Abstract
The capacity of any portion of the murine mammary gland to produce a complete functional mammary outgrowth upon transplantation to an epithelium-divested fat pad is unaffected by the age or reproductive history of the donor. Likewise, through serial transplantations, no loss of potency is detected when compared to similar transplantations of the youngest mammary tissue tested. This demonstrates that stem cell activity is maintained intact throughout the lifetime of the animal despite aging and the repeated expansion and depletion of the mammary epithelium through multiple rounds of pregnancy, lactation and involution. These facts support the contention that mammary stem cells reside in protected tissue locales (niches), where their reproductive potency remains essentially unchanged through life. Disruption of the tissue, to produce dispersed cells results in the desecration of the protection afforded by the “niche” and leads to a reduced capacity of dispersed epithelial cells (in terms of the number transplanted) to recapitulate complete functional mammary structures. Our studies demonstrate that during the reformation of mammary stem cell niches by dispersed epithelial cells in the context of the intact epithelium-free mammary stroma, non-mammary cells, including mouse and human cancer cells, may be sequestered and reprogrammed to perform mammary epithelial cell functions including those ascribed to mammary stem/progenitor cells.
Keywords: transplantation, microenvironment, mammary, development, reprogramming
1.1 Introduction
Schofield [1] was the first to propose the idea of a specific location defined by specific cells and cellular signals controlling stem cell function (a stem cell niche) for hematopoietic stem cells. This theory was proposed to explain why stem cells from aged mice were functionally as capable of long-term engraftment of young recipients as hematopoietic stem cells from young donors. His idea was that stem cells were essentially “immortal” so long as they resided in their niche but when removed from these sites then “immortality” was lost. He defined this stem cell “niche” as a specific anatomical site where stem cells were sustained and could reproduce; where differentiation of the stem cell was inhibited and most importantly a site where reversion to a stem cell phenotype might be induced in a more (slightly) mature cell type. Schofield’s concept remained hypothetical and without direct evidence until the late 1990’s when work from Spradling and his colleagues validated each of Schofield’s predictions about a stem cell niche in the ovary of Drosophila melanogaster [2–4]. A similar validation shortly followed from the study of the testes in Drosophila and later in C. elegans [5]. Even the most far-reaching of Schofield’s concept namely that a more mature cell could be induced to acquire stem cell attributes by interaction with the niche was validated in these invertebrate models.
1.2 Use of conditional reporter models to test for niche signals
We were inspired to test this final point of Schofield’s niche concept in the regenerating mammary gland because we had successfully rescued mammary stem/progenitor cells (Fig. 1) from transgenic mammary tissues where regenerative capacity had been obliterated by the ectopic expression of the transgene by simply mixing the incompetent epithelial cells with normal wild type mammary epithelium prior to introduction into the epithelium-free mammary fat pad [6]. In two models, (WAP-Notch4/Int3 X WAP-Cre/Rosa26R and WAP-TGFβ1 X WAP-Cre/Rosa26R), where lacZ-reporter marked cells (PI-MEC) were present in mammary epithelial populations incapable of growth and reconstitution of mammary epithelium in vivo, we found that interaction with normal wild type epithelial cells allowed them to produce progeny during mammary gland regeneration. These results suggested that the mammary epithelial cells themselves in combination with the mammary fat pad and its stroma, along with extrinsic growth and hormonal factors were components essential to the mammary stem cell niche.
Figure 1. Mouse mammary stem/progenitor functional hierarchy.
A multi-potent stem cell gives rise to two lineage limited progenitor cells, ductal and alveolar progenitors. Both lineage-limited progenitors are multi-potent as they are capable of producing both ER+/− and PR+/− luminal epithelial cells as well as myoepithelial cells. Ductal progenitors give rise to specialized cap cells in the terminal end buds, which in turn become the myoepithelial cells of the subtending ducts. Alveolar progenitors proliferate during pregnancy to produce the luminal and myoepithelial cells of developing secretory lobules.
It is known that signals from progesterone receptor positive (PR) mammary epithelial cells are essential to secretory alveolar development [7] and that signaling from estrogen receptor alpha-positive (ERα) epithelium is needed for mammary duct growth and expansion [8]. The growth factor, Amphiregulin (AR), has been identified as an important mediator of ERα+ signaling for duct elongation and development [9]. Gata3 has been shown to be essential for luminal epithelial differentiation in the ducts [10] and Beta1 integrin expression for full development of the secretory alveoli [11]. Other regulatory factors present or generated in the mammary stroma have also been identified such as transforming growth factor beta (TGFβ) [12], fibroblast growth factor (FGF), heregulin (HGF), insulin growth factors (IGFs) and the RANKL/RANK interaction [13–15]. Thus the mammary microenvironment that supports and maintains mammary epithelial homeostasis and the capacity for regeneration upon transplantation consists of local signals emanating from both the stroma and the existing epithelium and circulating host factors. Adhesiveness and cell-to-cell contact also plays an important role in mammary structure and function[16–18].
In parous females WAP-Cre/Rosa26-lacZ, lacZ expression marks cells that survive after lactation and involution (PI-MEC). Experiments where WAP-Cre/Rosa26-lacZ reporter glands from nulliparous females were incubated as explant fragments in various combinations of growth factors and hormones demonstrated that milk induction in the epithelial cells was not necessary to active the Rosa26-lacZ reporter [19]. This indicated that the PI-MEC were already present in nulliparous mammary tissue and were subsequently identified following pregnancy, lactation and involution. In other experiments, PI-MEC were marked by the expression of GFP in WAP-Cre/Chicken-actin gene promoter (CAG)-flox-stop-flox-GFP parous females. In these studies GFP+ PI-MEC were fluorescent activated cell sorted (FACS) and found to be virtually 100% present in the CD49fhi population. This population was shown earlier to possess essentially all of the mammary repopulating activity. Subsequent transplantation of GFP+/CD49fhi positive PI-MEC and the GFP−/CD49flo epithelial cells into epithelium-divested mammary fat pads indicated that all the repopulating activity was associated with the GFP+ fraction.
Encouraged by these observations, we set out to determine if cells from non-mammary tissues could be altered from their initial cell fate lineage to adopt mammary epithelial characteristics upon interaction with mammary epithelial cells during reconstitution of mammary epithelium in regenerating mammary tissue in vivo.
1.3 Reprogramming cells from Ectoderm-derived Tissues
Our experiments have shown that the mammary microenvironment described above is capable of re-directing both spermatogenic cells and neural stem cells from ectodermally derived non-mammary organs to produce progeny destined for mammary epithelial cell fates [20, 21]. This is not accomplished through cell-cell fusion but rather by adoption of the non-mammary cells into the reforming mammary epithelial niche(s) and the reformatting their previous cellular repertoire to one shared by other mammary epithelial cells. In this work we were only able to identify (by lacZ expression) those non-mammary cell progeny that expressed Cre from the WAP promoter during pregnancy and subsequently survived post lactation remodeling, principally cells with the properties of PI-MEC. Transplantation/mixing experiments with normal mammary epithelial cells were performed using multipotent neural stem cells (NSC) maintained as such under specific culture conditions and shown to be capable of differentiating into three distinct differentiated neural cell types in vitro. These NSC were derived from both adult and embryonic brain of WAP-Cre/Rosa26R mice. Our results show that they are capable of interacting with normal mammary epithelial cells and cooperate copiously in the generation of a fully differentiated functional mammary gland. NSC alone without cooperation from co-injected mammary epithelial cells fail to produce mammary epithelium. This indicates that signals, both paracrine and juxtacrine, from bona fide mammary epithelial cells are indispensable to their active participation in chimera formation. Once incorporated into mammary epithelial structures, NSC progeny behave similarly to PI-MEC, producing both luminal and myoepithelial progeny and actively proliferating in secondary transplants. LacZ+ NSC luminal progeny synthesize milk protein in pregnant hosts and along ducts, some express PR and ERα.
Most LacZ+ NSC progeny among the mammary chimera no longer express neural stem cell specific genes, however occasional cells continue to express Sox2 and nestin. The significance of this finding is not clear at present although this observation has been made both in primary chimeras and in secondary transplants.
The redirected NSC proliferated and contributed extensively to primary and secondary mammary outgrowth and produced both luminal epithelial progeny capable of synthesizing milk protein and myoepithelial cells in secretory acini. NSC progeny also included estrogen receptor (ER) and progesterone receptor (PR) positive epithelial offspring among the chimeric epithelium (Fig. 2). Mammary epithelial cells with these properties have been shown to be indispensable to complete duct and secretory alveolar development in murine mammary gland [7, 8]. In the chimeric mammary tissue NSC-derived mammary progeny, conditionally marked by LacZ expression due to WAP-Cre initiated recombination, behaved in all manner exactly like the PI-MEC described earlier in intact WAP-Cre/Rosa26R female mammary glands following parity [22–24]. In these experiments, we are not able to ascertain whether NSC-derived mammary epithelial progeny contributed to other aspects of mammary development because WAP-Cre activation of the reporter does not mark all mammary epithelium. Despite this, the widespread contribution of NSC-derived progeny to the mammary epithelial population prior to pregnancy (as determined by the uniform distribution of LacZ+ progeny along the mammary ducts in fully involuted primiparous females) suggest that NSC-derived progeny contribute robustly to the total mammary epithelial content in the chimeric outgrowths. In addition, the demonstration that a specific tissue locale comprised of stromal and epithelial factors can dictate the repertoire of bona fide somatic stem cells to its own purpose reinforces the concept of the inductive power of the tissue microenvironment in redirecting the intrinsic nature of a tissue-specific stem cell.
Figure 2. Mouse neuronal stem cells are incorporated into reforming mammary niches and reprogrammed to a mammary epithelial cell fate.
(A and B) When mixed with dispersed mammary epithelial cells, LacZ+ marked neuronal stem cells contribute to the resulting mammary outgrowth, and lacZ+ cells are present in second generation outgrowths (A = whole mount, B = cross-section). C–F) B-Gal expressing cells in second generation outgrowths co-express beta casein (C), smooth muscle actin (D), PR (E), and/or ERalpha (F).
1.4 Reprogramming cells from mesoderm-derived tissue
Bone marrow was isolated from the tibias of bitransgenic R26 R mice as described above and lineage negative (Lin−) cells were further isolated through a negative selection magnetic bead prep as described. Mammary cells mixed with Lin−, Lin−/cKit+, and Lin−/cKit+/Sca+ BMC at a 1:1 ratio were used in attempts to develop chimeric mammary outgrowths comprised of cells from tissue derived from differing germ layers. The cells were combined and immediately inoculated (10ul) directly into epithelium-divested mammary fat pads of 3-week-old Nu/Nu females. Mammary ductal growth proceeded for 6–8 weeks following injection, whereupon hosts were allowed to complete a full-term pregnancy (required for activation of the Rosa26 LacZ reporter gene via WAP-Cre expression). Pups were removed after birth. Complete glandular involution was allowed. Subsequently, the mammary outgrowths were removed and stained as whole mounts for LacZ activity by X-Gal. Only mesodermal cells contained both the WAP-Cre and the Rosa26 reporter transgene. Therefore, only BMC-derived cells, which survived tissue remodeling following lactation and possessed both the WAP-Cre and theRosa26 reporter transgene during pregnancy, will be lacZ+ [25]. Following Cre-induced recombination, lacZ expression is constitutive and subsequently acts as a lineal marker, which can be used to trace the subsequent fate of the activated, surviving lacZ+ cells and their progeny. The presence of lacZ+ (blue) cells signals the occurrence of mesodermal cell progeny within the mammary outgrowth. An important feature of the experimental design is the restriction of Cre expression from the WAP promoter to mammary epithelium during secretory differentiation. This conclusion is borne out by the absence of lacZ+ cells in the mammary outgrowths removed from control nulliparous hosts. When mixed with normal MEC, total Lin−, Lin−/ckit+, and Lin−/ckit+/sca1+ cellular fractions, all contributed to the resulting outgrowths in 6/11, 6/8, and 5/7 positive takes, respectively (Table 1). No LacZ+ cells appeared in outgrowths produced by injecting mammary epithelium alone, and no outgrowths resulted from inoculating mesodermal cells alone (Table 1). This result is consistent with the conclusion that the BMC-derived cells effectively competed for reforming mammary stem cell niches only when mammary stem cell numbers (and the frequency of positive takes) were reduced.
Table 1.
Results indicate the number of fat pads that contained mammary growth (takes) out of the total number of fat pads injected.
| TGEN 1 | TGEN 2 | |||
|---|---|---|---|---|
| Total Takes | LacZ+ | Total Takes | LacZ+ | |
| Lin− Alone | 0/6 | N/A | N/A | N/A |
| Lin− + MEC | 11/20 | 6/11 | 8/10 | 7/8 |
| Lin−/ckit+ + MEC | 8/10 | 6/8 | 6/6 | 6/6 |
| Lin−/ckit+/Sca+ + MEC | 7/10 | 5/7 | ND | ND |
Abbreviations: N/A, not applicable; ND, not determined.
1.5 Appearance of LacZ+ Cells in Second-Generation Transplants
Our previous studies of WAP-Cre activated, lacZ+ PI-MEC in intact primiparous, involuted chimeric mammary outgrowths indicated that they were capable of self-renewal upon transplantation. In addition, the lacZ-positive cells gave rise to epithelial cell progeny of both luminal and myoepithelial cell lineages [25]. Second generation transplantation of fragments from the BMC/MEC chimeric outgrowths confirmed that the lacZ+, mesoderm-derived epithelial cells were also capable of self-renewal and proliferation (Table 1), activities analogous to those displayed by the PI-MEC in intact R26R mouse mammary glands. The results clearly demonstrate that cells from bone marrow of adult mice interact with mammary epithelial cells upon inoculation into the epithelium divested mammary fat pad and proliferate to contribute cells analogous to PI-MEC in the resulting epithelial outgrowths.
1.6 Demonstration of Transgene and Y Chromosome-Specific Sequences in Chimeric Glands
PCR analysis of the DNA isolated from the chimeric outgrowths demonstrated the presence of both Wap-Cre and Rosa26R transgenes and sequences specific to the Y chromosome, verifying the presence of mesodermal-derived male cell DNA. Similar results were obtained when secondary outgrowths from BMC/MEC outgrowths were sectioned and analyzed by in situ fluorescent hybridization for male Y-chromosome associated DNA (Fig. 3).
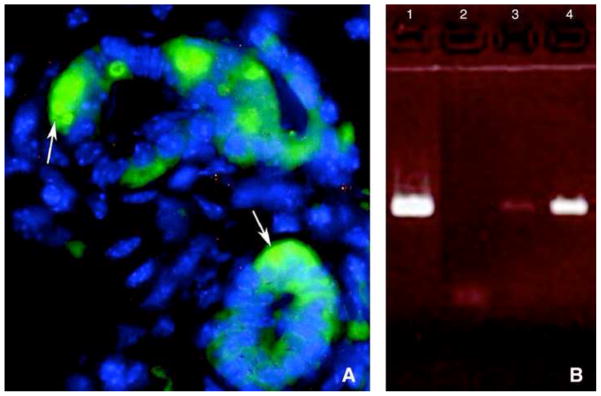
Figure 3. Presence of Y chromosome in chimeric outgrowths.
(A) Y chromosome FISH analysis performed on paraffin-embedded, 6 μm sections of mammary glands containing chimeric outgrowths. A mammary secretory structure is shown with the Y chromosome labeled in green (arrows), and the nuclei stained with DAPI (blue). Serial 1.0 μm slices photographed under 3-color confocal microscopy reveal the presence of male BM cell progeny juxtaposed with mammary cells in the same acinus. (B) DNA PCR of Y6 male primers. Lane 1 is male DNA control, lane 2 is negative female control, lane 3 is first-generation Lin− chimeric outgrowth, and lane 4 is DNA from a secondary chimeric outgrowth. Presence of male DNA is a result BM cell contribution.
1.7 Reprogramming Cancer Cells to Mammary Epithelial Cell Fates
We used two established tumor cell lines derived from mammary carcinomas that arose in WAP-Cre/Rosa26R/MMTV-neu triple transgenic female mice [26, 27]. One line was developed from one of the rare tumors that arose in a nulliparous female, while the second line was derived from one of the numerous tumors developing in parous females. Each tumor cell population is constitutively lacZ-positive (blue after X-gal staining). Thus, constitutive expression of lacZ is a lineal marker for neu tumor-derived mammary cells in our experiments. To establish the tumorigenic potential of these two cell lines, each was injected separately into the cleared mammary fat pads of 3-week old Nu/Nu mice at varying concentrations. Tumor cells inoculated by themselves at 3 separate concentrations (100K, 10K and 1.0K) produced 100% tumors with an average latency of 5.5, 7.5 and 7.5 months respectively. When inoculated together with 50K wild type FVB/N mammary epithelial cells at 2:1, 1:5 and 1:50 ratios respectively, tumors appeared in all groups, but in < 23% of the 1:50 ratio implants, with an intermediate latency (5–6 mos.), A significant reduction in tumor development (22.5%) was observed in the hosts inoculated with 1.0K tumor cells and 50K normal cells. The results are summarized in Table 3. Injection of tumor cells from either cell line alone resulted in mammary tumor formation at indistinguishable incidence and latency. Focal tumor development at numerous sites along the needle track was observed at early time points. Cross-sectional histological analysis showed that each small tumor was comprised entirely of lacZ+ cells indicating that the growths arose from the transplanted tumor cells. The small focal tumors eventually progressed to form large mammary tumors comprised of lacZ+ cells if left unattended (not shown).
Apparently normal mammary outgrowths comprised of lacZ-positive and lacZ-negative portions were observed when tumorigenic cells at ratios of 1:5 and 1:50 (10K, 1K) were mixed with normal mammary epithelial cells. The reconstituted glands contained both lacZ+ and lacZ− cells indicating that both normal and tumorigenic epithelial cells contributed to the outgrowth since only the tumorigenic cells express lacZ. The lacZ+ cells were located in luminal and occasionally basal positions along the ducts, side branches and in developing acinar structures. They were found in groups indicative of local clonal expansion of lacZ+ progeny and also juxtaposed to lacZ− epithelial cells suggesting interaction between tumor and normal mammary cells during duct development. In some cases, an entire ductal side-branch was composed of lacZ+ epithelial cells signifying that tumor cell progeny could independently generate a normal mammary epithelial structure. Both tumor-derived cell lines were able to contribute to the outgrowths with equivalent frequency when combined with normal mammary epithelial cells. Some mammary ducts in the chimeras did not contain lacZ+ cells consistent with the absence of lacZ staining in some portions of the X-gal stained whole mount preparations. In second transplantation generation chimeric glands no tumors formed after 12 months although lacZ+ cells were present (Table 2). In longer-term studies, no tumor formation was observed in a group of recipient females maintained for 18 months post-surgery bearing either first or second transplantation generation outgrowths.
Table 2. Summary of transplantation results for LacZ-marked MMTV/Neu tumor cells and FVB mouse mammary epithelial cells.
Results indicate the number of fat pads that contained mammary growth out of the total number of fat pads injected. Time until tumor formation range is given in months with average month in parenthesis.
| No. injected | Result | Normal growth | %Tumors | LacZ+ | Time (months) |
|---|---|---|---|---|---|
| 100K Tumor | 6/6 | No | 100 | Yes | 3–7(5.5) |
| 10K Tumor | 6/6 | No | 100 | Yes | 5–8(7.5) |
| 1K Tumor | 6/6 | No | 100 | Yes | 6–8(7.5) |
| 100K Tumor + 50K FVB/n | 6/6 | No | 100 | Yes | 5 |
| 10K Tumor + 50K FVB/N | 7/8 | Yes (5) | 62.5 | Yes | 5 |
| 1K Tumor + 50K FVB/N | 15/16 | Yes (15) | 6.67 | Yes | 6 |
| 10 K Tumor + 50K FVB/N second Tgen | 4/6 | Yes | 0 | Yes | ND |
| 1K Tumor + 40K FVB/N second Tgen | 12/14 | Yes | 0 | Yes | ND |
Abbreviations: Tgen, transplantation generation; ND, no tumors developed up to 18 months.
Our results demonstrated that some MMTV-neu-initiated tumor cells are capable of interacting with the normal epithelium such that they contribute to normal mammary gland growth and regeneration, and in the process produce both luminal and myoepithelial epithelial progeny. Nevertheless, the tumor cells (lacZ+) are incapable of generating ERα+ or PR+ progeny during either ductal or lobular development in the resulting chimeric gland and continue to express the MMTV-neu transgene. Second transplant generations from these chimeric outgrowths indicate that the tumor cells are capable of self-renewal and further contribution to normal mammary epithelial development (although they still fail to produce nuclear steroid positive offspring). Surprisingly, cells recovered from secondary tumor/normal cell outgrowths were incapable of forming tumors when inoculated into gland-free mammary fat pads even though they continue to express the neu transgene. When recovered cells were sorted for ErbB2 expression, tumor-initiating activity was re-established in some (tumors were 100% lacZ+), but not for all erbB2-positive cells. Inoculations of 50K and100K of erbB2-positive sorted chimeric cells gave rise to mammary outgrowths without tumor formation (6/8). These were mixed for lacZ-positive and negative mammary epithelial cells. No lacZ-positive cells or tumors were found in 8 fat pads inoculated with 50K or 100K erbB2 negative sorted chimeric cells (unpublished observations). This result suggested that some of the erbB2 transformed cells maintained a non-tumor phenotype after isolation from secondary generation chimeras.
1.8 Reprogramming Human Cancer Cells in Mouse Mammary Glands
Human cancer cells were reprogrammed in the mouse mammary gland and these were identified by human specific cytokeratin expression [28]. We found human cancer cell progeny that were positive for PR and ERα amongst the luminal epithelium and at basal locations where they expressed the cellular markers associated with myoepithelial cells. Therefore like PI-MEC these reprogrammed cells were multipotent and were able to give rise to diverse mammary epithelial subtypes including cells that were capable of synthesizing milk proteins, both mouse and human. Human embryonal carcinoma cells proliferate and produce differentiated mammary epithelial cell progeny when mixed with mouse mammary epithelial cells and inoculated into the epithelium-free mammary fat pads of athymic nude mice. FISH confirmed the presence of human cell progeny in the mammary outgrowths for human centromeric DNA, as well as immunochemistry for human specific breast epithelial cytokeratins and human specific milk proteins (Fig. 4) in impregnated transplant hosts. It was found that the number of human cells increased by 66–660 fold during mammary epithelial growth and expansion as determined by human cytokeratin expression. All features found in primary outgrowths were recapitulated in the secondary outgrowths from chimeric implants. These results demonstrate that human embryonal carcinoma-derived progeny interact with mouse mammary cells during mammary gland regeneration and are directed to differentiate into cells that exhibit diverse mammary epithelial cell phenotypes. This was the first demonstration that human cells are capable of recognizing the signals generated by the mouse mammary gland microenvironment present during gland regeneration in vivo.
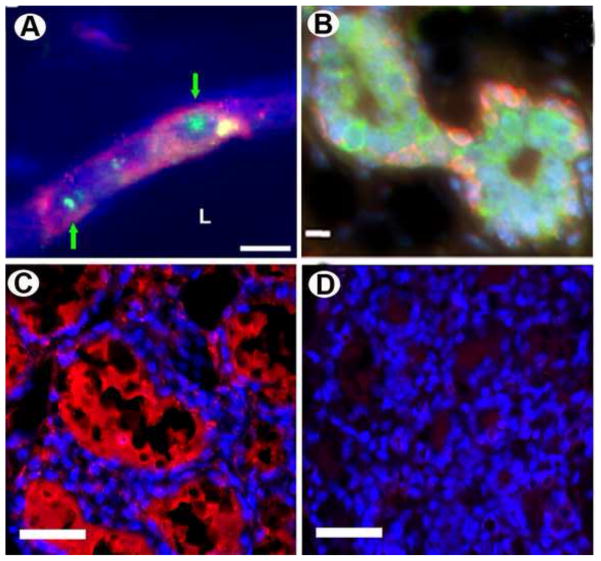
Figure 4. Human embryonal carcinoma cells (NT2) are incorporated into reforming mammary niches and reprogrammed to a mammary epithelial cell fate.
A) Human NT2 cells are present within the mammary outgrowths as determined by human-specific FISH (green, nuclear; identified with green arrows) and human-specific immunocytochemical staining for CD133 (red). B) NT2 cells incorporated into second generation mammary gland outgrowths express human cytokeratin 5 (red) and human cytokeratin 8 (green). C and D) 2nd generation NT2/MEC chimeric gland (C) and intact axillary mammary gland of the same animal were taken on day 2 of lactation and stained with a human specific alpha-lactalbumin (red) antibody. Note immunoreactivity only occurred in the chimeric gland, demonstrating production of human milk.
To determine if any of the preceding results were attributable to fusion between the NT2 and host mammary cells, cells were isolated from the second-generation chimeric gland and stained with propidium iodide. DNA content was determined by flow cytometry. As shown in Figure 5A, propidium iodide-stained cells isolated from chimeric glands were compared with similarly stained cells from NT2 and mammary epithelial cell cultures. The proportion of chimera cells with DNA content greater to 4N or intermediate between 2N and 4N did not differ from mouse epithelial cells or NT2 cells alone. This result is inconsistent with the presence of human/mouse cell fusion. For further confirmation of DNA content, metaphase spreads were generated from cells taken from the co-cultured human NT2 cells and mouse mammary epithelial cells, as well as from cultures generated from secondary chimeric outgrowths (Fig 5B–E). Human and mouse chromosomes can be easily distinguished by morphology and none of the metaphase spreads exhibited evidence of mouse/human cell fusion. Cells isolated from second-generation chimeric outgrowths were also analyzed (n=30), and were found to contain either all human (n=5) or mouse (n=25) chromosomes. Furthermore, of the nuclei containing chromosomes with mouse morphology, all but one had a euploid number (i.e. 40) of chromosomes, with one cell containing 80 chromosomes (all possessed normal mouse chromosome morphology). Cells with human chromosomes all had 45–59 chromosomes, which is within the chromosome range seen with metaphase spreads from NT2 cell cultures. These results demonstrate that the human gene activities detected in the second generation chimeric outgrowths are not the result of fusion between human and mouse cells and further demonstrate that human cells proliferated independently in the formation of primary and secondary chimeras.
Figure 5. Chimeric mammary outgrowths do not contain fused human and mouse cells.
A) NT2 cells, mouse mammary epithelial cells, and cells isolated from second-generation chimeric outgrowths were fixed and stained with propidium iodide, and DNA content was determined by flow cytometry. The chimeric gland did not have a greater proportion of cells with abnormal ploidy compared with cultures of mouse mammary epithelial cells (MEC) or NT2 cells. B–E) Metaphase spreads of (A) mouse mammary epithelial cells prior to transplantation, (B) epithelial cells from a chimeric outgrowth containing only mouse chromosomes, (C) NT2 cell prior to transplantation and (D) epithelial cell from chimeric outgrowth containing only human chromosomes. No evidence of human/mouse cell fusion was observed and all cells had the expected number of chromosomes.
Conclusions
To our knowledge, this was the first demonstration that cancer cells from a different mammalian species can be redirected to produce progeny capable of typical mammary epithelial cell function by interaction with a developing adult organ system in vivo. Totipotent human embryonal carcinoma cells readily interpreted the developmental signals generated in the mouse mammary gland during regeneration. The resulting interaction leads to the redirection of human NT2 cancer cell progeny to adopt a human mammary epithelial cell phenotype with an absence of tumorigenic activity. Thus, these results provide direct evidence that the developmental cues in the mouse mammary gland and those present during human breast development may be indistinguishable. Furthermore, the human cancer cell progeny differentiate down two distinctly different mammary epithelial cell pathways, i.e. luminal and basal (myoepithelial) during chimeric mammary regeneration. This indicates multi-potentiality in the cancer cell response to signals from the mouse mammary microenvironment. In the case of mouse stem/progenitor cell activities, evidence for distinct lobule-limited and duct-limited multipotent epithelial cells has been demonstrated [29–31]. No such evidence is yet available for human breast stem/progenitor cells. Therefore, isolation and characterization of the human cells present in the mouse/human mammary chimeras will be important in understanding the nature of the reprogrammed human cancer cell progeny. Studies are in progress to label NT2 cells with a selectable marker so that these investigations may be possible. The observation that human embryonal carcinoma cells are able to detect and respond to signals generated within the developing mammary tissue suggests that other human cancer cells may also be able to respond in a similar manner. We are currently investigating this hypothesis. In addition, these observations may lead to a new paradigm for control and treatment of cancer in situ.
Although it is clear from transplantation studies that the rodent mammary gland contains stem/progenitor cells in all parts of its epithelial tree throughout life, there is little evidence to define or delineate a locale or niche required for the maintenance of this “stem cell” activity.
The foregoing supports the concept that the tissue microenvironment can affect the cellular repertoire of an adult stem cell. The influence in the murine mammary gland appears to be manifest in signals emanating from the epithelial cells as well as the stromal elements of the mammary fat pad. Several questions remain to be answered. For example, what is the role if any of mammary stem/progenitor cells in this process? Does the mammary fat pad selectively support the reprogramming in conjunction with the mammary epithelial cells or can any fat pad in the female mouse demonstrate this activity? What are the cellular, genetic and molecular components that define the mammary epithelial-specific stem cell niche and how can these factors be utilized for developing new paradigms for stem cell control and cancer therapy? Our preliminary experiments have shed a small amount of light on some of these questions. First, enriching or depleting the mammary epithelial cells for cells expressing the currently accepted cell surface markers for mammary stem/progenitor cells (CD49f, CD29 or CD24) did not affect the efficiency of reprogramming non-mammary cells from the testes [32, 33]. Likewise enriching or depleting for Thy1 a marker associated with mammary myoepithelial cells [34], failed to have any significant effect on the redirection of seminiferous tubule cells to mammary epithelial cell fates. Testing mammary epithelial cell populations from various gene knockout models, including estrogen receptor (ER) beta, Progesterone receptor (PR), Amphiregulin (AR) and Prolactin (Prl), has thus far not revealed any particular gene product that is essential for reprogramming. However our recent findings have delineated at least one essential epithelial cell characteristic necessary for the process of reprogramming. The cells must be able to proliferate during pregnancy in order to redirect spermatogenic cells to mammary epithelial cell fates (manuscript in preparation).
Serial transplantation of the mammary epithelium inevitably leads to growth senescence, which has clearly been linked to the number of mitotic events required for stem cell activity to reach the outermost periphery of the regenerated gland. We have begun studies designed to determine whether growth senescent mammary epithelial cell populations that are unable to support in vivo mammary epithelial regeneration by themselves may be able to re-program non-mammary stem/progenitor cells. Thus far, those growth-deficient mammary populations that we have tested were able to reprogram non-mammary stem cells and in the process were able to generate full mammary outgrowths in cleared mammary fat pads, but each of these were successful in pregnant hosts of regenerating a mammary outgrowth by themselves, with the exception of lethally irradiated mammary cells. These findings have strong implications for recruitment of transformed cells to growth-deficient niches and neoplasia. Our present work demonstrates that signals from the mammary microenvironment in the context of the regenerating gland are capable of redirecting the repertoire of adult somatic stem cells from three non-mammary tissues and both mouse and human cancer cells. The only mammary epithelial cells that were found to fail to redirect testicular cells were ones that were unable to proliferate either in nulliparous mice or pregnant hosts, e.g., irradiated mammary epithelial cells or ERα-null epithelium. This leaves the molecular events involved in reprogramming so far unresolved.
Preliminary studies indicate that tissue extracts may be used in place of live mammary epithelial cells to reprogram adult mouse spermatogenic cells to adopt mammary epithelial cell fate. Likewise, it may be possible to redirect tumorigenic cells to normal cell function by exposure to substances present within normal tissues. Further efforts to extend these initial findings will elucidate at least some of the mechanisms involved.
Highlights.
Mammary stem cells reside in protective tissue locales (niches)
Niches are disrupted and must reform when dispersed epithelial cells are transplanted
Cells of non-mammary origin can be incorporated into reforming mammary niches
Non-mammary cells within the mammary niche adopt a mammary stem/progenitor cell fate
These results extend to mesoderm-derived cells and human and mouse cancer cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Yamashita YM, Fuller MT. Asymmetric stem cell division and function of the niche in the Drosophila male germ line. Int J Hematol. 2005 Dec;82(5):377–80. doi: 10.1532/IJH97.05097. [DOI] [PubMed] [Google Scholar]
- 3.Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002 Dec;3(12):931–40. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- 4.Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005 Oct;9(4):501–10. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Kimble J, Crittenden SL. WormBook. 2005. Germline proliferation and its control; pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno RD, Boulanger CA, Smith GH. Notch-induced mammary tumorigenesis does not involve the lobule-limited epithelial progenitor. Oncogene. Jun 13; doi: 10.1038/onc.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998 Apr 28;95(9):5076–81. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2196–201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci U S A. 2007 Mar 27;104(13):5455–60. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouros-Mehr H, Kim JW, Bechis SK, Werb Z. GATA-3 and the regulation of the mammary luminal cell fate. Curr Opin Cell Biol. 2008 Apr;20(2):164–70. doi: 10.1016/j.ceb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005 Nov 21;171(4):717–28. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulanger CA, Smith GH. Reducing mammary cancer risk through premature stem cell senescence. Oncogene. 2001 Apr 26;20(18):2264–72. doi: 10.1038/sj.onc.1204312. [DOI] [PubMed] [Google Scholar]
- 13.Soriano JV, Pepper MS, Orci L, Montesano R. Roles of hepatocyte growth/scatter factor and transforming growth factor-beta1 in mammary gland ductal morphogenesis. J Mammary Gland Biology and Neoplasia. 1998;3:133–50. doi: 10.1023/a:1018790705727. [DOI] [PubMed] [Google Scholar]
- 14.Stull MA, Rowzee AM, Loladze AV, Wood TL. Growth factor regulation of cell cycle progression in mammary epithelial cells. J Mammary Gland Biology and Neoplasia. 2004;9:15–26. doi: 10.1023/B:JOMG.0000023585.95430.f4. [DOI] [PubMed] [Google Scholar]
- 15.Lu P, Ewald AJ, Martin GR, Werb Z. Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis. Dev Biol. 2008 Sep 1;321(1):77–87. doi: 10.1016/j.ydbio.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008 Apr;14(4):570–81. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz E, Streuli CH. The extracellular matrix as an adhesion checkpoint for mammary epithelial function. Int J Biochem Cell Biol. 2007;39(4):715–26. doi: 10.1016/j.biocel.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Belmonte F, Yu W, Rodriguez-Fraticelli AE, Ewald AJ, Werb Z, Alonso MA, et al. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr Biol. 2008 Apr 8;18(7):507–13. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth BW, Boulanger CA, Smith GH. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol. 2007 Sep;212(3):729–36. doi: 10.1002/jcp.21071. [DOI] [PubMed] [Google Scholar]
- 20.Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci U S A. 2007 Mar 6;104(10):3871–6. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci U S A. 2008 Sep 30;105(39):14891–6. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002 Mar;129(6):1377–86. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- 23.Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005 Jan 20;24(4):552–60. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- 24.Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007 Mar 1;303(1):29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Boulanger CA, Bruno RD, Rosu-Myles M, Smith GH. The Mouse Mammary Microenvironment Redirects Mesoderm-derived Bone Marrow Cells to a Mammary Epithelial Progenitor Cell Fate. Stem Cells Dev. Jun 8; doi: 10.1089/scd.2011.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004 Sep 9;23(41):6980–5. doi: 10.1038/sj.onc.1207827. [DOI] [PubMed] [Google Scholar]
- 27.Booth BW, Boulanger CA, Anderson LH, Smith GH. The normal mammary microenvironment suppresses the tumorigenic phenotype of mouse mammary tumor virus-neu-transformed mammary tumor cells. Oncogene. Feb 10;30(6):679–89. doi: 10.1038/onc.2010.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bussard KM, Boulanger CA, Booth BW, Bruno RD, Smith GH. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. Aug 1;70(15):6336–43. doi: 10.1158/0008-5472.CAN-10-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39:21–31. doi: 10.1007/BF01806075. [DOI] [PubMed] [Google Scholar]
- 30.Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–60. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- 31.Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–86. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- 32.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006 Jan 5;439(7072):84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 33.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006 Feb 23;439(7079):993–7. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 34.Lennon VA, Unger M, Dulbecco R. Thy-1: a differentiation marker of potential mammary myoepithelial cells in vitro. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6093–7. doi: 10.1073/pnas.75.12.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]