Abstract
Endothelial cell (EC) adhesion, shear retention, morphology, and hemostatic gene expression on fibronectin (FN) and RGD fluorosurfactant polymer (FSP) coated expanded polytetrafluoroethylene (ePTFE) grafts were investigated using an in vitro perfusion system. ECs were sodded on both types of grafts and exposed to 8dyn/cm2 of shear stress. After 24h the EC retention on RGD FSP coated grafts was 59±14%, which is statistically higher than the 36±11% retention measured on FN grafts (p<0.02). Additionally, ECs on RGD-FSP exhibited a more spread morphology and oriented in the direction of shear stress, as demonstrated by actin fiber staining. This spread morphology has been previously observed in cells that are adapting to shear stress. Real time PCR for vascular cell adhesion molecule 1, tissue factor, tissue plasminogen activator, and inducible nitric oxide synthase indicated that the RGD FSP material did not activate the cells and that shear stress appears to induce a more vasoprotective phenotype, as shown by a significant decrease in VCAM-1 expression, compared with sodded grafts. RGD FSP coating allows for a cell layer that is more resistant to physiological shear stress, as shown by the increased cell retention over FN. This shear stable EC layer is necessary for in vivo endothelialization of the graft material, which shows promise to increase the patency of synthetic small diameter vascular grafts.
Keywords: Endothelial Cells, ePTFE Vascular Grafts, Surface Modification, Shear Stress
Introduction
Over 86 million Americans have cardiovascular disease and 16 million have coronary heart disease (CHD). This resulted in over 406,000 deaths and 408,000 coronary revascularization procedures in 2007, the most recent year for which data is available1. Autologous vessels, such as the saphenous vein and mammary artery, are most commonly used as the bypass conduit, however 20–40% of patients lack a suitable saphenous vein2,3. Expanded polytetrafluoroethylene (ePTFE) is used as a synthetic graft material in medium to large diameter vascular grafting. However, the long term patency of these grafts is much lower than vein grafts, especially in applications where the inner diameter is less than 6mm4–6. Consequently, they are not widely used in coronary artery bypass grafting.
One of the main failure mechanisms of ePTFE grafts is thrombosis7–11. A confluent layer of endothelial cells at the blood interface would help prevent thrombosis and could increase the patency of vascular grafts. Previous attempts to implant endothelialized ePTFE grafts have required long in vitro incubation times and have had only moderately improved outcomes, in comparison with non-endothelialized grafts12–15. In order to improve the outcome for small diameter vascular grafts, we have developed a self-assembling peptide biomimetic fluorosurfactant polymer (FSP) coating that facilitates adhesion of endothelial cells to ePTFE through the cell adhesive ligand, Arg-Gly-Asp (RGD)16. In this study, we have investigated the ability of RGD FSP coated ePTFE grafts to adhere and retain endothelial cells (ECs) under physiological shear stress.
Endothelial cells in the anti-coagulant phenotype produce vasoprotective factors and inhibit the production of factors that induce inflammation. Some of these factors include inducible nitric oxide synthase (iNOS), which produces NO and reduces platelet adhesion17, tissue factor (TF), which is a procoagulant cell-associated protein that combines with fVIIa to activate FX and ultimately produce thrombin18, tissue plasminogen activator (tPA), which activates plasminogen and is involved in fibrinolysis, the degradation of fibrin clots19, and vascular cell adhesion molecule 1 (VCAM-1), which has variable expression that is upregulated by tumor necrosis factor alpha and interleukin-1 and supports the adhesion of white blood cells, such as monocytes and lymphocytes20,21. We also investigated the phenotype of ECs on coated ePTFE grafts by measuring the expression of these factors in response to physiological shear stress.
This paper compares EC shear stability on fibronectin (FN) and RGD FSP coated ePTFE grafts, using an in vitro perfusion system. We also investigated the shear dependent gene expression of ECs on both surfaces. Overall we demonstrate that RGD FSP coated grafts support a more shear stable EC layer than FN coated grafts and therefore could be used to improve the patency of pre-endothelialized small diameter vascular grafts.
Methods
Peptide Fluorosurfactant Polymer Synthesis and Surface Modification
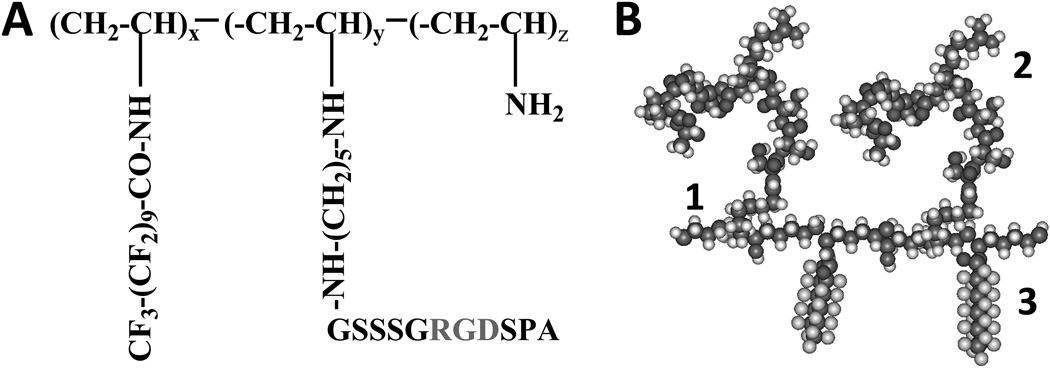
The RGD FSP (Figure 1) was synthesized as previously described16. Briefly, cell adhesive peptide (GSSSGRGDSPA) was purified and reacted with glutaraldehyde, though a Schiff’s base reaction. The glutaraldehyde-modified RGD peptide was attached to poly(vinyl amine). N-(perfluoroundecanoyloxy) succinimide was synthesized and combined with PVAm-Pep to create the final product.
Figure 1.
A. Chemical structure of RGD FSP. B. Molecular model of RGD FSP consisting of poly(vinyl amine) backbone (1), RGD peptide (2), fluorocarbon (3).
The surfactant polymer was dissolved in distilled water (1mg/mL) and adsorbed on ePTFE grafts (20–40 µm intermodal distance; ZEUS; Orangeburg, SC) for 24 h. The samples were removed from the solution and dried. For cell studies, the surfaces were sterilized with ethylene oxide gas, using the cold cycle with a 36h outgassing at University Hospitals Case Medical Center. Sterile grafts were stored at room temperature and protected from light until use. Grafts freshly coated with human fibronectin (10µg/cm2, Sigma) were used as a control.
Culture of Human Pulmonary Artery Endothelial Cells
Human pulmonary artery endothelial cells (ECs; Lonza; Walkersville, MD), obtained at passage 3, were grown to 80–90% confluence from a 1:3 split ratio in 75 cm2 tissue culture polystyrene flasks (Corning Life Sciences; Lowell, MA) in endothelial cell growth medium (ECGM) and trypsinized using 0.05% trypsin/EDTA (Invitrogen; Carlsbad, CA). ECGM was made by supplementing basal media (MCDB 131, Invitrogen) with 0.015% (w/v) EC growth factor supplement (ECGS, Core Facilities Laboratory, Cell Biology Department, Cleveland Clinic Foundation, Cleveland, OH), 0.0009% heparin (w/v, heparin activity, 16.3 U/mL; isolated from porcine mucosa, Sigma; St. Louis, MO) and 10% (v/v) fetal bovine serum (FSB, Sigma). ECs for experiments were used at passages 6–9.
In Vitro Perfusion System
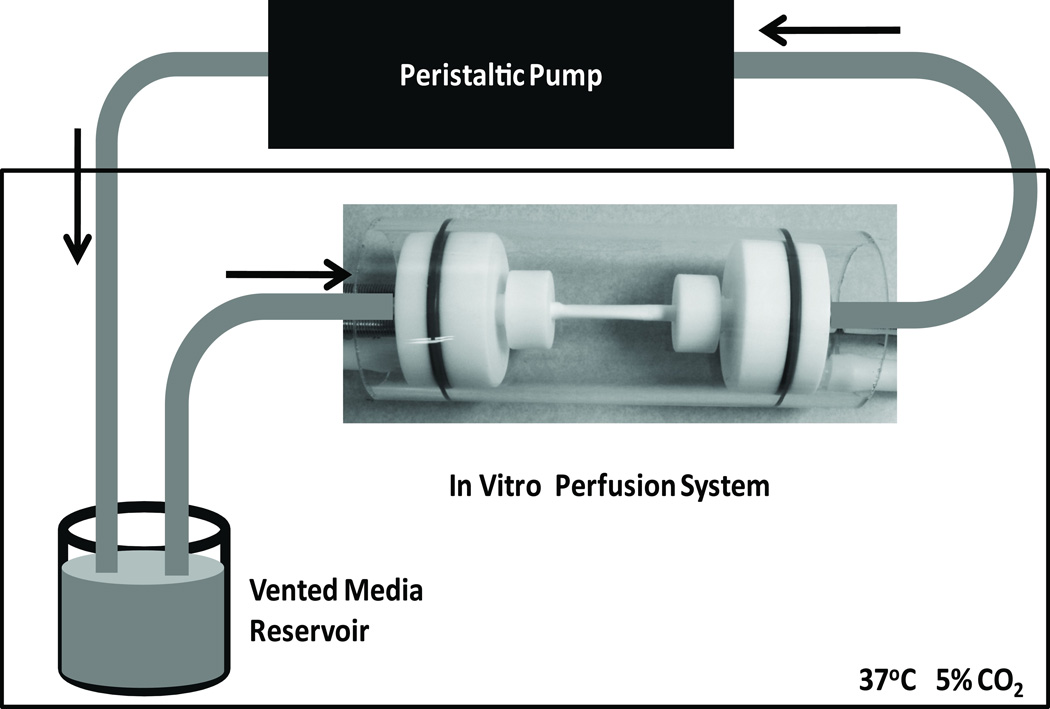
The in vitro perfusion system (Figure 2) consisted of a 4mm inner diameter (ID) coated graft held between Teflon connectors fit into an outer glass tube (51mm OD). Glass tubing (4mm OD) was inserted through the connectors to act as an adaptor for the silicone tubing used by the peristaltic pump (Cole-Parmer, Vernon Hills, IL) to the graft. Grafts were held on the glass tubing by an O-ring and nut that screwed onto the connector. The two connectors with the graft in between were placed in the outer glass tube and the outer chamber was filled with ECGM.
Figure 2.
Diagram of in vitro perfusion system used to apply shear stress (8dyn/cm2) to EC sodded grafts. The system consisted of holder for the ePTFE graft, vented media reservoir in a 37°C incubator with 5% CO2, and a peristaltic pump. EC growth media thickened with 2% (w/v) poly (ethelyene oxide) was used as the perfusate in the system.
ECs were pressured sodded on the grafts at 75,000 cells/cm2 as described by Jarrell et al.22. Cell suspension (62,831 cells/mL) was pumped across the pores of the graft by clamping one end of the perfusion system closed. Excess media flowed out of the outer glass tube. The grafts were sodded for 1 h at 37°C then placed on a Labquake rotisserie (Fisher; Waltham, MA) and rotated at 8 rpm in a 37°C incubator (New Brunswick Scientific; Edison, NJ) with 5% CO2 overnight.
ECGM was thickened with 2% w/v poly(ethylene oxide) (PEO; Polysciences Inc.; Worrington, PA) to achieve a viscosity of 2.5 mm2/sec. A cell viability assay using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; Promega; Madison WI) confirmed that this concentration of PEO was not toxic to ECs. This solution was pumped through the perfusion system, using a peristaltic pump at 120 mL/min to result in a shear stress of 8 dynes/cm2, which is half the maximum shear stress measured in bypass grafts by Shimizu et al.23. Shear stress was applied for 4 or 24 h. The retrieved grafts were examined with epifluorescent microscopy or adherent cells were lysed to examine gene expression.
For analysis by epifluorescent microscopy, the cells were fixed while in the in vitro perfusion system with 4% w/v paraformaldehyde for 20 min, then treated with 0.01% TritonX and stained with ethidium homodimer (Invitrogen) for cell nuclei and phalloidin-Alexaflour488 (Invitrogen) for actin filaments. The perfusion system was drained and the grafts were removed and carefully cut open longitudinally and mounted on glass slides. Epifluorescent images were taken with 10× and 40× objectives on a Nikon Diaphot 200. Images were taken at 1mm intervals along three longitudinal lines, spanning the lumen of the graft. Cell population was determined by semi-automatically counting the number of nuclei that were stained with ethidium homodimer in a 0.61mm2 region of interest using ImageJ (v1.43f, NIH). The average cell population was determined from at least 40 regions per graft. Actin stress fiber alignment was visualized with the phalloidin-Alexaflour488 stain and analyzed using Metamorph (Molecular Devices, Sunnyvale, CA) software to determine the angle of the long axis of traced cells.
In order to lyse the cells for examining gene expression, the inner and outer chambers of the perfusion system were drained and the graft was removed. The cells were lysed immediately by pipetting RLT Plus lysis buffer (Qiagen, Valencia, CA) over the cell surface for 5 min. The buffer containing the lysed cells was collected and homogenized using a QIAShredder column (Qiagen) according to manufacturer’s directions.
Real Time Reverse Transcriptase Polymerase Chain Reaction
RNA purification, reverse transcription, and PCR were performed as previously reported24. Briefly, RNA was purified from cell lysate using RNeasy spin columns (Qiagen). The total RNA yield was determined spectroscopically, and RNA was reverse transcribed using the Superscript First Strand Synthesis System (Invitrogen). The cDNA template was diluted in ultra pure water (4ng/PCR well) and was used for real-time PCR analysis with the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and an iCycler fluorescence detection system (Bio-Rad). Relative transcript abundance was normalized to Glyceraldehyde 3-phophate dehydrogenase (GAPDH) expression assuming 100% PCR efficiency. Primers for genes representing a variety of EC functions were designed using Primer-BLAST (NIH). The forward and reverse primers were: tissue plasminogen activator: TGATGGGCTAATATGGCATT and TCCACGTCTCACCCAGTTAT; tissue factor: GCACTGTGACCGAGAACTTT and AGGTGCCATGGTGTTAAAAA; inducible nitric oxide synthase: CCTGCACTATGGAGTCTGCT and GAACACCAAAGTCATGGGAG; vascular cell adhesion molecule 1: GGCAGAGTACGCAAACACTT and CCCCAGAATCTTCCATTTTT; GAPDH: ACGAATTTGGCTACAGCAAC and GTGAGGAGGGGAGATTCAGT.
Statistical Analysis
Statistical analysis was done using Microsoft Excel and Minitab 15. Data are represented as mean ± standard deviation. Analysis of variance (ANOVA) followed by Tukey’s post hoc test was used for data sets with multiple comparisons. Statistical analysis of real-time PRC data was performed in the logarithmic domain. A value of p < 0.05 was considered significant.
Results
Peptide fluorosurfactant polymer surface modification
Peptide fluorosurfactant polymer, consisting of a poly(vinyl amine) backbone with fluorocarbon side chains to allow for stable adherence to ePTFE grafts and RGD peptide ligands to adhere endothelial cells, was used to coat ePTFE grafts with an EC binding peptide. RGD FSP was adsorbed from aqueous solution onto the grafts for 24h. Water contact angles were used to evaluate the success of the coating. Table 1 shows the contact angles on ePTFE grafts before and after coating. Unmodified surfaces have a high contact angle, indicating a highly hydrophobic surface. RGD FSP coated grafts had a lower contact angle, which demonstrates that the coating procedure was successful.
Table 1.
Water Contact Angles for Unmodified and FSP Coated Grafts
| Surface | Advancing Contact Angle (θ,°) | Receding Contact Angle (θ,°) |
|---|---|---|
| ePTFE | 122 ± 5 | 119 ± 4 |
| RGD FSP coated ePTFE | 107 ± 2 | 44 ± 9 |
EC attachment and shear stability
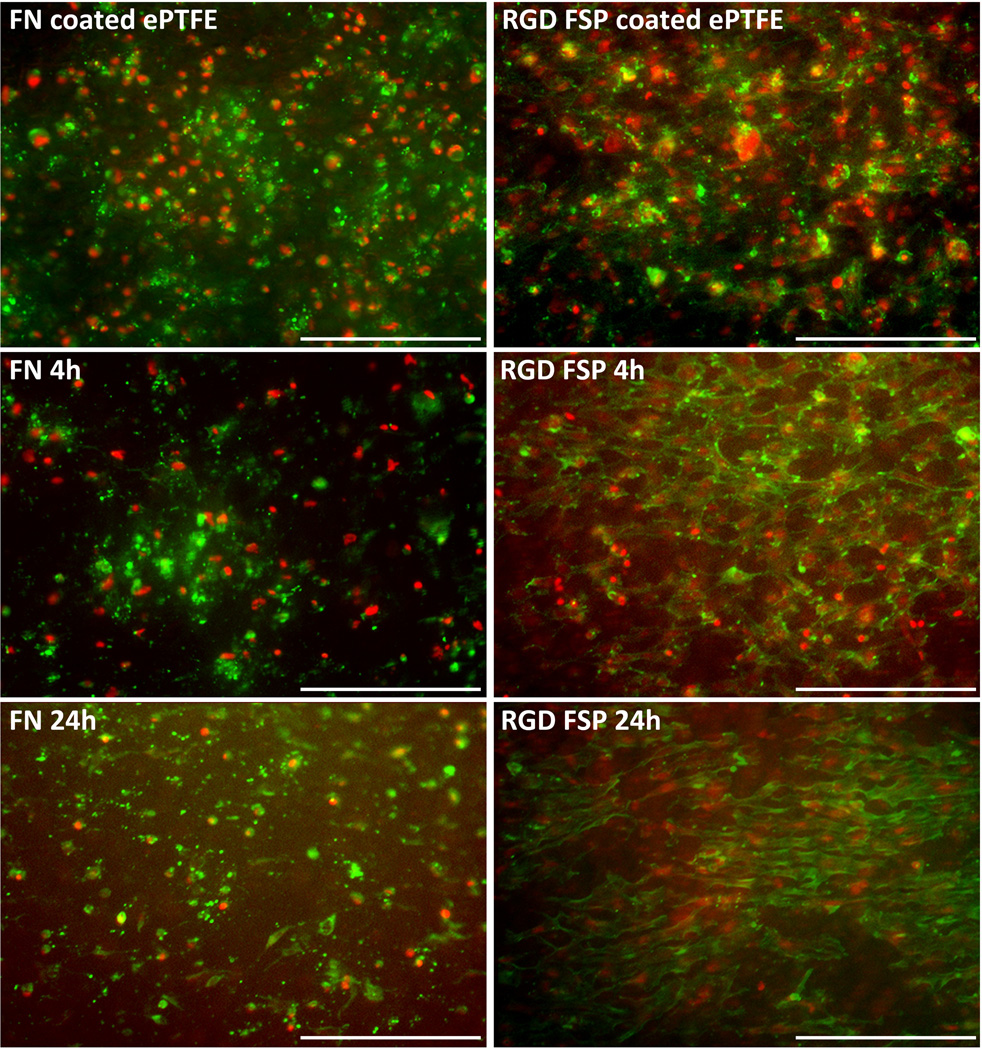
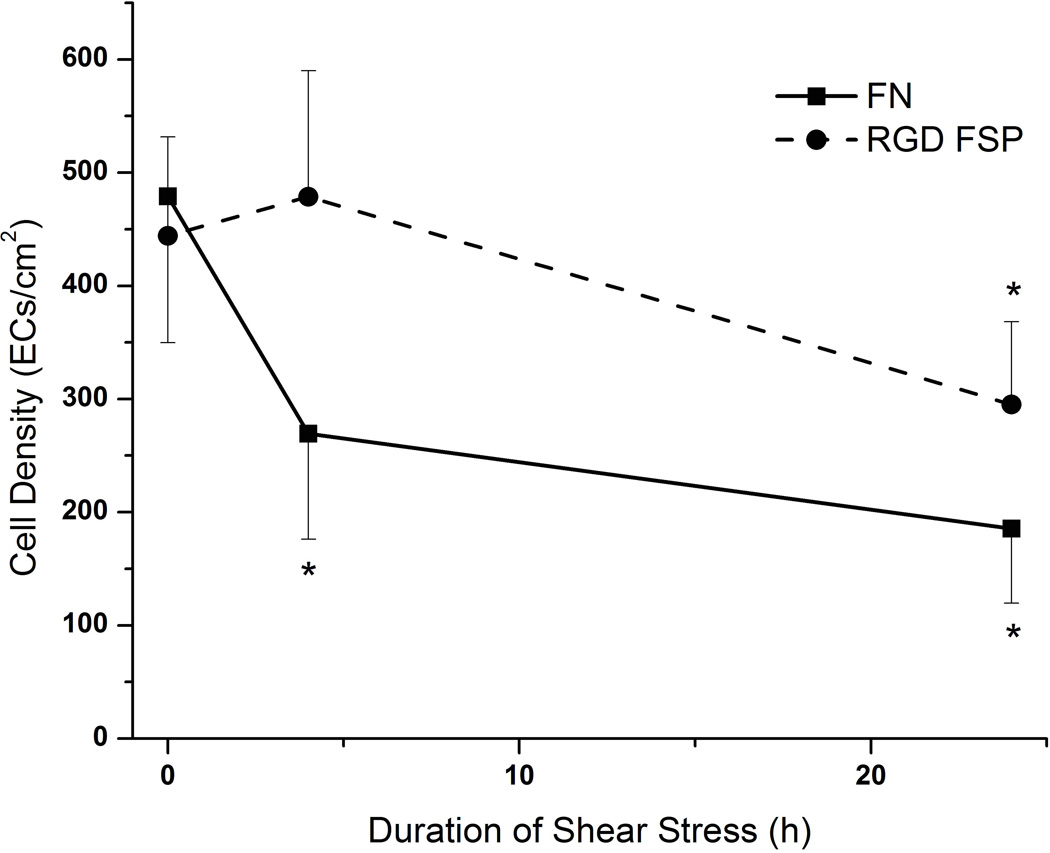
In native vasculature, ECs are exposed to shear stress. These cells must be shear stable in order to maintain their vasoprotective properties. Therefore, the ability of these coated grafts to maintain a confluent layer of cells was examined. Endothelial cells were sodded to FN and RGD FSP coated ePTFE grafts. After sodding, the cell population was determined by counting the number of cells in multiple areas of the lumen of the graft. The populations were similar for both coatings (n=9 for FN and n=10 for RGD FSP) (Figures 3 and 4). After 4 hours of 8 dyn/cm2 shear stress, the number of cells on FN grafts (n=3) was significantly decreased from the initial population on sodded grafts. At this time point, the number of cells on the RGD FSP coated grafts (n=4) had not decreased from baseline. After 24 hours of shear stress, the number of cells on FN coated grafts (n=6) continued to decrease and had a cell retention of 36±11%. The cell population on the RGD FSP grafts (n=6) was also significantly lower than that on grafts which had not been exposed to shear stress. However, the retention on RGD-FSP was 59±14%, which was statistically higher than the retention on FN coated grafts (p<0.02). Additionally, ECs on RGD FSP exhibited a more spread morphology and were oriented in the direction of shear stress, as demonstrated by actin fiber staining. In order to quantify these changes, higher magnification images of the cytoskeleton were examined.
Figure 3.
Representative epifluorescent images of ECs on FN and RGD FSP coated grafts. Cells were stained for nuclei (red) and actin filaments (green). EC population was higher on RGD FSP coated grafts compared with FN coated grafts. ECs on RGD FSP were more spread than cells on FN, as demonstrated by epifluorescent images of the grafts after 8 dyn/cm2 shear stress exposure for 4 or 24h. Scale bars = 250 µm.
Figure 4.
Graph of EC population on FN and RGD FSP coated grafts exposed to 8 dyn/cm2 shear stress for 0,4, or 24h. EC retention on RGD FSP coated grafts was significantly higher than retention on FN coated grafts at both 4 and 24h. * p< 0.05 compared to sodded graft
EC morphology
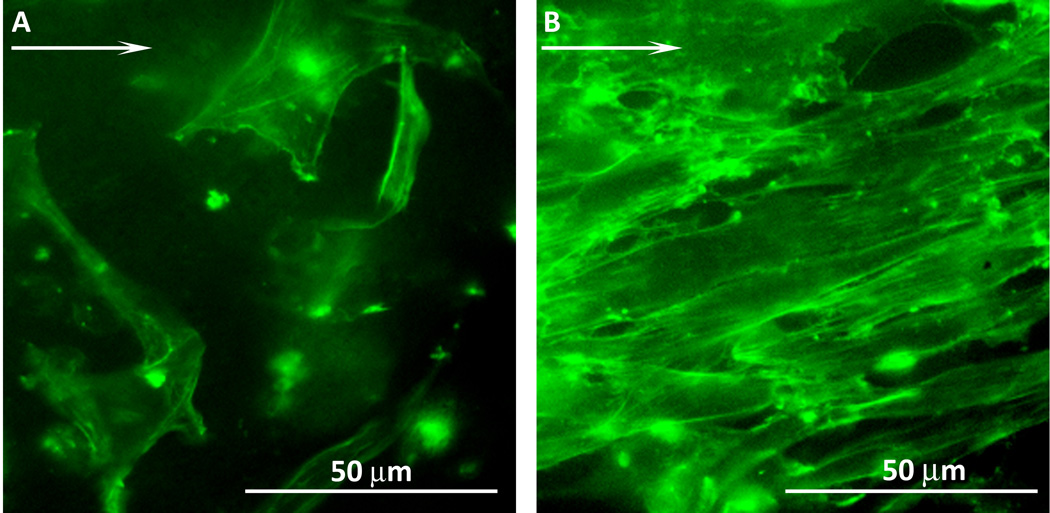
The morphology of ECs on FN and RGD FSP coated grafts were examined after 24h of shear stress. At least 20 cells from three FN and three RGD FSP coated grafts were studied. Actin fibers were stained and epifluorescent images were taken at 40× (Figure 5). The actin fibers were analyzed to determine the percent of cells that had aligned to within 20° of the direction of shear stress, indicated by the arrow in Figure 5. On FN grafts, 53% of the cells aligned with the shear stress. In comparison, 86% of the cells on RGD FSP grafts were aligned. This spread and oriented morphology on RGD FSP coated grafts has been observed previously in cells that are adapting to shear stress25,26.
Figure 5.
Representative epifluorescent images of EC actin filaments after 24h shear stress on FN (A) and RGD FSP (B) coated ePTFE grafts. Arrow indicates direction of shear stress. 86% of cells on RGD FSP coated grafts aligned within ±20° of the direction of flow, while only 53% of cells on FN were aligned.
Gene Expression
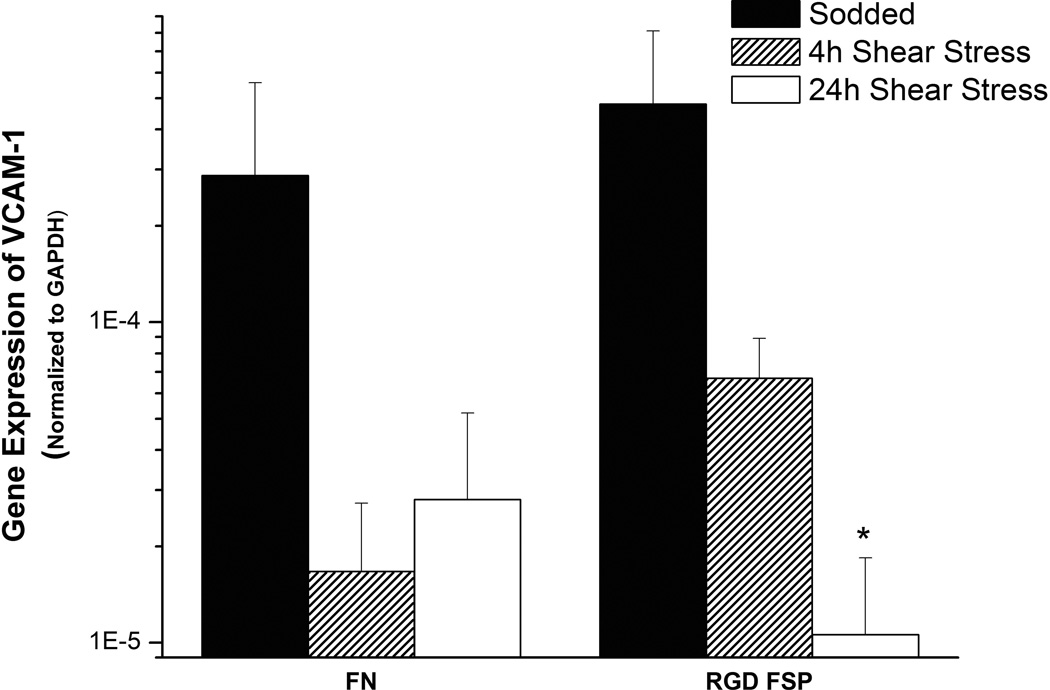
Endothelial cells in an anti-coagulant phenotype express factors that reduce platelet activity, induce fibrinolysis, and limit the recruitment of white blood cells. To check the phenotype of ECs on RGD FSP and FN coated grafts, real time PCR was performed before and after shear stress on a variety of markers for EC functions. No significant changes with shear stress or between FN and RGD FSP coated grafts were observed in the expression of TF, iNOS, and tPA. The initial expression of VCAM-1 normalized to GAPDH expression (Figure 6) was similar on FN and RGD FSP grafts. With shear stress the expression decreased on both types of grafts. This decrease was statistically lower on the RGD FSP grafts after 24h of shear stress compared with sodded grafts (p<0.001).
Figure 6.
Graph of VCAM-1 mRNA expression normalized to GAPDH, measured by real time PCR, by ECs on FN and RGD FSP coated grafts after sodding and 4 or 24h of 8 dyn/cm2 shear stress. VCAM-1 mRNA was reduced after shear stress, compared with sodded grafts with the same coating. TF, iNOS, and tPA mRNA did not change significantly with shear stress. *p< 0.05 compared to sodded graft
Discussion
This study examined EC retention and characteristics on vascular graft material under physiological shear stress. We have reported previously on the behavior of ECs in response to RGD FSP on fluorosilane self assembled monolayers, but not on tubular ePTFE graft materials. Furthermore, the use of the in vitro perfusion systems allows for testing of EC stability under shear stress with flow conditions that are similar to those in the native vessel. By combining the ePTFE graft material with an in vitro perfusion system, we are able to mimic the shear environment that would be experienced by an implanted bypass vessel.
We investigated the differences between fibronectin and RGD FSP coated grafts. RGD FSP adsorbs onto the ePTFE graft by means of hydrophobic interactions between the pendant fluorocarbon groups and fluorocarbon chains in the ePTFE surface. We have reported previously that the expected peptide density for this material is 0.11–0.19 nmol/cm2 16. Considering that a 4 cm long piece of graft has a surface area of about 5cm2, it is expected that an RGD FSP coated graft would have 3.3–5.7× 1017 RGD ligands. For these experiments, grafts were coated with fibronectin at 10µg/cm2. Assuming the graft absorbed all of the fibronectin, the theoretical maximum number of FN molecules in a monolayer on the graft is 5.2×1016. This is an order of magnitude less than the number of expected RGD ligands on RGD FSP coated grafts. These differences in the number of adhesion sites may contribute to the differences in cell retention between the grafts. Protein denaturation of adsorbed FN, caused by the underlying hydrophobic ePFTE surface, likely makes the actual number of accessible RGD sites much less than the theoretically calculated number. This factor may also contribute to the decreased cell retention on FN coated grafts in that the FN protein itself may be removed by shear stress. It follows that the cells on the FN coated ePTFE grafts may lack sufficient integrin adhesion sites to allow for spreading in the direction of shear stress and are removed soon after the onset of fluid flow. ECs on the RGD FSP coated grafts have many more accessible adhesion sites so are more able to align in the direction of fluid flow. This alignment has been shown to result in a decreased z axis profile of the cell, making it more shear stable25–29.
In order to provide vasoprotective benefits, ECs must be in the anti-coagulant phenotype. When expressing this phenotype, ECs limit platelet activity, reduce the production of thrombin, induce fibrinolysis, and diminish inflammatory cell adhesion. Expression of mRNA for inducible nitric oxide synthase, tissue factor, tissue plasminogen activator, and vascular cell adhesion molecule 1 was compared between FN or RGD FSP coated grafts at baseline and after 4 or 24h of shear stress. The results from the real time PCR did not show any differences between FN and RGD FSP coated grafts under the same shear stress conditions, indicating that the RGD FSP did not cause an activation of the cells. Additionally, shear stress appears to induce a more vasoprotective phenotype, as shown by the decrease in VCAM-1 expression (Figure 6). Bergh et al. demonstrated a reduction in VCAM-1 expression in human umbilical vein ECs grown on glass with increasing shear stress30. This result is in agreement with the decrease we observed on coated ePTFE. However the same group also observed significantly decreasing tPA expression when shear stress was increased from 1 to 12.5 or 25 dyn/cm2 30. Another group observed a significant increase in tPA gene expression for human saphenous vein ECs seeded fibrin glue coated ePTFE grafts exposed to 12 dyn/cm2 shear stress for 4h compared to the static control31. In this study, no change was observed in ECs tPA gene expression for human pulmonary artery with 8 dyn/cm2 of shear stress. Variations in cell type and level and duration of shear stress could account for the differences in gene response to shear stress reported here and from other groups. The lack of change in gene expression of tPA, TF, and iNOS and decrease in VCAM-1 with shear stress on both FN and RGD FSP coated ePTFE grafts indicates that the RGD FSP coating does not cause a pro-coagulant phenotype, which is a critical benefit for preventing thromobogenesis.
Conclusion
In this study, we describe a coating for vascular grafts that provides a high density of cell adhesive ligands and a system for examining EC retention and characteristics on ePTFE graft materials. ePTFE grafts coated with RGD fluorosurfactant polymer and fibronectin are capable of attaching ECs. The RGD FSP coating allowed for a cell layer that is more resistant to physiological shear stress, as shown by the increased cell retention over FN. Furthermore, gene expression profiles on RGD FSP were similar to FN both before and after shear stress, indicating that RGD FSP does not alter the cells’ phenotype, which is critical for preventing thrombogenesis. A shear stable EC layer is necessary for in vivo endothelialization of the graft material. RGD FSP coated ePTFE shows promise to promote endothelialization and should be studied further as a method to increase the patency of synthetic small diameter vascular grafts.
Acknowledgements
The project described was supported by Grant Number 5R01EB002067 for the National Institute of Biomedical Imaging and Bioengineering and Grant Number 1R01HL087843 for the National Heart, Lung, and Blood Institute.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Works Cited
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chew DK, Owens CD, Belkin M, Donaldson MC, Whittemore AD, Mannick JA, Conte MS. Bypass in the absence of ipsilateral greater saphenous vein: safety and superiority of the contralateral greater saphenous vein. J Vasc Surg. 2002;35(6):1085–1092. doi: 10.1067/mva.2002.124628. [DOI] [PubMed] [Google Scholar]
- 3.Taylor LM, Jr, Edwards JM, Porter JM. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg. 1990;11(2):193–205. doi: 10.1067/mva.1990.17235. discussion 205-6. [DOI] [PubMed] [Google Scholar]
- 4.Kannan RY, Salacinski HJ, Butler PE, Hamilton G, Seifalian AM. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B Appl Biomater. 2005;74(1):570–581. doi: 10.1002/jbm.b.30247. [DOI] [PubMed] [Google Scholar]
- 5.Aracil-Sanus E, Mendieta-Azcona C, Cuesta-Gimeno C, Chinchilla-Molina A. Infragenicular bypass graft for limb salvage using polytetrafluoroethylene and distal vein cuff as the first alternative in patients without ipsilateral greater saphenous vein. Ann Vasc Surg. 2005;19(3):379–385. doi: 10.1007/s10016-004-0130-6. [DOI] [PubMed] [Google Scholar]
- 6.Pereira CE, Albers M, Romiti M, Brochado-Neto FC, Pereira CA. Meta-analysis of femoropopliteal bypass grafts for lower extremity arterial insufficiency. J Vasc Surg. 2006;44(3):510–517. doi: 10.1016/j.jvs.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 7.Canizales S, Charara J, Gill F, Guidoin R, Roy PE, Bonnaud P, Laroche G, Batt M, Roy P, Marois M, et al. Expanded polytetrafluoroethylene prostheses as secondary blood access sites for hemodialysis: pathological findings in 29 excised grafts. Can J Surg. 1989;32(6):433–439. [PubMed] [Google Scholar]
- 8.Jenkins AM, Buist TA, Glover SD. Medium-term follow-up of forty autogenous vein and forty polytetrafluoroethylene (Gore-Tex) grafts for vascular access. Surgery. 1980;88(5):667–672. [PubMed] [Google Scholar]
- 9.Mehta RI, Mukherjee AK, Patterson TD, Fishbein MC. Pathology of explanted polytetrafluoroethylene vascular grafts. Cardiovasc Pathol. 2011;20(4):213–221. doi: 10.1016/j.carpath.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Shuhaiber JH, Evans AN, Massad MG, Geha AS. Mechanisms and future directions for prevention of vein graft failure in coronary bypass surgery. Eur J Cardiothorac Surg. 2002;22(3):387–396. doi: 10.1016/s1010-7940(02)00253-1. [DOI] [PubMed] [Google Scholar]
- 11.Chiesa R, Marone EM, Tshomba Y, Logaldo D, Castellano R, Melissano G. Aortobifemoral bypass grafting using expanded polytetrafluoroethylene stretch grafts in patients with occlusive atherosclerotic disease. Ann Vasc Surg. 2009;23(6):764–769. doi: 10.1016/j.avsg.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Laube HR, Duwe J, Rutsch W, Konertz W. Clinical experience with autologous endothelial cell-seeded polytetrafluoroethylene coronary artery bypass grafts. J Thorac Cardiovasc Surg. 2000;120(1):134–141. doi: 10.1067/mtc.2000.106327. [DOI] [PubMed] [Google Scholar]
- 13.Leseche G, Ohan J, Bouttier S, Palombi T, Bertrand P, Andreassian B. Above-knee femoropopliteal bypass grafting using endothelial cell seeded PTFE grafts: five-year clinical experience. Ann Vasc Surg. 1995;9(3 Suppl):S15–S23. doi: 10.1016/s0890-5096(06)60447-0. [DOI] [PubMed] [Google Scholar]
- 14.Meinhart JG, Deutsch M, Fischlein T, Howanietz N, Froschl A, Zilla P. Clinical autologous in vitro endothelialization of 153 infrainguinal ePTFE grafts. Ann Thorac Surg. 2001;71(5 Suppl):S327–S331. doi: 10.1016/s0003-4975(01)02555-3. [DOI] [PubMed] [Google Scholar]
- 15.Stroncek JD, Ren LC, Klitzman B, Reichert WM. Patient-derived endothelial progenitor cells improve vascular graft patency in a rodent model. Acta Biomater. 2012;8(1):201–208. doi: 10.1016/j.actbio.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen CC, Kligman F, Kottke-Marchant K, Marchant RE. The effect of RGD fluorosurfactant polymer modification of ePTFE on endothelial cell adhesion, growth, and function. Biomaterials. 2006;27(28):4846–4855. doi: 10.1016/j.biomaterials.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 18.Kato H. Regulation of functions of vascular wall cells by tissue factor pathway inhibitor: basic and clinical aspects. Arterioscler Thromb Vasc Biol. 2002;22(4):539–548. doi: 10.1161/01.atv.0000013904.40673.cc. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Yasui H, Brzoska T, Mogami H, Urano T. Surface-retained tPA is essential for effective fibrinolysis on vascular endothelial cells. Blood. 2011;118(11):3182–3185. doi: 10.1182/blood-2011-05-353912. [DOI] [PubMed] [Google Scholar]
- 20.Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol. 2009;53(3):317–333. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 22.Jarrell BE, Williams SK, Rose D, Garibaldi D, Talbot C, Kapelan B. Optimization of human endothelial cell attachment to vascular graft polymers. J Biomech Eng. 1991;113(2):120–122. doi: 10.1115/1.2891225. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu T, Ito S, Kikuchi Y, Misaka M, Hirayama T, Ishimaru S, Yamashina A. Arterial conduit shear stress following bypass grafting for intermediate coronary artery stenosis: a comparative study with saphenous vein grafts. Eur J Cardiothorac Surg. 2004;25(4):578–584. doi: 10.1016/j.ejcts.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 24.Beamish JA, Fu AY, Choi AJ, Haq NA, Kottke-Marchant K, Marchant RE. The influence of RGD-bearing hydrogels on the re-expression of contractile vascular smooth muscle cell phenotype. Biomaterials. 2009;30(25):4127–4135. doi: 10.1016/j.biomaterials.2009.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagnella S, Kligman F, Marchant RE, Kottke-Marchant K. Biometric surfactant polymers designed for shear-stable endothelialization on biomaterials. J Biomed Mater Res A. 2003;67(3):689–701. doi: 10.1002/jbm.a.10035. [DOI] [PubMed] [Google Scholar]
- 26.Murugesan G, Ruegsegger MA, Kligman F, Marchant RE, Kottke-Marchant K. Integrin-dependent interaction of human vascular endothelial cells on biomimetic peptide surfactant polymers. Cell Commun Adhes. 2002;9(2):59–73. doi: 10.1080/15419060214148. [DOI] [PubMed] [Google Scholar]
- 27.Franke RP, Grafe M, Schnittler H, Seiffge D, Mittermayer C, Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984;307(5952):648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- 28.Mott RE, Helmke BP. Mapping the dynamics of shear stress-induced structural changes in endothelial cells. Am J Physiol Cell Physiol. 2007;293(5):C1616–C1626. doi: 10.1152/ajpcell.00457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40(4):317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Bergh N, Ulfhammer E, Glise K, Jern S, Karlsson L. Influence of TNF-alpha and biomechanical stress on endothelial anti- and prothrombotic genes. Biochem Biophys Res Commun. 2009;385(3):314–318. doi: 10.1016/j.bbrc.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez P, Bourget C, Bareille R, Daculsi R, Bordenave L. Gene response in endothelial cells cultured on engineered surfaces is regulated by shear stress. Tissue Eng. 2007;13(7):1607–1614. doi: 10.1089/ten.2006.0399. [DOI] [PubMed] [Google Scholar]