Abstract
Limited functional imaging evidence suggests increased beta-amyloid deposition is associated with alterations in brain function, even in healthy older adults. However, the majority of these findings report on resting-state activity or functional connectivity in adults over age 60. Much less is known about the impact of beta-amyloid on neural activations during cognitive task performance, or the impact of amyloid in young and middle-aged adults. The current study measured beta-amyloid burden from PET imaging using18Florbetapir, in a large continuous age sample of highly-screened, healthy adults (N = 137; aged 30–89 years). The same participants also underwent fMRI scanning, performing a memory encoding task. Using both beta-amyloid burden and age as continuous predictors of encoding activity, we report a dose-response relationship of beta-amyloid load to neural function, beyond the effects of age. Specifically, individuals with greater amyloid burden evidence less neural activation in bilateral dorsolateral prefrontal cortex, a region important for memory encoding, as well as reduced neural modulation in areas associated with default network activity: bilateral superior/medial frontal and lateral temporal cortex. Importantly, this reduction of both activation and suppression as a function of amyloid load was found across the lifespan, even in young- and middle-aged individuals. Moreover, this frontal and temporal amyloid-reduced activation/suppression was associated with poorer processing speed, verbal fluency, and fluid reasoning in a subgroup of individuals with elevated amyloid, suggesting that it is detrimental, rather than compensatory in nature.
1. Introduction
Beta-amyloid plaque (Aβ) deposition is a hallmark characteristic of Alzheimer’s Disease (AD) pathology. Until recently, Aβ deposition could only be quantified at autopsy, precluding our understanding of its in vivo developmental trajectory and how differing levels of Aβ affected neural and cognitive function. Recently, radiolabeled agents have been developed such as Pittsburgh-Compound-B (PiB) and [18F]-AV-45 (Florbetapir) that permit measurement of fibrillar Aβ deposition in vivo using PET imaging (Klunk et al., 2004; Wong et al., 2010). Strikingly, 20–30% of cognitively normal older adults appear to harbor Aβ accumulation at levels associated with AD (Aizenstein et al., 2008; Pike et al., 2007; Rodrigue et al., 2009; Rodrigue et al., 2012; Rowe et al., 2007). Recently, the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s Disease (Sperling et al., 2011) suggested that significant Aβ deposition in asymptomatic healthy adults might be evidence of “preclinical Alzheimer’s disease,” while noting that research was critically needed in healthy adults to better understand the relationship of Aβ to neural and cognitive function. Thus, the focus of the present study is on understanding the effects of Aβ deposition on neural function in healthy adults across the adult lifespan from age 30 to 89, as well as the relationship between that altered neural function and cognitive performance.
To date, existing knowledge regarding amyloid’s effect on neural function in normal adults has focused almost entirely on adults over age 60 and has largely been based on studies of resting-state intrinsic connectivity and default mode network (DMN) function (Hedden et al., 2009; Sheline et al., 2010; Sperling et al., 2009; Vannini et al., in press). Functional imaging (fMRI) studies of intrinsic functional connectivity at rest in healthy older adults showed that Aβ accumulation had an adverse effect, decreasing neural connectivity in regions of the default mode network (Hedden et al., 2009; Sheline et al., 2010). The default mode network is a large-scale brain network that is active during rest and typically appears as task-related deactivation during functional imaging studies. When young subjects are presented with a cognitive challenge or cognitive task, they actively suppress activity in the default sites and activate neural circuitry utilized for the cognitive task. Interestingly, unlike young adults, older adults have difficulty suppressing or modulating this default activity in response to cognitive challenge (Damoiseaux et al., 2008). Thus far, there is evidence that Aβ negatively affects modulation of the default network in healthy older adults, adding to age-related difficulties in efficient modulation. Recent studies have shown that when older adults were presented with name-face pairs to encode (Sperling et al., 2009), those with elevated Aβ had increased difficulty suppressing default activity during encoding and also showed less habituation of neural signal to repetition of name-face pairs (Vannini et al., in press). Sperling et al (2009) have reported increased hippocampal activation with increased Aβ but only in PiB-positive cognitively impaired (CDR 0.5) subjects.
Although relatively little is known about the effect of Aβ on connectivity and default activity, even less is known about how Aβ affects activation during cognitive engagement. A recent study has shown enhanced activation during encoding in left occipital cortex and right hippocampus in older adults who had elevated Aβ deposition, but no effects were found in regions of the default mode network (Mormino et al., in press).
The present study asks a different question about the impact of Aβ than has been raised to date. Thus far, our understanding of the relationship of Aβ deposition in healthy adults to neural function has been limited almost entirely to older adults with levels of Aβ above some critical cutoff score. In the present study we were interested in understanding the incremental effects of Aβ deposition (independent of any cutoff level) on neural function. We examined the effect of Aβ during encoding (task-positive activity) as well as its effect on activity in task-negative regions (baseline minus encoding, which typically isolates the default mode network) across the adult lifespan. We recently reported that amyloid deposition shows some variance in individuals aged 30–59, as well as in older adults (Rodrigue et al., 2012). Therefore we hypothesized that increasing Aβ burden would affect neural activity across the lifespan, regardless of one’s age (e.g., even in middle-aged adults). Our approach allows us to address several open questions: (a) Controlling for age, does Aβ load relate to neural function during an encoding task and what is the nature of the relationship (i.e., does it correspond to increased or decreased activation)? (b) Does Aβ burden exert an effect on both task-positive and task-negative activity? (c) Does the impact of Aβ burden interact with age? In other words, does amyloid load predict neural function across the adult lifespan or is its effect limited to older age? (d) Are the alterations in neural activity associated with amyloid deposition related to poorer cognitive performance?
We addressed these questions in subjects from the Dallas Lifespan Brain Study (a large lifespan study of healthy neurocognitive aging), using PET imaging of 18F-Florbetapir, which displays strong agreement with Aβ plaque labeling in an autopsy study (Clark et al., 2011). Subjects performed an fMRI scene-encoding task developed in our laboratory (Gutchess et al., 2005; H. Park et al., under revision) to assess the impact of Aβ on memory encoding. We focused on precuneus Aβ deposition as our index of amyloid burden because the precuneus is a critical component of the memory system (Cabeza and Nyberg, 2000; Rugg and Henson, 2003), it displays the greatest metabolic abnormality with AD progression (Buckner et al., 2008), and it is also the brain region with the highest amyloid deposition in both AD and normal aging (Aizenstein et al., 2008; Mintun et al., 2006; Rodrigue et al., 2012). We note that our sample size combined with a wide age distribution allowed us to examine Aβ as a continuous variable rather than relying on cutoff scores that dichotomize participants as either Aβ positive or Aβ negative.
2. Methods and Materials
2.1. Participants
Participants were paid, healthy volunteers selected from the larger Dallas Lifespan Brain Study (DLBS; N = 350, aged 20–89), which is a comprehensive lifespan study on cognitive function and neuroimaging. DLBS includes detailed and comprehensive measures of brain structure and function using MRI as well as multiple measures of a broad range of neuropsychological and cognitive indices. DLBS participants were recruited through media advertisements and flyers and underwent health history screening via a health questionnaire as well as telephone and personal interviews. We invited all DLBS participants who were 30–90 years old and who had completed functional MRI and cognitive testing within the last 12 months to return for a beta-amyloid PET scan. We contacted 215 participants and received 196 replies (91%). Of these, 90% accepted the PET scan invitation (177), and the first 137 participants scanned are reported herein. Participants were healthy adults ranging in age from 30–89 (mean age 64.2 ± 16.3 years), including 82 women and 55 men. All participants were screened against cardiovascular, neurological and psychiatric disorders, head injury with loss of consciousness > 10 min, and drug/alcohol abuse. Participants were native English speakers and strongly right-handed (on the Edinburgh Handedness Questionnaire; Oldfield, 1971). The participants were well-educated (mean 16.4 ± 2.7 years) and scored highly (29.3 ± .9) on the Mini Mental State Examination (MMSE; Folstein et al., 1975). All participants provided written informed consent and were debriefed in accord with university human investigations committee guidelines. Table 1 details sample demographics, including APOE ε4 distribution and cognitive score means.
Table 1.
Sample demographic information
| Age | N | Education | MMSE | APOE ε4 | PS | WM | VF | FR | Hits | d’ |
|---|---|---|---|---|---|---|---|---|---|---|
| 30–49 | 30 | 16.63 | 29.47 | 27% | 61.47 | 24.63 | 36.87 | .88 | .47 | .10 |
| 50–69 | 46 | 16.97 | 29.50 | 28% | 55.53 | 25.02 | 37.09 | .85 | .50 | .24 |
| 70–89 | 61 | 15.85 | 29.02 | 20% | 43.28 | 18.67 | 32.57 | .70 | .55 | −.23 |
| Total | 137 | 16.40 | 29.28 | 24% | 51.35 | 22.11 | 35.03 | .79 | .52 | 0 |
Note. APOE ε4 – percent heterozygous or homozygous for ApolipoproteinE ε4; PS – processing speed (number items completed); WM – working memory (total span); VF – verbal fluency (number words generated); FR – fluid reasoning (proportion correct); Hits – proportion of high confidence hits on the subsequent memory recognition task; d’ – d-prime as standardized z-scores = z[High Confidence hits] – z[High Confidence false alarms]
2.2. Procedures
All participants completed four visits for this study in the following order: two 2-hr visits to the lab for cognitive and neuropsychological testing, one 2-hr visit to the UT Southwestern Advanced Imaging Research Center for structural and functional MRI scanning, and one 2-hr visit to the UT Southwestern PET imaging center for amyloid imaging. Average time between MRI visit and PET visit was 7±4 months.
2.2.1. Neuropsychological Testing
The full battery included measures of processing speed, verbal fluency, working memory, episodic memory, and fluid reasoning. For this study Processing Speed was measured by Digit Symbol (from Wechsler Adult Intelligence Scale III; Wechsler, 1997), Verbal Fluency was measured with the Controlled Oral Word Association Test (Spreen and Benton, 1977), Working Memory was assessed with Operation Span (Turner and Engle, 1989), and Reasoning was measured with Raven’s Progressive Matrices (Raven, 1976). Table 1 displays sample means for these cognitive measures.
2.2.2. PET Protocol
2.2.2.1. PET Acquisition
All participants were injected with a 370 MBq (10 mCi) bolus of 18F-Florbetapir as described in Rodrigue et al (2012). At 30min post injection subjects were positioned on the imaging table of a Siemens ECAT HR PET scanner. Soft Velcro straps and foam wedges were used to secure the participant’s head and the participant was positioned using laser guides. A 2min scout was acquired to ensure the participant’s brain was completely in the field-of-view and there was no rotation in either plane. A 2-frame by 5-min each dynamic emission acquisition was started 50min post injection and immediately after an internal rod source transmission scan was acquired for 7min. The transmission image was reconstructed using backprojection and a 6mm FWHM Gaussian filter. The emission images were processed by iterative reconstruction, 4 iterations and 16 subsets with a 3mm FWHM ramp filter.
2.2.2.2. PET Data Processing
PET data were processed as described previously (Rodrigue et al., 2012). Each participant’s PET scan was spatially normalized to a Florbetapir template (2×2×2mm3 voxels) registered to MNI space using SPM8 and in-house MATLAB scripts and visually inspected for registration quality. Regions of Interest (ROIs) in template space were created in-house to obtain anatomically defined amyloid counts. ROIs were overlaid onto template-normalized subject scans, and exclusion masked to minimize white matter. To develop an appropriate white matter mask, we averaged across SUVR maps from the youngest participants scanned, setting an SUVR threshold (1.2; selected on the basis of visual observation) that successfully segmented white from gray matter on their average map (Rodrigue et al., 2012). The resulting white matter binary mask was used to erode the ROIs so that the regional uptake estimates were affected minimally by white matter uptake (within the limits of the resolution of the PET imaging technique). We recognize that this approach relies on the assumption that our youngest controls (age 30–34) were free of amyloid plaques and that our threshold selection accurately represented the boundary between non-specific and specific binding. Florbetapir counts were extracted for each ROI, and then normalized by binding counts in the cerebellar gray matter to produce standardized uptake value ratios (SUVR) that represent regional beta-amyloid burden for each region for each subject.
2.2.3. MRI Protocol
2.2.3.1. MRI Acquisition
All participants were scanned on a single 3T Philips Achieva scanner equipped with an 8-channel head coil. High-resolution anatomical images were collected with a T1-weighted MP-RAGE sequence with 160 axial slices, 1×1×1mm3; 256×256×160 matrix, TR=8.18ms, TE=3.76ms, flip-angle=12°, FOV=220mm. Blood Oxygen Level Dependent (BOLD) fMRI data were acquired using a T2*-weighted echo-planar imaging sequence (with SENSE encoding) with 43 interleaved axial slices per volume acquired parallel to the AC-PC line, 64×64×43 matrix (3.4×3.4×3.5mm3), FOV=220mm, TE=25ms, TR=2sec, FA=80°. Five dummy volumes were discarded at scan time to allow for T1 stabilization.
2.2.3.2. fMRI Task
Using an incidental encoding paradigm, participants viewed images of outdoor landscape scenes and determined (via yes or no button press) whether there was water present in the scene (Gutchess et al., 2005). Images were presented for 3s each in an event-related design, and jittered so that ITIs ranged between 4 and 14s. Three runs of 32 outdoor scenes were presented. Total scan time was 20min. Responses were recorded using a fiber-optic button box held in the right hand. Visual stimuli were presented using E-prime software projected through the back of the scanner and viewed though a mirror attached to the head coil. During an off-line recognition task 20min later, participants were shown the 96 target scenes interspersed with 96 closely matched lure scenes (to ensure the task was difficult enough) and were instructed to respond whether they had seen the picture at encoding or not, and if they had, how confident were they in this recollection (remembered with high confidence or remembered with low confidence).
2.2.3.3. fMRI Data Processing
SPM5 was used for data preprocessing and statistical analyses. For preprocessing, functional images were corrected for slice acquisition time followed by motion correction. Using the T1-weighted anatomical image for each subject as a coregistered intermediary, functional images were normalized to standard MNI template space and resampled into 3 mm3 voxels. Normalized images were smoothed with an isotropic 8mm FWHM Gaussian kernel.
Statistical analyses were performed using a mixed-effects General Linear Model (GLM). To analyze subsequent memory effects, offline recognition data were used to backsort the encoding trials into three groups: items remembered with high confidence, items remembered with low confidence, and forgotten items. Neural activity for each condition was modeled as a 3000ms event and convolved with a canonical hemodynamic response function. In addition, six motion regressors were included. An AR(1) model was used to handle time-series autocorrelations.
Like others (e.g., Sperling et al., 2009), we focused on a contrast of activity on High Confidence remembered trials vs. activity on Baseline (crosshair fixation) trials to examine the effects of beta-amyloid burden on activation level.† This contrast image for each subject was entered into a second-level multiple regression analysis that included chronological age and precuneus Aβ SUVR as continuous predictors. Because the primary goal of the study was to investigate the independent, additional effects of amyloid on functional activation after age effects were accounted for, we used a less conservative threshold of p < .005, uncorrected, k = 6 to delineate the age-adjusted dose-response effect of amyloid burden on activation patterns, in those voxels remaining after applying an inclusion mask for main effect of task (p < .005). Following this, we then tested associations between significant clusters from this effect and cognitive performance.
3. Results
3.1. Behavioral Data from the Functional Task
Out-of-the-scanner recognition data were used to assess item memory. As a measure of recognition accuracy we computed d-prime (d’ = z[High Confidence hits] – z[High Confidence false alarms]) which yielded a standardized measure of memory sensitivity. Age was negatively- associated with d’ (r = −.20, p < .02) indicating a small but significant decrease in memory with age. Similarly, precuneus Aβ was negatively associated with d’ (r = −.18, p < .04). Proportion of High Confidence hits and d-prime are reported in Table 1.
3.2. Effects of Age on Amyloid Deposition
An examination of amyloid deposition across eight regions showed linear increases with age (Rodrigue et al., 2012). The greatest age-related increase was observed in the precuneus (r = .41, p < .001), and this was the region selected as the predictor for further analysis in the present study.
3.3. Functional Results
3.3.1. Age Effects on Task Activity
We examined the main effect of age for the contrast of High Confidence Remembered vs. Baseline, treating age as a continuous variable‡. We first utilized an inclusion mask from the main effect of task positive regions (High Confidence remembered – Baseline) to investigate regions that showed age differences in neural activity, both increases and decreases with age. As expected, older age was related to greater activation in many regions including precentral and postcentral gyri and cerebellum (response-related), superior, middle, and inferior temporal cortex, and also frontal (superior, middle, and inferior gyri) and parietal (angular and supramarginal gyri) cortices. Areas of age-related decreased activation were observed in posterior regions (primary and association occipital cortices and cerebellum). We then utilized an inclusion mask from the main effect of task negative regions (Baseline - High Confidence remembered). The results of the age main effect for this reverse contrast revealed only increases in activation with age (i.e., reduced suppression) in a set of regions typically associated with the default mode network (superior/medial frontal, precuneus, angular gyrus). Thus, with increased age there was less suppression of default mode network activity, in agreement with previous findings (e.g., Damoiseaux et al., 2008).
3.3.2. Independent Beta-Amyloid Effects on Task Activity after Controlling for Age
The remainder of our functional analyses focused on a series of questions that addressed the effect of amyloid on neural activity, after controlling for the effect of age. In other words, beyond the effects of age, does amyloid exert an additional effect on functional activations? Each question is addressed in turn.
3.3.2.1. Does Aβ load impact neural activity after age is controlled and what is the nature of the relationship (increased or decreased activation)?
We addressed this question by entering age and precuneus amyloid load as continuous variables into a GLM on High Confidence remembered vs. Baseline contrast. The use of amyloid as a continuous variable provided greater power than the use of categorical cutoffs of high or low amyloid which has typified the literature to date, and allowed us to evaluate the independent incremental impact of amyloid on brain function, beyond the effects of normal aging. After controlling for age, we found a significant main effect of precuneus amyloid load on task activity. Amyloid burden was associated with dampened activity in all areas that were significant. We found decreased activity as a function of increased precuneus amyloid burden in bilateral superior frontal, middle frontal, right inferior frontal, left insula and bilateral middle and right inferior temporal gyri, as well as left cuneus, lingual/calcarine and right cerebellum (Table 2 and Figure 1). No regions showed increased activation as a function of amyloid level. To assess whether the effect was driven exclusively by individuals with the highest amyloid burden, we reran these analyses after removal of individuals with marked Aβ elevation (n = 18), and the incremental effect of Aβ remained, indicating the findings were not driven by the high amyloid individuals.
Table 2.
Coordinate locations for main effect of precuneus amyloid on encoding, whether the region was part of the task-positive or task-negative network, and portion of variance explained
| Cluster | x y z peak (mm) | k | t | network | R2 |
|---|---|---|---|---|---|
| R mid frontal | 42 44 22 | 20 | 3.53 | + | 7.0 |
| L mid frontal | −42 50 4 | 6 | 3.13 | + | 4.4 |
| R inf frontal | 51 29 25 | 6 | 2.83 | + | 5.1 |
| R sup frontal | 15 59 28 | 7 | 3.20 | − | 6.1 |
| L sup frontal | −27 38 40 | 12 | 3.32 | − | 6.6 |
| R mid temporal | 45 −67 4 | 17 | 2.95 | + | 5.2 |
| L mid temporal (lateral) | −48 −55 16 | 20 | 3.00 | − | 5.4 |
| L mid temporal | −42 −67 13 | 7 | 3.06 | + | 5.1 |
| R inf temporal | 54 −46 −11 | 6 | 3.36 | + | 5.3 |
| L insula | −42 5 −8 | 28 | 3.04 | + | 6.6 |
| L cuneus | −15 −79 34 | 14 | 2.92 | + | 5.9 |
| L lingual/calcarine | −6 −61 1 | 12 | 2.82 | + | 5.2 |
| R cerebellum | 9 −46 −32 | 8 | 3.53 | + | 6.2 |
Note. R2 -- percent variance in encoding activity accounted for by amyloid amount (beyond effects of age); p < .005, uncorrected; k threshold = 6; controlled for age effect (p < .005)
Figure 1.
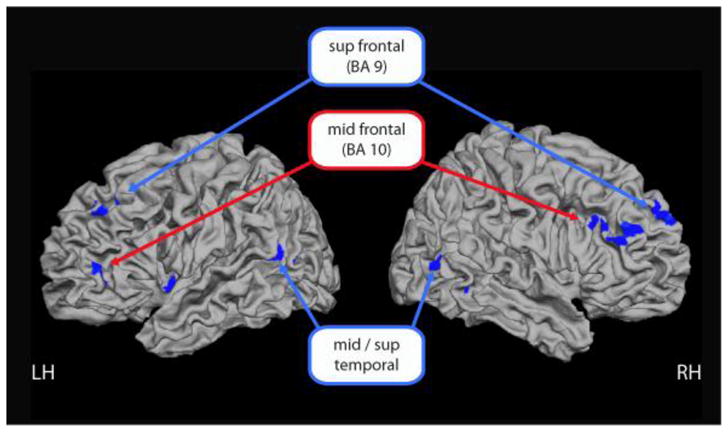
Dose-response effect of Aβ burden: increasing precuneus Aβ is associated with reduced activation and suppression during scene encoding in task positive (red labels) and task negative regions (blue labels).
3.3.2.2. In which networks does amyloid exert an effect (task-positive and/or task-negative)?
We isolated the task-positive and task-negative networks by using an inclusion mask that encompassed areas from the main effect of task (Supplemental Figure 1). We note that all voxels that showed an effect of amyloid were contained within either a task-positive or a task-negative cluster (Table 2). The task-positive analysis yielded evidence that increased amyloid burden was associated with decreased activity in bilateral middle frontal gyri (BA 10), right inferior frontal, bilateral middle temporal, right inferior temporal, left insula, cuneus, lingual/calcarine and right cerebellum (red labels Fig 1). The task-negative analysis, which primarily encompassed the default mode network, showed that greater amyloid burden resulted in reduced suppression in default regions, including bilateral superior frontal (BA 9) and left middle temporal gyri (the lateral cluster) (blue labels Fig 1).
3.3.2.3 Does the impact of Aβ burden interact with age? In other words, does amyloid load predict neural function across the adult lifespan or is its effect limited to older age?
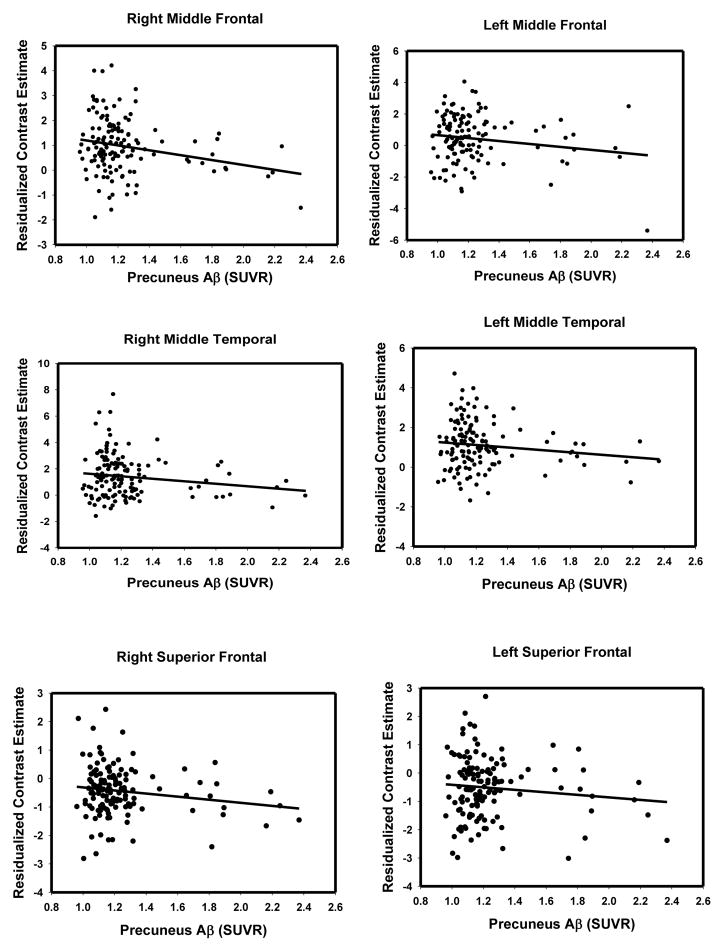
Before examining the interaction, we first examined the overall dose-response effect of amyloid load on neural function (both task-positive and task-negative regions). To do this, we extracted the contrast estimates (residualized to remove the effect of age) for each participant from the clusters associated with the main effect of amyloid and plotted them against precuneus Aβ SUVR. Table 2 provides the portion of variance in encoding activation explained by amyloid amount (R2). As Table 2 indicates, approximately 6% of total variance (range of 4.4% to 7.0%) was independently explained by amyloid after controlling for age. Scatterplots in Figure 2 clearly illustrate that as amyloid load increases, activation decreases in representative frontal and temporal regions. To confirm the linearity of these effects, we also fit a quadratic age function for all of the regions in Table 2, and only the insula cluster was better described by a quadratic fit (ΔR2= .37, p < .02).
Figure 2.
Scatterplots illustrating dose-response effect of increasing amyloid and decreasing activation/suppression in the whole sample for representative frontal and temporal regions. Contrast estimates controlled for age.
To determine whether the observed incremental alterations in neural function were primarily driven by older adults for the regions presented in Table 2, we examined the Age × Amyloid interaction (with age and precuneus amyloid SUVR as continuous variables). Contrast estimates for these analyses were extracted from the main effect of task (High Confidence remembered vs. Baseline without age or Aβ as predictors). In nine of the 13 regions, there was no significant interaction, indicating that the amyloid effect was equivalent across the lifespan. For four of the regions, however, the interaction was significant: right superior frontal (R2 = 14%, p < .05), left middle temporal (lateral) (R2 = 14%, p < .002), left insula (R2 = 14%, p < .005), and left cuneus (R2 = 12%, p < .03), with stronger Aβ-activation associations in the older adults. This suggests that, in general, the incrementing effects of Aβ on neural activity are at play at all ages, though in some regions this effect may be exacerbated by age.
3.3.2.4. Are the alterations in neural activity associated with amyloid deposition related to poorer cognitive performance?
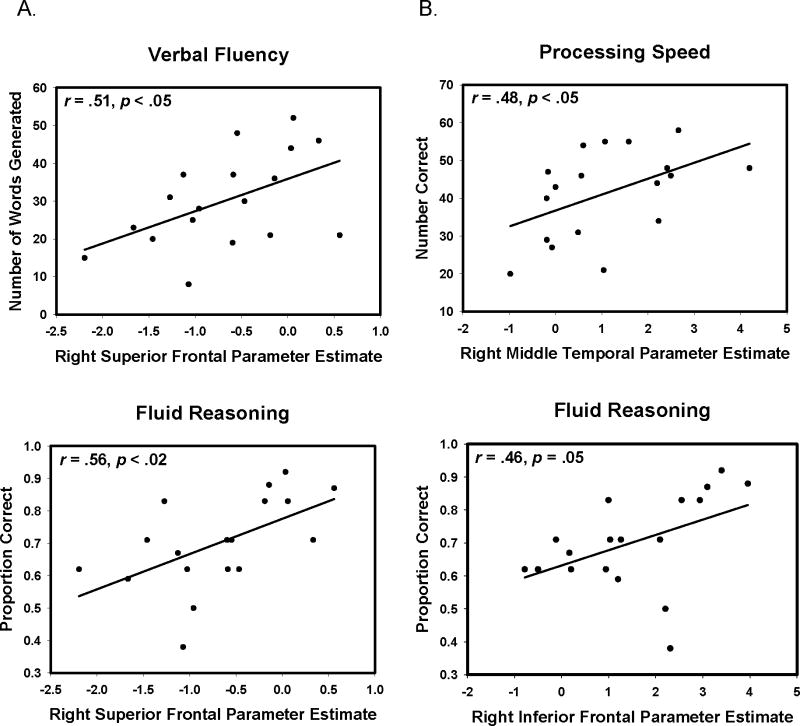
We now address our final question. To investigate the potential cognitive consequences of amyloid-related reduction in neural function, we examined whether activation/suppression differences at encoding were associated with cognitive performance on measures from the neuropsychological battery. We extracted mean beta-weights from the nine significant frontal and temporal clusters shown in Table 2 and examined their association with cognitive performance on measures that have a known frontal component: processing speed, verbal fluency, working memory and reasoning. An analysis of the whole sample yielded no significant relationships between activation/suppression in these clusters and cognition (all p > .30). We also examined the relationship between reduced activation/suppression and cognition in the elevated amyloid subgroup (participants above the age of 60 who exceeded the 95% confidence interval on precuneus amyloid burden; n = 18). We found a significant relationship between amyloid-modified activation and cognition. Impaired suppression in the right superior frontal cluster was associated with poorer performance on tests of verbal fluency (r = .51, p < .05) and fluid reasoning (r = .56, p < .02) as was reduced activation in the right inferior frontal cluster (r = .46, p = .05). Reduced activation in the right middle temporal cluster was associated with slower processing speed (r = .48, p < .05). See Figure 3 for plots of these associations.
Figure 3.
Beta-Amyloid-altered neural function predicts poorer cognitive performance. Panel A indicates the effect of Aβ-associated reduced suppression in superior frontal region on verbal fluency and fluid reasoning. Panel B indicates the effect of Aβ-associated reduced activation in temporal and frontal regions on processing speed and fluid reasoning.
4. Discussion
In this study we examined the relationship between beta-amyloid burden and neural function during an encoding task in a large lifespan sample of adults, treating both age and Aβ burden as continuous variables. We addressed four related questions which are discussed below.
4.1. Does Aβ load relate to neural activity during an encoding task (controlling for age) and what is the nature of the relationship (increased or decreased activation)?
Greater Aβ load had a detrimental effect on neural activity across a range of both task positive and task-negative regions, even after controlling for age. Specifically, a dose-response relationship between precuneus Aβ and neural function was observed.
As Aβ increased, activation decreased in multiple regions of the prefrontal cortex (bilateral middle and superior frontal and right inferior frontal) and temporal cortices (bilateral middle temporal and right inferior temporal), which are part of a fronto-temporal memory network. Interestingly, most of this reduced activity was found in bilateral, homologous frontal and temporal regions, suggesting that amyloid’s effects are systemic, rather than lateralized.
Our results also suggest that there is a dose-response relationship of increased amyloid to decreased neural activity. Only a small subset of our participants had what might be considered clinically significant amyloid burden (e.g., the 18 individuals aged 60 and over who were above the 95% confidence interval) and none of our participants had any symptoms of cognitive dysfunction. Nevertheless, increasing amyloid burden led to a continuously decreasing level of neural activity across the age span, suggesting that increased amyloid at any age, even at subthreshold levels, may be associated with reduced neural activation. To date, we are unaware of any study that has isolated the importance of subthreshold levels of amyloid and its effect on neural activity in healthy adults.
4.2. Does amyloid burden exert an effect on both task-positive and task-negative activity?
We observed that this functional disruption arises from decreased activation in regions activated in the service of encoding (task-positive activity) as well as decreased suppression in regions typically associated with default activity (task-negative regions). These regions of decreased suppression included bilateral superior frontal and lateral temporal cortices. Our findings are in accord with and expand upon previous studies. As in Sperling et al., (2009) and Vannini et al., (in press) we find amyloid-related impairments in the task-negative (default mode) network during encoding of confidently-remembered items. However, we additionally find impaired function of the task-positive network during encoding, indicating that amyloid deposition impacts neural function both in support of the task and in suppression of the default mode network. We note that these results differ from Mormino et al., (in press) who showed increased activity with Aβ in occipital and hippocampal regions. However, these regions were not areas isolated as ROIs in the present or other previous studies, so the results are not directly inconsistent. Our findings also differ somewhat from Sperling et al (2009) perhaps due to differences in task, analytic approaches (ROI vs whole-brain), or brain regions evaluated. It is noteworthy that while they reported increased hippocampal activation with respect to increasing Aβ (the opposite direction of our findings for non-hippocampal brain regions), there was no effect of Aβ on hippocampal activation in their CDR 0 subjects. Hyperactivation of the hippocampus was only seen in PiB positive CDR 0.5 subjects. Hippocampal (hyper)activation, especially in more pathological groups, may have a different biological origin (e.g., excitotoxicity or apoptosis). Further research will undoubtedly yield a better understanding of these relationships.
4.3. Does the impact of Aβ burden interact with age? In other words, does amyloid load predict neural function across the entire adult lifespan or is its effect limited to older age?
To ensure that our observation of increasing amyloid and decreasing neural function was not primarily driven by older adults, we examined the age × amyloid interaction. In most regions, the relationship of amyloid to neural activity was not dependent on age, lending further support to the idea that increasing amyloid is associated with reduced neural function at any age, although in some regions this effect may be exacerbated in older adults. Thus, an important aspect of the present work is evidence that Aβ exerts effects at early ages and that increased deposition relative to one’s age peers may be an early event in a cascade toward possible clinical AD expression later in life (Jack et al., 2009; Mormino et al., 2009; Rodrigue et al., 2009). This is a particularly important issue as it is becoming increasingly clear that interventions will likely be most effective if they occur well before cognitive symptomatology appears (e.g., in middle age) (Sperling et al., 2011).
4.4. Are the alterations in neural activity associated with amyloid deposition related to poorer cognitive performance?
Importantly, in the group of individuals with the most elevated Aβ, altered prefrontal and temporal activation is associated with poorer performance on offline cognitive tests, particularly processing speed, verbal fluency and fluid reasoning, all tasks that heavily rely on prefrontal function. This finding suggests that the amyloid-related functional reductions are detrimental rather than compensatory in nature, at least for those older adults with marked amyloid elevations. Interestingly, prefrontal regions that exhibited reduced activation as a function of amyloid are those commonly found to be over-recruited by older adults during more difficult cognitive tasks in the scanner, which has been interpreted as a compensatory strategy (Cabeza, 2004; Cappell et al., 2010). Given that prefrontal cortex function is altered by Aβ, our results suggest that beta-amyloid may interfere with compensatory recruitment (Park and Reuter-Lorenz, 2009), particularly in light of our finding that reduced prefrontal cortex activation/suppression was associated with poorer cognitive performance. The aging brain undergoes numerous alterations to its structure (Rodrigue & Kennedy, 2011) and function (Reuter-Lorenz and Park, 2010), even without frank pathology. Beta-amyloid deposition may be but one additional neural insult the healthy brain endures with age, in addition to atrophy, accumulation of white matter lesions and degraded connectivity. Because aging is characterized by both losses and gains, only longitudinal investigations will determine if elevated Aβ burden necessarily represents a prodromal hallmark of neurodegenerative disease.
We note that our continuous age and amyloid modeling approach allowed us to specifically examine dose-response relationships across the adult lifespan, which provided sufficient power to allow us to use amyloid burden as a continuous variable, and assess its incremental effects on neural activity. We were thus able to avoid the two-step approach common to small sample studies in which functional activations are first isolated, and then in a separate model interrogated for correlations with variables of interest. We also note that the study participants showed no evidence of cognitive dysfunction at the time of test, and that longitudinal follow-up will be essential to understanding the predictive role that amyloid burden plays in subsequent cognitive decline over time. Only longitudinal study of these participants will reveal whether Aβ deposition always predicts a conversion to MCI and AD, and at what rate progression occurs. The study of amyloid deposition in normal adults of all ages will be of great importance in understanding when and on whom to intervene as more treatments for AD become available.
4.5. Conclusion
In sum, the major finding is that precuneus Aβ deposition is associated with altered neural function across the adult lifespan, regardless of an individual’s age. Amyloid load is associated with neural alterations even in younger and middle-aged individuals. The dose-response effect of amyloid on neural activation/suppression further suggests that not only markedly elevated amyloid deposition, but also incremental amyloid load, is associated with reduced neural response. Finally, this reduced neural function is associated with poorer cognitive performance in older individuals with marked amyloid deposition, suggesting that these functional changes are detrimental, rather than compensatory in nature.
Acknowledgments
This study was supported in part by NIH grants 5R37AG-006265-25, 3R37AG-006265-25S1, and Alzheimer’s Association grant IIRG-09-135087. KMK was supported in part by NIA grant 1K99-AG-0368180-2. The radiotracer was provided by Avid Radiopharmaceuticals. We thank Dr. Dana Mathews and Michael Viguet for assistance with PET scanning and Jenny Rieck and Prasanna Tamil for participant recruitment, scheduling and testing.
Footnotes
We chose this contrast rather than the traditional High Confidence – Forgotten contrast to ensure a robust set of activations to examine the modifying effect of amyloid. Similar contrasts were used in other studies (Sperling et al., 2009; Vannini et al., in press).
When we examined the main effect of task independent of age, we observed significant neural activity, encompassing most areas of the brain (see Supplemental Figure 1).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, Dekosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of Neurology. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Task-independent and Task-specific Age Effects on Brain Activity during Working Memory, Visual Attention and Episodic Retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, Krautkramer MJ, Kung HF, Coleman RE, Doraiswamy PM, Fleisher AS, Sabbagh MN, Sadowsky CH, Reiman EP, Zehntner SP, Skovronsky DM. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. Journal of cognitive neuroscience. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Brandel MG, Madison CM, Marks S, Baker SL, Jagust WJ. Aβ Deposition in Aging Is Associated with Increases in Brain Activation during Successful Memory Encoding. Cerebral Cortex. doi: 10.1093/cercor/bhr255. (in press) doi:10.1093/cercor/bhr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kennedy KM, Rodrigue KM, Hebrank AC, Park DC. Episodic Encoding across the Lifespan: Task-positive Activity Declines Earlier than Task-negative Activity. (in revision) Manuscript under revision. [Google Scholar]
- Pike K, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain: A Journal of Neurology. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Raven J. Raven’s Coloured Progressive Matrices. San Antonio: Psychological Corporation; 1976. [Google Scholar]
- Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2010;65:405–415. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM. The cognitive consequences of structural changes to the aging brain. In: Schaie KW, Willis SL, editors. Handbook of the Psychology of Aging. 7. Ch 5. New York: Elsevier; 2011. pp. 73–92. [Google Scholar]
- Rodrigue KM, Kennedy KM, Park DC. Beta-Amyloid Deposition and the Aging Brain. Neuropsychology Review. 2009;19:436–450. doi: 10.1007/s11065-009-9118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC. β-Amyloid Burden in Healthy Aging: Regional Distribution and Cognitive Consequences. Neurology. 2012;78:387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O'Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA. Episodic memory retrieval: an (event-related) functional neuroimaging perspective. London: Psychology Press; 2003. [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Neurosensory center comprehensive examination for aphasia. Victoria, B. C: University of Victoria, Neuropsychology Laboratory; 1977. (rev. ed.) [Google Scholar]
- Turner M, Engle R. Is working memory capacity task dependent? Journal of Memory and Language. 1989;28:127–154. [Google Scholar]
- Vannini P, Hedden T, Becker JA, Sullivan C, Putcha D, Rentz D, Johnson KA, Sperling RA. Age and amyloid-related alterations in default network habituation to stimulus repetition. Neurobiology of Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.01.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, Dannals RF, Nandi A, Brasic JR, Ye W, Hilton J, Lyketsos C, Kung HF, Joshi AD, Skovronsky DM, Pontecorvo MJ. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir F 18) Journal of Nuclear Medicine. 2010;51(63):913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]