Abstract
Relatively little is known about the behavioral effects of the neurosteroids compared with other drugs that modulate the γ-aminobutyric acid A (GABAA) receptor complex. This study examined the acute effects of pregnanolone and dehydroepiandrosterone (DHEA) in male rats responding under a differential-reinforcement-of-low-rate schedule of reinforcement. For comparison, three positive modulators of the GABAA receptor (lorazepam, ethanol, and pentobarbital), one negative modulator (β-CCM), and one neutral modulator (flumazenil) were tested. Pregnanolone was also administered in combination with DHEA to test for antagonism between these substances. Pregnanolone, lorazepam, and pentobarbital produced increases in responding at intermediate doses, and ethanol and pentobarbital produced decreases in responding at the highest doses tested. However, all four drugs dose-dependently decreased reinforced responding by decreasing inter-response times. DHEA, β-CCM, and flumazenil did not increase responding at intermediate doses or decrease reinforced responding. DHEA did not competitively antagonize the disruptive effects of pregnanolone. In summary, pregnanolone and DHEA produced effects on differential-reinforcement-of-low-rate responding that are similar to other positive and negative GABAA modulators, respectively, and do not produce these effects through a single binding site.
Keywords: γ-aminobutyric acid receptor, dehydroepiandrosterone, differential reinforcement of low rate, pregnanolone
Introduction
Neurosteroids are being studied as potential therapeutics because of their sedative, anxiolytic, anticonvulsant, and muscle relaxant effects (Monaghan et al., 1997; Hamilton, 2001; Legrain and Girard, 2003; Belelli and Lambert, 2005; Morrow, 2007; Mitchell et al., 2008). Many of these effects are thought to be mediated through allosteric sites on the γ-aminobutyric acid A (GABAA) receptor complex (Rupprecht and Holsboer, 1999; Belelli and Lambert, 2005; Akk et al., 2007, 2008; Wegner et al., 2007). Although there are similarities between different classes of GABAA receptor modulators, there are also reported differences at both the cellular and behavioral level (Costa, 1998). For example, Costa (1998) found that the partial allosteric modulator imidazenil could attenuate the proconvulsant effects of bicuculline in rats, but did not produce tolerance, dependence, or ataxia, unlike other allosteric modulators of the GABAA receptor complex (e.g. diazepam, alprazolam).
The purpose of this study was to determine the effects of the neurosteroids, pregnanolone and dehydroepiandrosterone (DHEA), on responding under a differential-reinforcement-of-low-rate (DRL) schedule, and compare those effects with the effects of drugs known to positively or negatively modulate the GABAA receptor complex. Schedule-controlled behavior can be remarkably stable and reproducible over long periods of time and can serve as a sensitive baseline to study a variety of drug interactions. Establishing a sensitive baseline is also critical in an interaction study where the repeated assessment of the effects of two or more compounds, both alone and in combination, is required (e.g. Winsauer and Riley, 1988). More specifically, the DRL schedule was chosen because it allowed for the comparison of these drugs over several dependent measures of responding (e.g. response rate, percentage of reinforced responses and the temporal pattern of responding), and being sensitive to both the rate-increasing and rate-decreasing effects of the positive GABAA modulators. A DRL schedule of 18 s was chosen because responses under this interval produces an intermediate response rate that can be either increased or decreased by administration of drug. The positive GABAA modulators lorazepam, pentobarbital, and ethanol were administered as a comparison with pregnanolone. The negative GABAA modulator β-CCM and the neutral GABAA modulator flumazenil (i.e. an antagonist at the benzodiazepine site on the GABAA receptor) were administered as a comparison with DHEA. Although the neurosteroids have been reported to have multiple binding sites on the GABAA receptor complex (Majewska et al., 1990; Hosie et al., 2006, 2007), very few studies have administered neurosteroids in combination to determine whether they might produce a physiological or pharmacological antagonism. Obtaining a dose-dependent pharmacological antagonism would indicate that these neurosteroids produced their effects by binding to the same site.
At present, only one neurosteroid (alphaxalone) is used clinically and it is approved for use as a general anesthetic. If neurosteroids do produce their behavioral effects in a manner similar to other GABAA modulators, there may be an increased therapeutic potential for these compounds. Several disorders (i.e. epilepsy, pain syndromes, anxiety, depression, and schizophrenia) exhibit perturbations of GABAergic transmission and some of these conditions have been associated with abnormal levels of certain endogenously occurring neurosteroids (Mitchell et al., 2008). There is also evidence that tolerance may not develop as readily during chronic neurosteroid administration, and in contrast to other GABAA modulators may be advantageous in treating long-term disorders (Reddy and Rogawski, 2000).
Methods
Subjects
Sixteen male Wistar rats (Charles River Laboratories, Wilmington, Delaware, USA) served as subjects. All subjects were housed singly in a colony room maintained at 21±2°C with 50±10% relative humidity on a 14-h light/10-h dark cycle (lights on at 06: 00 h CST). Nine of the 16 rats were exposed to three 30-s shocks 45 days before this experiment. The behavioral data from these nine subjects, however, were not significantly different from the seven naive subjects and they were subsequently grouped. Each subject earned 45 mg food pellets (TestDiets, a division of LabDiet, Richmond, Indiana, USA) during the experimental sessions and, when necessary, were provided standard rodent chow (Rodent Diet 5001, PMI Inc., Brentwood, Missouri, USA) in the home cage after the test sessions to maintain them at 90% of their free-feeding weight. Water was freely available in their home cage. This study was carried out in accordance with the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and the guidelines of the Committee on Care and Use of Laboratory Animal Resources, as adopted and promulgated by the US National Institutes of Health.
Apparatus
Nine identical modular test chambers (Coulbourn Instruments, Allentown, Pennsylvania, USA, Model E10-10TC) configured specifically for rodents were used. Located on the front wall of each chamber were a house light, speaker, auditory feedback relay, pellet trough (2 cm above the floor and 5 cm to the left of the response lever), and one response lever (6.5 cm above the floor and centered) with three-colored stimuli above the lever. The three stimuli above the lever were aligned horizontally and they could be transilluminated by three Sylvania 28ESB (Billerica, Massachusetts, USA) indicator lamps, one with a red plastic cap, one with a yellow cap, and one with a green cap. The response lever required a minimum force of 0.15N for activation and responses produced an audible click of the feedback relay. Each chamber was enclosed within a sound-attenuating cubicle equipped with a fan for ventilation, and a white-noise generator to mask extraneous sounds. All test chambers were connected to a computer programmed in MED-PC for Windows, Version IV (MED Associates, Inc., St Albans, Vermont, USA).
Behavioral procedures
A DRL schedule of food presentation was used to maintain responding. During the initial training sessions, the house light and the yellow stimulus above the lever were illuminated and every response on the lever resulted in the illumination of the pellet trough (0.4 s) and the delivery of a 45-mg food pellet. After rats reliably pressed the lever under this continuous reinforcement schedule, a DRL 18-s schedule of food presentation was instituted. Under this spaced-response schedule, responses were reinforced only if they occurred at least 18 s after the previous response or reinforcement; responses emitted before 18 s reset the interval. Responding was considered stable when each subject had at least 10 consecutive days in which the percentage of reinforced responses did not vary by more than 20%. Each session was 1 h in duration.
Drugs and drug administration
The acute effects of pregnanolone (1–18 mg/kg), lorazepam (0.01–3.2 mg/kg), ethanol (0.32–2.4 g/kg), pentobarbital (1.8–18 mg/kg), β-CCM (0.32–5.6 mg/kg), DHEA (32–240mg/kg), and flumazenil (3.2–18 mg/kg) were determined after responding under the 18-s DRL schedule stabilized. All the drugs were administered intraperitoneally 15 min before the session and the injection volume was always 0.1 ml/100 g, with the exception of ethanol, which was injected as a 15% (v/v) solution and required different volumes for each dose. Lorazepam (Wyeth Laboratories Inc., Marietta, Pennsylvania, USA) was dissolved in a vehicle containing polyethylene glycol (18%), benzyl alcohol (2%), and propylene glycol (80%). Pentobarbital sodium (Hart-Delta, Inc., Zachary, Louisiana, USA) was dissolved in a vehicle containing propylene glycol (40%), ethanol (10%), and sterile water (50%). β-CCM (methyl β-carboline-3-carboxylate, Research Biochemicals Inc., Natick, Massachusetts, USA) was dissolved in a vehicle containing polyethylene glycol (11%), benzyl alcohol (2%), propylene glycol (50%), and sterile water (37%). Flumazenil (Ascent Scientific Ltd., Princeton, New Jersey, USA) was dissolved in a vehicle containing polyethylene glycol (10%), benzyl alcohol (2%), propylene glycol (44%), dimethyl sulfoxide (11%), and sterile water (33%). Pregnanolone (5β-pregnan-3α-ol-20-one; Steraloids Inc., Newport, Rhode Island, USA) and DHEA (dehydroepiandrosterone; Sigma-Aldrich Corporation, St Louis, Missouri, USA) were dissolved in 45% 2-hydroxypropyl-γ-cyclodextrin (Sigma-Aldrich Corporation) in saline. Drug injections generally occurred on Tuesdays and Fridays, with control (vehicle or saline) injections occurring on Thursdays. Baseline sessions (no injections) occurred on the remaining days to ensure behavior had not been altered by drug injection. Higher dosages of all drugs were only administered on Wednesdays with no other drug injections occurring that week to avoid any carryover drug effects. Doses of each drug were administered in a mixed order and at least 10 days of testing without drug occurred between the different drugs.
Statistical analysis
A one-way analysis of variance (ANOVA) using SigmaStat (SYSTAT Software, Inc., Point Richmond, California, USA) was used to determine the effects of each drug on response rate and percentage of reinforced responses, whereas a two-way ANOVA was used to determine the effects of the DHEA and pregnanolone combinations on response rate and percentage of reinforced responses. Holm–Sidak post-hoc tests were used to analyze differences between control and drug data. The area-under-the-curve (AUC) values were calculated for the frequency-distribution curves using a transform provided by SigmaPlot (SYSTAT Software, Inc.). The transform provided was an algorithm that integrates the AUCs using the trapezoidal rule, which can be used for equal or unequally spaced × values. A one-way ANOVA was then used to determine the effects of each drug on the AUC values. Median effective dose (ED50) values were determined by linear regression using two or more data points reflecting the slopes of the descending portions of the curve for each drug. For response rate, the ED50 represented the dose of drug that decreased response rates to 50% of the control value. For percentage of reinforced responses, the ED50 represented the dose of drug that decreased the percentage of reinforced responses to 50% of the control value. If the curves did not fall below 50% of control, ED50 values were not calculated.
Results
Positive γ-aminobutyric acid A modulators
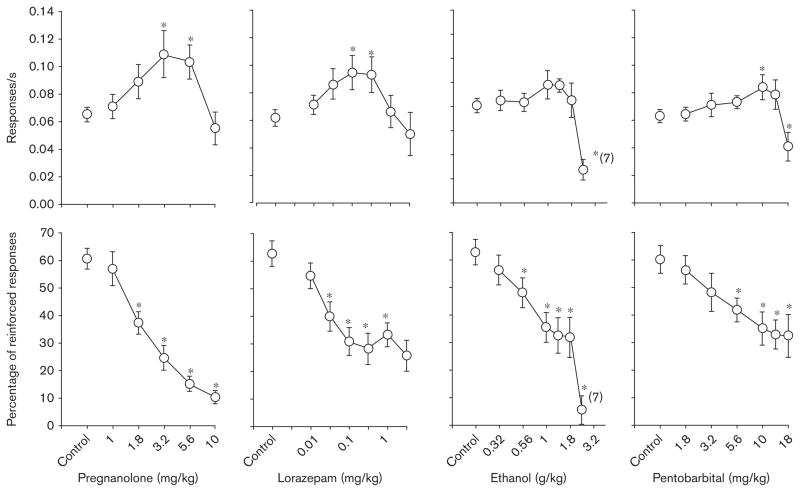
Changes in response rate produced by pregnanolone, lorazepam, ethanol, and pentobarbital were indicated statistically by a one-way ANOVA in which there was a significant main effect of dose for each drug [F(5,40)= 11.80, P<0.001; F(6,48)=4.56, P<0.001; F(6,44)=7.19, P<0.001; F(7,44)=6.82, P<0.001, respectively]. Holm–Sidak post-hoc tests also confirmed that specific doses of each drug were significantly different from the control data (Fig. 1, top panels); however, there were some differences among the four drugs. For example, while pregnanolone, lorazepam, and pentobarbital produced significant increases in response rate at intermediate doses, the increases obtained with ethanol at intermediate doses did not achieve statistical significance. Furthermore, only ethanol and pentobarbital produced significant rate-decreasing effects; the doses of pregnanolone and lorazepam tested were not large enough to decrease response rate significantly.
Fig. 1.
Effects of pregnanolone (n=9), lorazepam (n =9), ethanol (n =8), and pentobarbital (n=7) on response rate (top row) and percentage of reinforced responses (bottom row) in rats responding under a differential-reinforcement-of-low-rate 18-s schedule. Data points and error bars above ‘control’ indicate the mean and SEM for 3–10 control sessions in which vehicle was administered. Data points and error bars in the dose–effect curves represent 2–3 determinations in each subject that were averaged to obtain the overall or grand mean. Asterisks reflect doses that were significantly (P ≤ 0.05) different from control as determined by a one-way analysis of variance for an effect of dose and Holm–Sidak post-hoc tests. Values in parentheses adjacent to the data point indicate the number of subjects represented by that point when the number was different from that for the entire group.
In contrast to the effects on response rate, all four drugs dose-dependently decreased the percentage of reinforced responses (Fig. 1, bottom panels). This was verified by a significant main effect of dose for each drug [F(5,40)= 42.56, P<0.001; F(6,48)=20.93, P<0.001; F(6,44)= 20.15, P<0.001; F(7,44)=7.23, P<0.001, respectively], and post-hoc comparisons indicating that 4–5 doses of each drug were significantly different from the effects obtained after control injections.
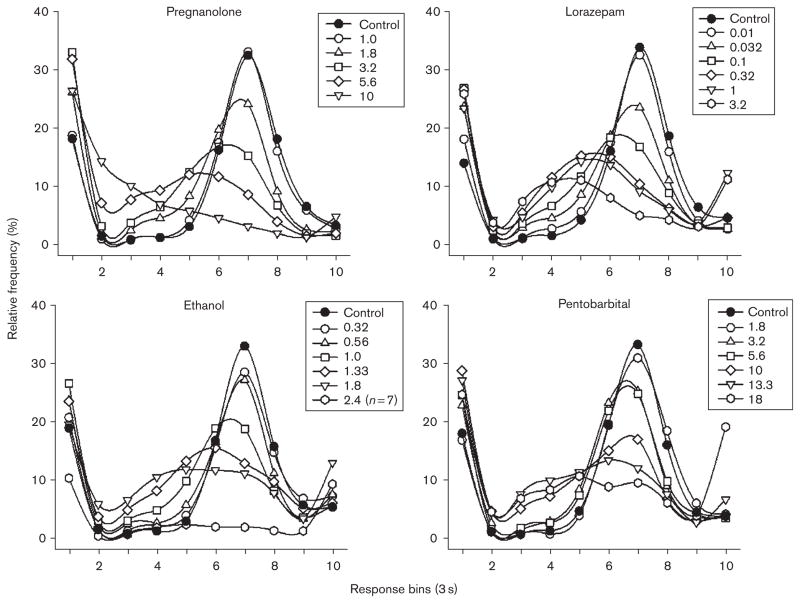
As shown in Fig. 2, when responding during the 18-s interval was separated into 3-s response intervals or bins (i.e. nine 3-s bins and a tenth bin for all responses that occurred after 27 s), the overall pattern of responding was similar for all of the subjects, as was the pattern of disruption produced by each of the four drugs. More specifically, under control conditions, there were relatively high levels of responding during the first bin [representing very short inter-response times (IRTs)], followed by very low levels in bins 2–5, and then graded increases from bins 6–7; responding then decreased in graded fashion from bins 8–10. This overall pattern of responding was altered by increasing doses of each drug, which increased responding in bin 1, increased responding in bins 2–5, and dose-dependently reduced the peak levels of reinforced responding in bin 7. This change in the pattern of responding after the administration of each of the four drugs was also indicated by an AUC analysis that excluded responding from bin 1 (Table 1). Although this analysis does not indicate the manner in which the temporal pattern of responding was altered, it does indicate that lorazepam significantly decreased AUC over a broad range of doses when compared with control data, and that the largest doses of ethanol and pentobarbital (2.4 g/kg and 18 mg/kg, respectively) produced a significant decrease in responding when compared with the curve obtained after control injections.
Fig. 2.
Effects of pregnanolone, lorazepam, ethanol, and pentobarbital on frequency of responding under a differential-reinforcement-of-low-rate18-s schedule in rats. Responses were plotted in 3-s intervals or bins. The tenth 3-s bin reflects all responses that occurred with an inter-response time greater than 27 s.
Table 1.
Area-under-the-curve analyses of the response–distribution curves for all of the drugs tested
| Drug | Control | Dose (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| Lorazepam | 0.01 | 0.032 | 0.1 | 0.32 | 1.0 | 3.2 | |
| 83.4 | 80.3 | 74.0 | 70.7* | 69.7* | 68.5* | 55.8* | |
| Pregnanolone | 1.0 | 1.8 | 3.2 | 5.6 | 10.0 | – | |
| 79.7 | 79.4 | 71.9 | 64.5 | 58.1* | 42.0* | – | |
| Ethanol | 0.32 | 0.56 | 1.0 | 1.33 | 1.8 | 2.4 | |
| 77.5 | 75.3 | 71.8 | 69.1 | 72.2 | 70.0 | 13.9* | |
| Pentobarbital | 1.8 | 3.2 | 5.6 | 10.0 | 13.3 | 18.0 | |
| 80.1 | 80.9 | 76.6 | 73.1 | 67.3 | 67.8 | 63.8* | |
| DHEA | 32.0 | 56.0 | 100.0 | 180.0 | 240.0 | – | |
| 84.7 | 81.2 | 83.1 | 82.7 | 81.3 | 67.8* | – | |
| β-CCM | 0.32 | 0.56 | 1.0 | 1.8 | 3.2 | – | |
| 80.5 | 81.1 | 81.5 | 78.2 | 77.9 | 67.3* | – | |
| Flumazenil | 1.8 | 3.2 | 5.6 | 10.0 | – | – | |
| 78.4 | 78.6 | 80.7 | 82.5 | 79.5 | – | – | |
DHEA, dehydroepiandrosterone.
P <0.05.
Negative γ-aminobutyric acid A modulators
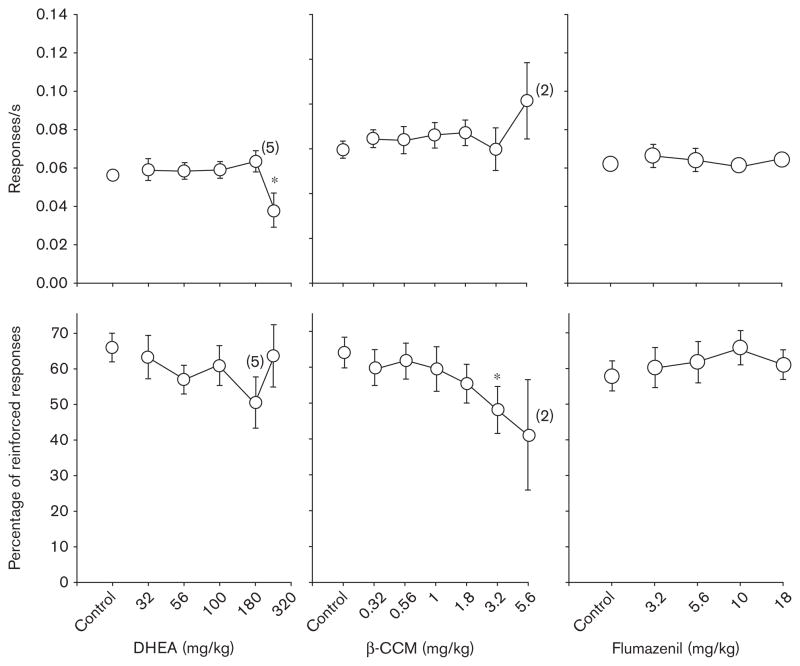
Figure 3 shows the dose–effect curves obtained after the administration of DHEA, β-CCM, and flumazenil. In general, each of these drugs had little or no effect on response rate with the exception of the largest dose of DHEA (240 mg/kg), which was confirmed by one-way ANOVA [F(5,25)=6.34, P<0.001; F(6,25)=1.02, not significant (NS); F(4,16)=0.48, NS, respectively] and Holm–Sidak post-hoc tests that compared the data obtained after each dose with the data obtained after control (vehicle) injections. With respect to the percentage of reinforced responses that occurred after drug administration, DHEA, β-CCM, and flumazenil also had little or no effect except at one dose of β-CCM (3.2mg/kg) that produced convulsions; the effect at this dose was confirmed by a one-way ANOVA and post-hoc tests [F(5,25)=1.32, NS; F(6,25)=3.57, P<0.02; F(4,16)= 0.73, NS, respectively].
Fig. 3.
Effects of dehydroepiandrosterone (DHEA) (n=6), β-CCM (n=6), and flumazenil (n=6) on response rate (top row) and percentage of reinforced responses (bottom row) in rats responding under a differential-reinforcement-of-low-rate 18-s schedule. For additional details, see legend of Fig. 1.
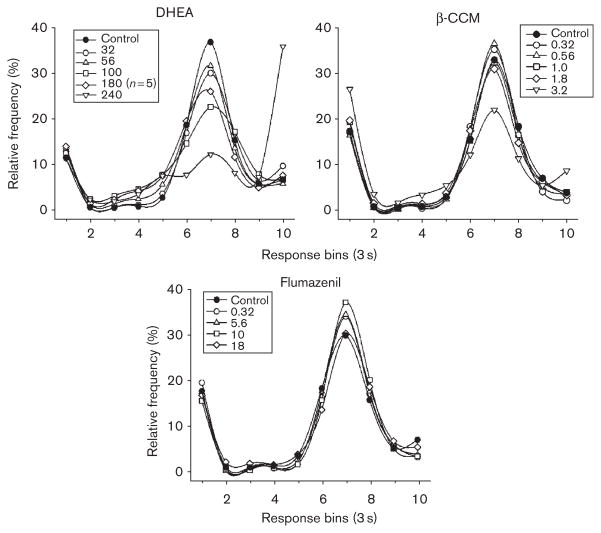
The temporal patterns of responding obtained after increasing doses of DHEA, β-CCM, and flumazenil are shown in Fig. 4. Unlike the patterns obtained after pregnanolone, lorazepam, ethanol, and pentobarbital, the temporal patterns obtained after administration of DHEA, β-CCM, and flumazenil were not substantially different from the pattern obtained after control injections. The only exceptions occurred after the highest dose of DHEA (240mg/kg) and β-CCM (3.2mg/kg). Thus, DHEA and the negative modulator β-CCM reduced the peak levels of responding that occurred in bin 7, but these drugs did not markedly increase the number of unreinforced responses that occurred in bins 1–6 (i.e. the response-distribution curves were not shifted leftward in a dose-dependent manner as with the positive modulators). An AUC analysis of the data for bins 2–10 (Table 1) also indicated that only the largest doses of DHEA and β-CCM (240 and 3.2mg/kg, respectively) significantly decreased the AUC when compared with control data. Flumazenil did not significantly decrease AUC at the doses tested (3.2–18mg/kg).
Fig. 4.
Effects of dehydroepiandrosterone (DHEA), β-CCM, and flumazenil on frequency of responding under a differential-reinforcement-of-low-rate18-s schedule in rats. Responses were plotted in 3-s intervals or bins. The tenth 3-s bin reflects all responses that occurred with an inter-response time greater than 27 s.
Dehydroepiandrosterone/pregnanolone
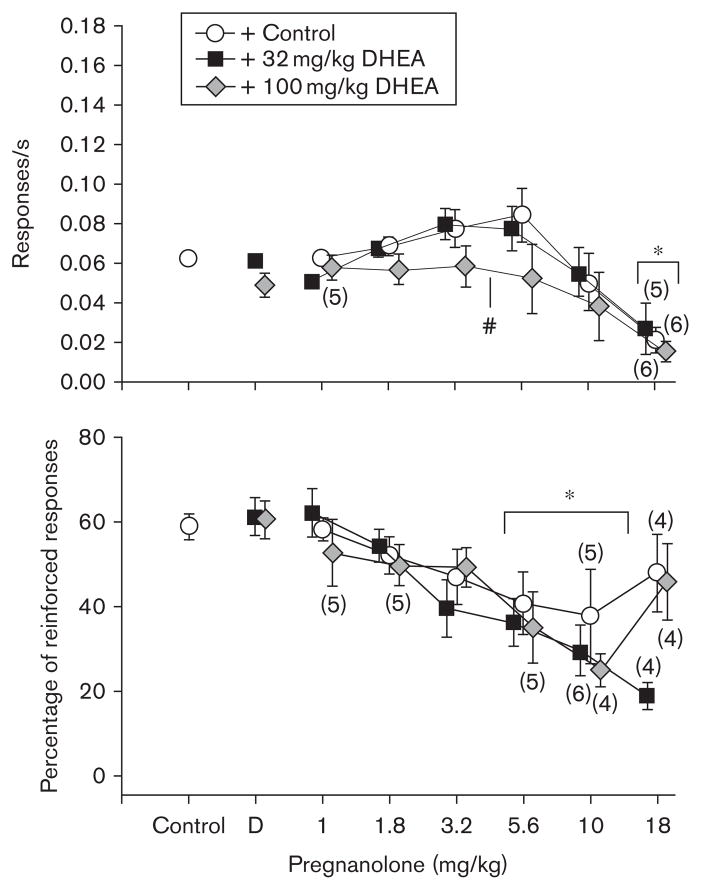
Figure 5 shows the effects of pregnanolone alone and in combination with 32 or 100 mg/kg of DHEA on response rate and the percentage of reinforced responses. As indicated by a two-way ANOVA, there were significant main effects of dose [F(6,63)=8.01, P<0.001] and of DHEA treatment (vehicle, 32, or 100 mg/kg DHEA) [F(2,62)=8.20, P<0.005] on response rate, but no significant interaction between dose and treatment [F(12,63)=1.01, NS]. Holm–Sidak post-hoc tests revealed that 18 mg/kg of pregnanolone was significantly different from control and that 100 mg/kg of DHEA in combination with pregnanolone was different than pregnanolone alone and pregnanolone in combination with 32 mg/kg of DHEA. A two-way ANOVA was also conducted on pregnanolone (1–10 mg/kg) alone and in combination with 32 or 100 mg/kg DHEA for percentage of reinforced responses. There was a significant main effect of dose on percentage of reinforced responses [F(6,41)=9.45, P<0.001]; however, there was no significant effect of treatment [F(2,41)=0.001, NS] and no significant interaction between dose and treatment [F(8,41)=1.21, NS] on percentage of reinforced responses. Holm–Sidak post-hoc tests revealed that two doses of pregnanolone (5.6 and 10 mg/kg), alone or in combination with DHEA, were significantly different from control. The absence of an effect of treatment was also indicated by the similarity of the ED50 values, that is, 13.2 for pregnanolone alone, 16.2 for pregnanolone and 32 mg/kg of DHEA, and 15.1 for pregnanolone and 100mg/kg of DHEA. The ED50 for the percentage of reinforced responses was 8.13 when pregnanolone was administered with 32 mg/kg of DHEA and 9.12 when pregnanolone was administered with 100 mg/kg of DHEA. The ED50 for pregnanolone alone could not be calculated because data points did not fall below 50% of the control value.
Fig. 5.
Effects of pregnanolone (n =7) in combination with two doses of dehydroepiandrosterone (DHEA) on response rate and percentage of reinforced responses in rats responding under a differential-reinforcement-of-low-rate18-s schedule. Data points and error bars above ‘control’ indicate the mean and SEM for 26–30 control sessions per subject in which two injections of vehicle were administered, whereas data points and error bars above ‘D’ indicate the mean and SEM for 2–3 injections of DHEA in combination with vehicle. Data points and error bars in the dose–effect curves represent 2–3 determinations in each subject that were averaged to obtain the overall or grand mean. Any points without error bars indicate instances in which the SEM is encompassed by the data point. For additional details, see legend of Fig. 1. Pound sign reflects treatments that were significantly (P ≤ 0.05) different from control as determined by a two-way analysis of variance (ANOVA) for an effect of treatment. Asterisks reflect doses that were significantly (P ≤ 0.05) different from control as determined by a two-way ANOVA for an effect of dose.
Discussion
This study examined the effects of positive and negative modulators of the GABAA receptor complex in rats responding under a DRL 18-s schedule of reinforcement. Although there were small differences in the effects of the drugs on response rate, administration of pregnanolone, lorazepam, ethanol, and pentobarbital produced changes in the temporal patterns of responding that were similar. Of particular interest were the significant increases in rate when subjects were administered intermediate doses of the positive modulators lorazepam and pentobarbital (drugs with sedative/hypnotic effects), and the similarity of pregnanolone’s effects to these drugs. Ethanol also produced small increases in the mean rate of responding above control values, but these effects were not significant. Despite the lack of significant rate-increasing effects for ethanol, there was an increase in responses with IRTs less than 18-s for all the drugs, which resulted in significant decreases in the percentage of reinforced responses. DHEA, β-CCM, and flumazenil also produced patterns of responding that were similar to each other, but distinct from the positive modulators (i.e. there were no dose-dependent increases in the frequency of IRTs less than 18 s and reductions in the percentage of reinforced responses occurred only at doses that produced other untoward effects).
The disparity in the rate-increasing effects of ethanol when compared with the other positive GABAA modulators points to the differences that can occur among the different classes of GABAA modulators. These differences were also highlighted in two recent drug discrimination studies in which increasing doses of ethanol only partially substituted for pregnanolone in rats discriminating between pregnanolone and saline (Gerak et al., 2008), and increasing doses of pregnanolone only partially substituted for ethanol in rats discriminating between ethanol and saline (Gurkovskaya and Winsauer, 2009). This partial substitution was also reminiscent of the partial substitution that has been reported between ethanol and the benzodiazepines (De Vry and Slangen, 1986). Given the pharmacological specificity of drug discrimination assays, these are strong behavioral data to indicate that ethanol has overlapping, but not identical, effects with the neurosteroids and other positive GABAA modulators. Unlike ethanol, the effects of pregnanolone on DRL responding were similar to those of lorazepam and pentobarbital, which suggests a similar GABA-mediated mechanism of action for its effects on schedule-controlled responding. Interestingly, the neurosteroids allopregnanolone and tetrahydrodeoxycorticosterone are capable of opening GABAA ion channels alone (similar to a barbiturate), whereas pregnanolone does not have this capacity and has been reported to require GABA binding to effectively open the channel (Hosie et al., 2006). This would suggest that the mechanism of action for pregnanolone may be more similar to that for the benzodiazepines than other positive GABAA modulators and could explain the highly comparable effects in rats responding under the DRL schedule. This argument is also supported by drug discrimination studies in which the benzodiazepines lorazepam, triazolam, and flunitrazepam substituted for pregnanolone (Vanover, 2000; Gerak et al., 2008).
With respect to responding under DRL schedules, Jarbe and Hiltunen (1988) reported small increases in response rate after administration of 0.3 and 0.56 g/kg of ethanol under a DRL 72-s schedule. In a study by Stephens and Voet (1994), two benzodiazepines (diazepam and lorazepam) and a β-carboline (abecarnil) all produced significant increases in response rate under a DRL 15-s when administered to rats. Similarly, Stretch and Dalrymple (1968) also found that when rats responding under a DRL 15-s schedule were administered 2.5 and 5 mg/kg of pentobarbital, response rate increased and this increase was characterized by rapid responding alternating with periods of inactivity. The periodic bursts in responding (Sidman, 1956) followed by periods of inactivity described by Stretch and Dalrymple (1968) could be indicative of extinction and provide a possible explanation for the increase in responding produced by the positive GABAA modulators. In this study, responding before 18 s was not reinforced, which could mimic extinction if responding remained elevated and produced IRTs less than 18 s (Sidman, 1956; Ferraro et al., 1965). This suggests that the increases in responding under a DRL schedule may have been partly because of extinction bursting, as suggested by the data in bin 1 of the response–distribution curves. However, responses occurring at IRTs between 3 and 18 s (response bins 2–6) also increased in frequency and are not characteristic of burst responding. If the effects of the GABAA modulators on IRTs between 0 and 3 s (bursting) are considered to be independent of their effects on IRTs between 3 and 18 s, then there is the possibility that these drugs affect two different types of unreinforced responding. Sanger et al. (1974) found that chlordiazepoxide and d-amphetamine both produced shorter IRTs when administered to rats responding under a DRL 15-s schedule; however, only chlordiazepoxide increased responding significantly at very short (burst) IRTs. They also noted that these bursts occurred mostly after nonreinforced IRTs and that chlordiazepoxide increased burst responding after these ‘misses.’
There are very limited data in the literature pertaining to DHEA, β-CCM, or flumazenil and their effects on responding under DRL schedules. These three drugs produced little or no effects on response rate and percentage of reinforced responses, except at the highest dose of DHEA (240 mg/kg), or proconvulsant doses of β-CCM (3.2 and 5.6 mg/kg). The absence of an effect on responding with flumazenil is also consistent with the fact that it is a neutral modulator of the benzodiazepine binding site on the GABAA receptor complex and does not have benzodiazepine-like effects (Nakamura and Carney, 1984). Although the lack of effect with flumazenil could also have resulted from the limited range of doses tested, Nakamura and Carney (1984) tested doses as large as 32 mg/kg and found no effect on DRL responding. Moreover, a dose of 5.6 mg/kg of flumazenil was shown to shift the dose–effect curve for flunitrazepam 30-fold to the right in a drug discrimination study (Gerak, 2009), indicating that flumazenil was effectively binding to the GABAA receptor complex. DHEA and the known negative GABAA modulator, β-CCM (Forster et al., 2001), should resemble neuroexcitatory compounds given their capacity to decrease chloride influx (Imamura and Prasad, 1998; Forster et al., 2001) and GABA-induced membrane currents (Park-Chung et al., 1999). However, the behavioral profiles of DHEA and β-CCM do not resemble those of other neuroexcitatory drugs such as the CNS stimulants whose effects on subjects responding under DRL schedules have been studied extensively.
The fact that DHEA did not shift the ED50 for the pregnanolone dose–effect curves to the right suggests the absence of a competitive antagonism, and that these two neurosteroids likely act by binding to different sites on the GABAA receptor complex or possibly different subunit conformations of the GABAA receptor complex. These data are also consistent with receptor binding studies in vitro, which suggest that there are at least two distinct neurosteroid binding sites (Park-Chung et al., 1999; Hosie et al., 2006, 2007). For example, Hosie et al. (2007) and others have suggested that the pharmacological effects of neurosteroids vary with stereochemical configuration and ionic charge, specifically the C-3 esters, and that the charge at that position determines binding specificity (Grant et al., 2008). Unfortunately, there is no direct evidence to indicate that systemically administered DHEA reaches the brain without first being metabolized or conjugated to its sulfated derivative. In support of this data though, several in-vivo studies have shown that acute systemic administration of DHEA can produce effects similar to other negative GABAA modulators on behaviors, such as sleep and aggression (Friess et al., 1995; Miczek et al., 2003), suggesting that DHEA may be binding to the GABAA receptor in vivo.
In summary, administration of the positive GABAA modulators lorazepam, pentobarbital, and the putatitive positive modulator pregnanolone to rats responding under a DRL schedule produced a significant increase in response rate at intermediate doses, whereas administration of ethanol did not. The difference in rate observed between ethanol and the other three positive GABAA modulators may be attributable to several variables, including the comparatively weak modulatory capacity of ethanol at the GABAA receptor complex, effects at different receptor subtypes, or effects at other receptors. Nevertheless, all four positive modulators dose-dependently decreased IRTs resulting in a decrease in the percentage of reinforced responses. These data also suggest that the neurosteroid pregnanolone may have effects more similar to lorazepam than pentobarbital. The negative modulator β-CCM and DHEA produced similar patterns of responding that were distinct from the positive modulators, whereas the neutral modulator flumazenil had no effect on responding in the dose range tested. Finally, DHEA did not competitively antagonize the disruptive effects of pregnanolone under a DRL schedule, showing that the two drugs have distinct sites of action.
Acknowledgments
This study was supported by USPHS AA09803 from the National Institute on Alcohol and Alcoholism, and DA019625 from the National Institute on Drug Abuse.
References
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorurnski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABA(A) receptors. Pharmacol Therap. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. Mutations of the GABA-A receptor alpha 1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol Pharmacol. 2008;74:614–627. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Costa E. From GABAA receptor diversity emerges a unified vision of GABAergic inhibition. Annu Rev Pharmacol Toxicol. 1998;38:321–350. doi: 10.1146/annurev.pharmtox.38.1.321. [DOI] [PubMed] [Google Scholar]
- De Vry J, Slangen JL. Effects of training dose on discrimination and cross-generalization of chlordiazepoxide, pentobarbital and ethanol in the rat. Psychopharmacology (Berl) 1986;88:341–345. doi: 10.1007/BF00180836. [DOI] [PubMed] [Google Scholar]
- Ferraro DP, Schoenfeld WN, Snapper AG. Sequential response effects in the white rat during conditioning and extinction on A DRL schedule. J Exp Anal Behav. 1965;8:255–260. doi: 10.1901/jeab.1965.8-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster IC, Harvey RJ, Darlison MG, Benson JA. Functional pharmacology of GABA(A) receptors containing the chicken brain gamma 4 subunit. Eur J Pharmacol. 2001;419:1–7. doi: 10.1016/s0014-2999(01)00964-5. [DOI] [PubMed] [Google Scholar]
- Friess E, Trachsel L, Guldner J, Schier T, Steiger A, Holsboer F. DHEA administration increases rapid eye movement sleep and EEG power in the sigma frequency range. Am J Physiol. 1995;268:E107–E113. doi: 10.1152/ajpendo.1995.268.1.E107. [DOI] [PubMed] [Google Scholar]
- Gerak LR. Antagonism of benzodiazepines by flumazenil in rats discriminating midazolam: child analyses. FASEB J. 2009;23:743.1. [Google Scholar]
- Gerak LR, Moerschbaecher JM, Winsauer PJ. Overlapping, but not identical, discriminative stimulus effects of the neuroactive steroid pregnanolone and ethanol. Pharmacol Biochem Behav. 2008;89:473–479. doi: 10.1016/j.pbb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Helms CM, Rogers LS, Purdy RH. Neuroactive steroid stereospecificity of ethanol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2008;326:354–361. doi: 10.1124/jpet.108.137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkovskaya OV, Winsauer PJ. Discriminative stimulus effects of ethanol, pregnanolone, and dehydroepiandrosterone (DHEA) in rats administered ethanol or saline as adolescents. Pharmacol Biochem Behav. 2009;93:82–90. doi: 10.1016/j.pbb.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NM. Therapeutic potential of steroids for CNS disorders. Expert Opinion on Therapeutic Patents. 2001;11:1523–1531. [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA(A) receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Imamura M, Prasad C. Modulation of GABA-gated chloride ion influx in the brain by dehydroepiandrosterone and its metabolites. Biochem Biophys Res Commun. 1998;243:771–775. doi: 10.1006/bbrc.1998.8177. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Hiltunen AJ. Ethanol and Ro 15-4513: behaviour maintained by operant procedures (DRL-72s and PTZ-drug discrimination) in rats. Drug Alcohol Depend. 1988;22:83–90. doi: 10.1016/0376-8716(88)90041-5. [DOI] [PubMed] [Google Scholar]
- Legrain S, Girard L. Pharmacology and therapeutic effects of dehydroepiandrosterone in older subjects. Drugs Aging. 2003;20:949–967. doi: 10.2165/00002512-200320130-00001. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Demirgoren S, Spivak CE, London ED. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990;526:143–146. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–257. doi: 10.1016/j.yhbeh.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of GABA(A) receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Monaghan EP, Navalta LA, Shum L, Ashbrook DW, Lee DA. Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia. 1997;38:1026–1031. doi: 10.1111/j.1528-1157.1997.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL. Recent developments in the significance and therapeutic relevance of neuroactive steroids introduction to the special issue. Pharmacol Therap. 2007;116:1–6. doi: 10.1016/j.pharmthera.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Carney JM. Antagonism by CGS 8216 and Ro 15-1788, benzodiazepines antagonists, of the action of chlordiazepoxide on a timing behavior in rats. Pharmacol Biochem Behav. 1984;21:381–385. doi: 10.1016/s0091-3057(84)80099-4. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000;295:1241–1248. [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Key M, Blackman DE. Differential effects of chlordiazepoxide and d-amphetamine on responding maintained by a DRL schedule of reinforcement. Psychopharmacologia. 1974;38:159–171. doi: 10.1007/BF00426110. [DOI] [PubMed] [Google Scholar]
- Sidman M. Time discrimination and behavioral interaction in A free operant situation. J Comp Physiological Psychol. 1956;49:469–473. doi: 10.1037/h0041892. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Voet B. Differential effects of anxiolytic and non-anxiolytic benzodiazepine receptor ligands on performance of a differential reinforcement of low rate (DRL) schedule. Behav Pharmacol. 1994;5:4–14. doi: 10.1097/00008877-199402000-00001. [DOI] [PubMed] [Google Scholar]
- Stretch R, Dalrymple D. Effects of methylphenidate, pentobarbital, and reserpine on behavior controlled by a schedule of interresponse time reinforcement. Psychopharmacologia. 1968;13:49–64. doi: 10.1007/BF00401618. [DOI] [PubMed] [Google Scholar]
- Vanover KE. Effects of benzodiazepine receptor ligands and ethanol in rats trained to discriminate pregnanolone. Pharmacol Biochem and Behav. 2000;67:483–487. doi: 10.1016/s0091-3057(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Wegner F, Rassler C, Allgaier C, Strecker K, Wohlfarth K. Auto-modulation of neuroactive steroids on GABA(A) receptors: a novel pharmacological effect. Neuropharmacology. 2007;52:672–683. doi: 10.1016/j.neuropharm.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Riley AL. Cholecystokinin potentiates the rate-decreasing effects of morphine on schedule-controlled behavior in rats. Pharmacol Biochem Behav. 1988;30:569–575. doi: 10.1016/0091-3057(88)90067-6. [DOI] [PubMed] [Google Scholar]