need to fix, process, section, and stain the sample.7,8,21,22 SHG signals can be obtained from sclera and used to study collagen organization.23,24 In this report we use the confocal two-photon microscopy to investigate femtosecond-laser-created partial-thickness drainage channels in the sclera of human cadaver eye.
2. Material and Methods
A femtosecond laser of wavelength 1700 nm was employed to create partial-thickness drainage channels in the sclera of a human cadaver eye. A confocal and two photon microscope was used to investigate the dimensions and location of these channels.
2.1. Femtosecond Laser System
A titanium:sapphire femtosecond laser system and an optical parametric amplifier (OPA) system were employed in this study. This laser is a femtosecond all-solid-state laser based on passive mode locking and chirped pulse amplification (CPA). This output laser beam from the femtosecond laser system was used to pump OPA system (Opera, Coherent, Santa Clara, CA). The output laser beam used in our experiment had a wavelength of 1700 nm, repetition rate of 5 KHz, and laser energy of up to 60 micro joule per pulse. In order to achieve the best ablation effect inside the sclera, the maximum laser pulse energy was employed to create the AH drainage channel in the sclera.
2.2. Sample Preparation
Ten human cadaver eyes were tested. The eyes were obtained from the San Diego eye bank. The cadaver eyes were shipped in Styrofoam containers packed in wet ice packs. Experiment was done as soon as possible after the cadaver eyes were taken out from organ culture medium.
The enucleated eyes were placed on a three-axis scanner stage driven by three stepper motors, one along each axis (Newport MM1000 and stepper motors; Newport Corp., Irvine, CA). The three scanning axes compose a Cartesian coordinate system. The stepper motors were controlled by a computer using Labview 7.1 (National Instruments) through electronic controller (ESP 300, Newport Corp.). In order to minimize light scattering on the surface of the tissue, a microscope slide was placed on the cadaver eye at the proposed location of the channel. For eliminating nonlinear effects like self focusing, a 0.5 numerical aperture (NA) objective was used to focus the laser beam into the tissue. The scanner stages were moved along programmed patterns with predetermined velocity to create the partial thickness AH drainage channel in the sclera. All remaining surfaces of the globe were covered with wet paper in order to prevent their surfaces from dehydration. Perfusion experiment was done before and after the laser ablation to investigate the effect of drainage channels on the outflow rate of aqueous humor. After perfusion experiment, the ablation area was cut to sample and fixed with formalin for future confocal and two-photon microscope imaging.25
2.3. Two Photon and Confocal Microscope
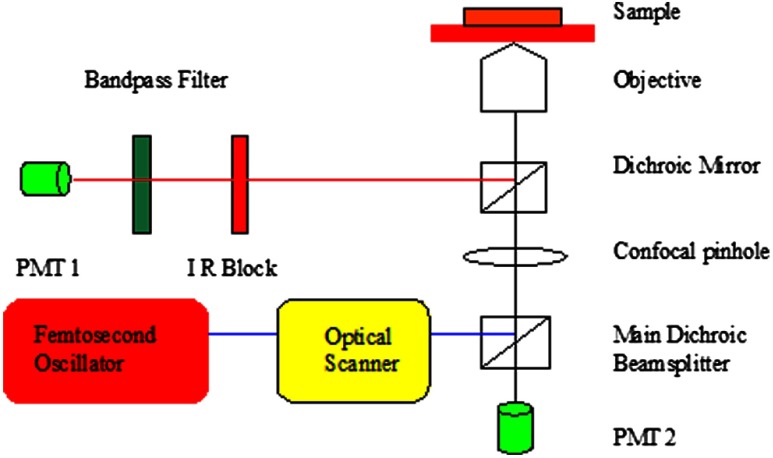
In this study, second harmonic generation signals (SHG) imaging and confocal imaging were performed on an inverted laser scanning multiphoton microscope (Zeiss LSM 510 META, Carl Zeiss, Jena, Germany) equipped with a mode-locked titanium:sapphire laser (Chameleon, Coherent, Santa Clara, CA, USA), which has a center wavelength of 800 nm for the experiments.26–28 A schematic drawing of this microscope is shown at Fig. 1. An Acoustic Optic Modulator (AOM) (Carl Ziess, Jena, Germany) was employed to attenuate the intensity of the illumination laser. The femtosecond oscillator produces a 150 femtoseconds pulse with a 90-MHz repetition rate. An illumination laser beam was circularly polarized with a quarter wave plate interposed between the oscillator and the microscope. Specimens were imaged with , , and oil-immersion objective lenses (Carl Ziess, Jena, Germany). The confocal pinhole was set to the maximal open setting for SHG application. The backward SHG signals were collected from the same objective and detected over the wavelengths from 377 to 430 nm with the detector on microscope (META detector on the model 510, Carl Ziess, Jena, Germany). Maximum input laser power was used to create the maximum SHG signal from tissue for the optimal SHG image quality. The pinhole was used for confocal application, and the reflected/backscattered laser signals were collected through the same objective and were directed by a main dichroic beam splitter to the photomultiplier tube detector (PMT2). Some samples were scanned using 5-micron vertical step size to generate 3-D data to a depth of 200 micron within the sclera. All images were recorded as 12-bit, images. 3-D data sets were reconstructed using the LSM Image Examiner (Carl Zeiss, Jena, Germany).
Fig. 1.
Schematic drawing of the two-photon confocal microscope experiment setup.
3. Results
During laser irradiation, the sclera is ablated at the laser focus. This is because of the formation of cavitation bubbles, where the sclera tissue is transformed into gas and vapors. The gas or vapor either diffuses out of the tissue or is resorbed within hours. After femtosecond laser treatment, the eyeball was kept moist with 4% phosphate-buffered saline (PBS, pH 7.4) for about two hours to wait for the ablation plume to diffuse. Then the sclera was excised and fixed with freshly prepared paraformaldehyde (4% in PBS, pH 7.4) for future imaging process.
3.1. Confocal Imaging
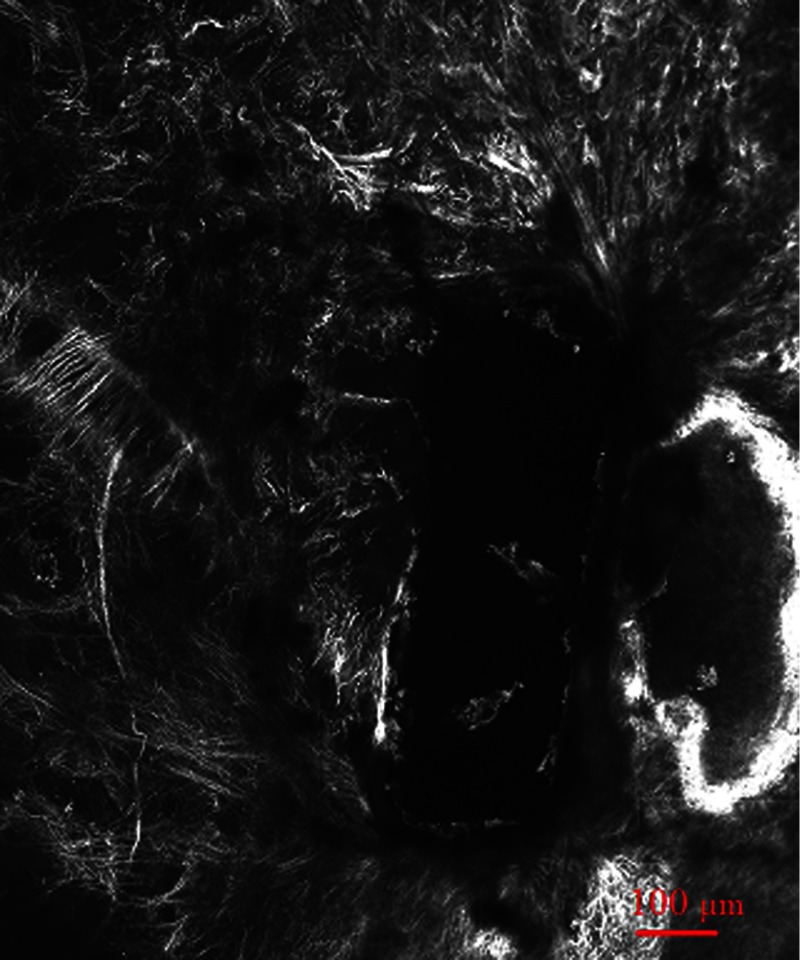
A rectangle intrascleral channel was created by a femtosecond laser 100 micron under the surface at a depth of 200 micron. Figure 2 shows a clear view of this subsurface channel at 110-micron depth count from the surface by confocal imaging. The image was obtained by illumination laser beam at wavelength 543 nm with oil-immersion objective. The resolutions at both axes are 0.9 micron. The stack size has a width of 2303 micron and a length of 2764 micron. Most laser ablation surfaces are smooth and are not coagulated or damaged by thermal effect. There is still a little tissue remaining inside the channel, which illustrates the difficulty of subsurface laser ablation, and more research should be done focusing on the ablation laser energy and ablation scanning pattern.
Fig. 2.
Confocal imaging of subsurface channel at 110-micron depth.
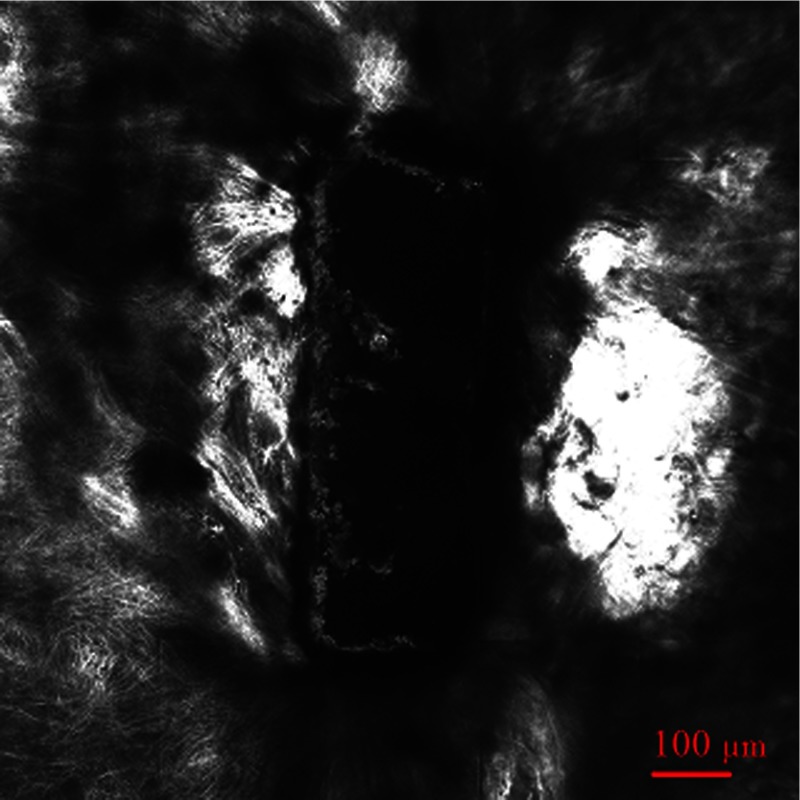
Figure 3 shows the same intrascleral channel at 205-micron depth. All parameters are the same as in Fig. 2. Subsurface incision was cut without damaging the region adjacent to treated volume.
Fig. 3.
Confocal imaging of subsurface channel at 205-micron depth.
A video of the whole channel was shown as Video 1 to show the total dimensions of AH drainage channel in the sclera.
Video 1.
3.2. SHG Imaging
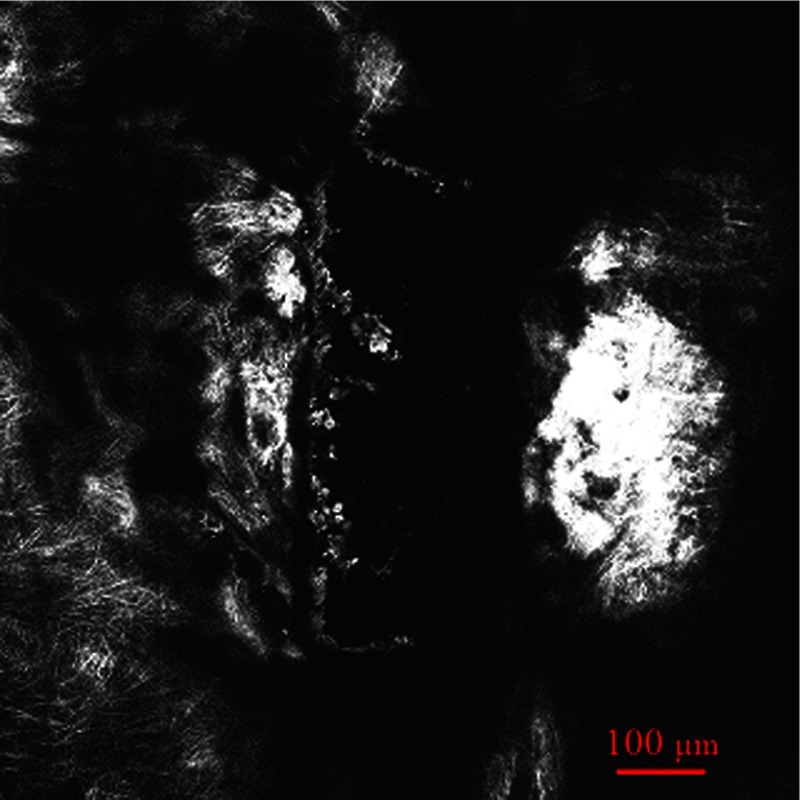
As a comparison with Fig. 3, a SHG imaging was observed at the same plane. All other parameters are the same except the wavelength of illumination laser beam is 800 nm. Figure 4 shows the SHG imaging.
Fig. 4.
SHG imaging of subsurface channel at 205-micron depth.
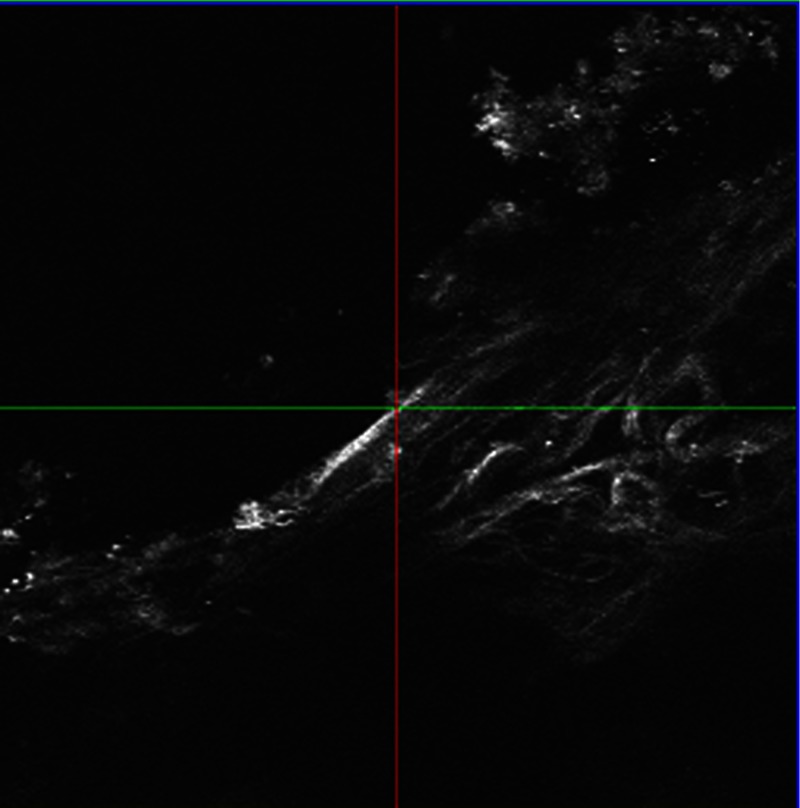
A oil-immersion objective was employed to investigate the edge of the intrascleral channel. The resolutions at both axes are 0.45 micron since a high-NA objective was involved. A series of SHG imaging was obtained to reconstruct the 3-D data sets. As shown in Fig. 5, SHG imaging is excellent in revealing intrascleral channel edge. The border region can be clearly recognized. The collagens adjacent to treated volume were untouched, and there is no indication of thermal damage.
Fig. 5.
SHG imaging of subsurface channel edge.
4. Discussion
Two-photon and confocal microscopy excel at high-resolution imaging hundreds of microns deep in the sclera of the human cadaver eye. It can generate confocal imaging or SHG imaging without processing and sectioning procedures. Because it avoids the shrinkage and distortion associated with conventional processing and sectioning procedures, it provides an important approach to evaluate dimensions and location of femtosecond-laser-induced subsurface channel in the sclera of human cadaver eye under the condition close to its physiological state. Such information will be important in understanding the femtosecond ablation effect in sclera on IOP reduction and helpful for the future of femtosecond laser surgical treatments of glaucoma.
As shown in Figs. 2 and 3, confocal microscopy provides a clear view. They demonstrated an excellent vertical ablation quality. The smooth ablation interface and high ablation precision confirmed the deterministic result from femtosecond laser ablation.
A lot of work has already been published concerning SHG imaging of the cornea.7,8,22,24,27,28 It is well known that the forward-scattered SHG is much more intensive than the backward-scattered SHG from the cornea. However, previous studies show that the intensity of the backward-scattered SHG from sclera is comparable to the forward-scattered SHG.24 Our results also show that backward-scattered SHG imaging is possible to investigate the femtosecond laser intrascleral channel. Figure 4 shows that after femtosecond laser ablation, most collagen fibers adjacent to the intrascleral channel have remained nearly intact, and a minimal invasive surgery process was obtained. In a clinical situation, forward-scattered SHG imaging seems to be unrealistic for diagnostic purposes; backward-scattered SHG imaging may be a possible method to explore the femtosecond laser intrascleral channel.
Figure 4 shows SHG imaging from the same position as Fig. 3. The imaging is blurred in Fig. 4 compared with Fig. 3. It is difficult to acquire distinct imaging at the depth of the sclera. Two-photon microscopy penetrated less deeply into the sclera than the cornea, which is consistent with previous studies.23 In contrast with corneal tissue, the sclera scatters visible light because Bragg scattering.29 By proper preparation with dehydrating and a suitable wavelength chosen, the intrascleral channel can be introduced.15,16,30,31 It is reasonable to hypothesize that we can use the same wavelength of 1700 nm as the femtosecond intrascleral channel created to generate SHG imaging of wavelength 850 nm in the sclera. Both wavelengths are more transparent for the sclera after proper preparation, and a distinct imaging will be obtained in deep depth of the sclera. The oscillator output at 800 nm is suitable to generate SHG imaging as commercial two-photon microscopy. Minimal invasive surgery and noninvasive diagnostics can be integrated in one femtosecond laser system for potential glaucoma femtosecond laser surgery. On the one hand, an amplified laser beam can produce an intrascleral channel to increase the outflow rate resulting in reduced IOP; one the other hand, an attenuated laser beam can introduce backward SHG imaging of such intrascleral channel. Therefore, therapeutic and diagnostic applications can be combined into one femtosecond laser and two-photon confocal microscope systems.
Acknowledgments
This work was supported by the National Institutes of Health, Grant R01 EY014456.
References
- 1.Schmier J., Halpern M., Jone M., “The economic implications of glaucoma: a literature review,” Pharmacoeconomics 25(4), 287–308 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Brusini P., Johnson C. A., “Staging functional damage in glaucoma: review of different classification methods,” Surv. Ophthalmol. 52(2), 156–179 (2007). 10.1016/j.survophthal.2006.12.008 [DOI] [PubMed] [Google Scholar]
- 3.Yee Richard W, “The effect of drop vehicle on the efficacy and side effects of topical glaucoma therapy: a review,” Curr. Opt. Ophthalmol. 18(2), 134–139 (2007). 10.1097/ICU.0b013e328089f1c8 [DOI] [PubMed] [Google Scholar]
- 4.Juhasz T., et al. , “Corneal refractive surgery with femtosecond lasers,” IEEE J. Selec. Top. Quam. Electron. 5, 902–910 (1999). 10.1109/2944.796309 [DOI] [Google Scholar]
- 5.Bille J. F., Harner C. F. H., Loesel F., New Frontiers in Vision and Aberration-Free Refractive Surgery, Springer Press, Heidelberg, Germany: (2002). [Google Scholar]
- 6.Juhasz T., et al. , “The femtosecond blade: applications in corneal surgery,” Opt. Photo. News 13, 24–29 (2002). 10.1364/OPN.13.1.000024 [DOI] [Google Scholar]
- 7.Han M., et al. , “Mini-invasive corneal surgery and imaging with femtosecond lasers,” Opt. Express 12, 4275–4281 (2004). 10.1364/OPEX.12.004275 [DOI] [PubMed] [Google Scholar]
- 8.Sun H., et al. , “Femtosecond laser ablation threshold: dependence on tissue depth and laser pulse width,” Lasers Surg. Med. 39(8), 654–658 (2007). 10.1002/lsm.20538 [DOI] [PubMed] [Google Scholar]
- 9.Loesel F. H., et al. , “Laser-induced optical breakdown on hard and soft tissues and its dependence on the pulse duration: experiment and model,” IEEE J. Quantum Electron. 32, 1717–1722 (1996). 10.1109/3.538774 [DOI] [Google Scholar]
- 10.Teng P., et al. , “Acoustic studies of the role of immersion in plasma mediated laser ablation,” IEEE J. Quant. Electron. 23, 1845–1852 (1987). 10.1109/JQE.1987.1073233 [DOI] [Google Scholar]
- 11.Stern D., et al. , “Corneal ablation by nanosecond, picosecond and femtosecond lasers at 532 and 625 nm,” Arch. Ophthalmol. 107, 587–592 (1989). 10.1001/archopht.1989.01070010601038 [DOI] [PubMed] [Google Scholar]
- 12.Niemz M. H., Klancnik E. G., Bille J. F., “Plasma mediated ablation of corneal tissue at 1053 nm using a Nd:YLF oscillator/regenerative amplifier laser,” Lasers Surg. Med. 11, 426–431 (1991). 10.1002/(ISSN)1096-9101 [DOI] [PubMed] [Google Scholar]
- 13.Niemz M. H., Laser-Tissue Interactions—Fundamentals and Applications, 3rd ed., Springer Press, Heidelberg, Germany: (2003). [Google Scholar]
- 14.Juhasz T., et al. , “Applications of femtosecond lasers in corneal surgery,” Laser Phys. 10(2), 495–500 (2000). [Google Scholar]
- 15.Sacks Z. S., et al. , “High precision subsurface photodisruption in human sclera,” J. Biomed. Opt. 7(3), 442–450 (2002). 10.1117/1.1482381 [DOI] [PubMed] [Google Scholar]
- 16.Sacks Z. S., et al. , “Subsurface photodisruption in human sclera: wavelength dependence,” Ophthalmol. Surg. Laser Im. 34(2), 104–113 (2003). [PubMed] [Google Scholar]
- 17.Juhasz T., “The effect of femtosecond laser scleral treatments on the outflow of aqueous humor,” Invest. Ophtha. Vis. Sci. 46, 1052 Suppl. S (2005). [Google Scholar]
- 18.Dongyul C., et al. , “3-D finite element model of aqueous outflow to predict the effect of femtosecond laser created partial thickness scleral channels on the IOP,” Laser Surg. Med. 39(Suppl. 19), 41–41 128 Suppl. 19 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Patel D. V., MRCOphth, McGhee Charles NJ, “Contemporary in vivo confocal microscopy of the living human cornea using white light and laser scanning techniques: a major review,” Clin. Expe. Ophthalmol. 35(1), 71–78 (2007). 10.1111/ceo.2007.35.issue-1 [DOI] [PubMed] [Google Scholar]
- 20.Jester J. V., “Extent of corneal injury as a biomarker for hazard assessment and the development of alternative models to the Draize rabbit eye test,” Cutan. Ocular Tox. 25(1), 41–54 (2006). 10.1080/15569520500536626 [DOI] [PubMed] [Google Scholar]
- 21.Denk W., Strickler J. H., Webb W. W., “Two-photon laser scanning fluorescence microscopy,” Science 248, 73–76 (1990). 10.1126/science.2321027 [DOI] [PubMed] [Google Scholar]
- 22.Sun H., et al. , “Ultrafast all-solid-state lasers for mini-invasive eye surgery,” Invest. Ophthalmol. Vis. Sci. 45: U139–U139 180 Suppl. 1 (2004). [Google Scholar]
- 23.Teng S. W., et al. , “Multiphoton autofluorescence and second-harmonic generation imaging of the ex vivo porcine eye,” Invest. Ophthalmol. Vis. Sci. 47(3), 1216–1224 (2006). 10.1167/iovs.04-1520 [DOI] [PubMed] [Google Scholar]
- 24.Han M., Giese G., Bille J. F., “Second harmonic generation imaging of collagen fibrils in cornea and sclera,” Opt. Exp. 13(15), 5791–5797 (2005). 10.1364/OPEX.13.005791 [DOI] [PubMed] [Google Scholar]
- 25.Chai D., et al. , “3-D finite element model of aqueous outflow to predict the effect of femtosecond laser created partial thickness drainage channels,” Las. Surg. Med. 40, 188–195 (2008). 10.1002/(ISSN)1096-9101 [DOI] [PubMed] [Google Scholar]
- 26.Brown D. J., et al. , “Application of second harmonic imaging microscopy to assess structural changes in optic nerve head structure ex vivo,” J. Biomed. Opt. 12, 024029 (2007). 10.1117/1.2717540 [DOI] [PubMed] [Google Scholar]
- 27.Morishige N., et al. , “Noninvasive corneal stromal collagen imaging using two-photon-generated second-harmonic signals,” J. Cat. Refrac. Surg. 32(11), 1784–1791 (2006). 10.1016/j.jcrs.2006.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morishige N., et al. , “Second-harmonic imaging microscopy of normal human and keratoconus cornea,” Invest. Ophthalmol. Vis. Science 48, 1087–1094 (2007). 10.1167/iovs.06-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaezy S., Clark J. I., “A quantitative analysis of transparency in human sclera and cornea using Fourier methods,” J. Micro. 163, 85–94 (1991). 10.1111/jmi.1991.163.issue-1 [DOI] [PubMed] [Google Scholar]
- 30.Tuchin V. V., et al. , “Light propagation in tissues with controlled optical properties,” J. Biomed. Optics 2(4), 401–417 (1997). 10.1117/12.281502 [DOI] [PubMed] [Google Scholar]
- 31.Zimnyakov D. A., et al. , “In vitro human sclera structure analysis using tissue optical immersion effect,” Proc. SPIE 2673(44), 233–242 (1996). 10.1117/12.240070 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.