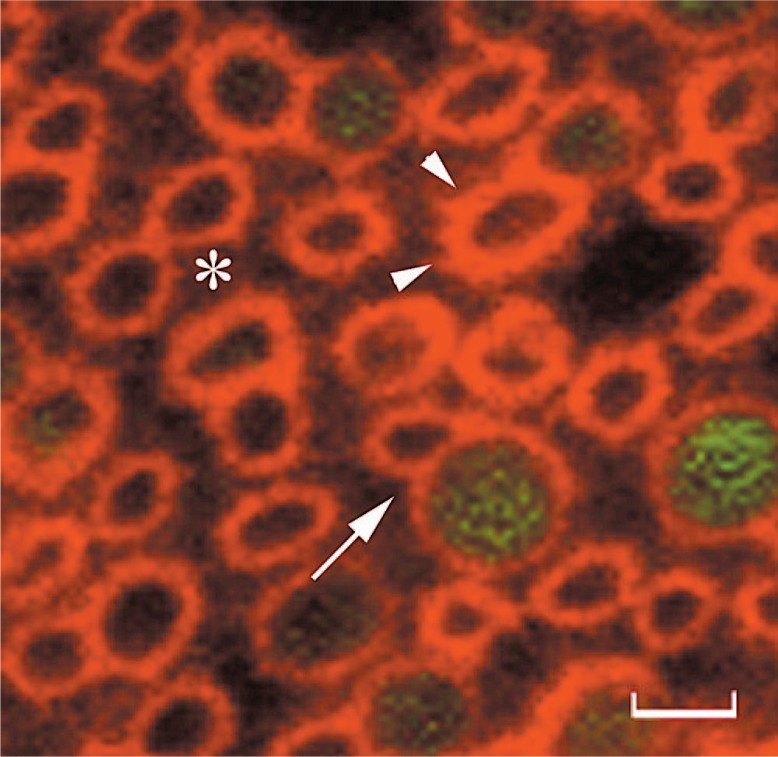
Although it is the major soluble protein component of the extracellular matrix bathing the photoreceptors, little is known about the structure and function of interphotoreceptor retinoid-binding protein (IRBP). The critical anatomic compartment it occupies cannot be overemphasized as through the involution of the optic vesicle, the retinal pigmented epithelium (RPE) comes in contact with the neural retina.1 The zonula occludens and adherens of the RPE and external limiting membrane respectively restrict the interphotoreceptor matrix (IPM) to the “subretinal space”. Given its presence at this interface and early expression patterns, IRBP could have a function in retinal development,2,3 and recent studies point to a role in eye growth4. Early studies assumed that IRBP does not interact with components of the IPM or retina, as it is easily extracted from detached retinas by only gentle saline wash. This notion, recently revisited experimentally,5 has shown not to be completely true as saline wash leaves behind IRBP tightly bound to specific regions, particularly a domain surrounding the cone outer segments, presumably the cone matrix sheath (Fig. 1). An important question, which has received insufficient attention, is what are the binding partners that IRBP interacts with in the IPM and/or on the cell surface?
Figure 1.
IRBP interacts with the cone matrix sheath. This photomicrograph of chicken cones in cross-sectional orientation shows the distribution of IRBP by indirect immunofluorescence following saline wash. The retinas were washed three times, flat mounted, and probed with mAb F7 anti-IRBP, followed by goat anti-mouse IgG-647. The saline wash removed IRBP from the regions between cone matrix sheaths (asterisk) leaving behind that bound to a matrix domain rimming the outer segments (arrow heads). Represented here are merged confocal fluorescence images at 633 nm (red, IRBP) and 488 nm (green, oil droplet autofluorescence). Arrow, double cone; scale bar = 3.3 μm. Adapted from Garlip et al. J. Comp. Neurol. (in press) with permission from Wiley-liss, Inc.
Its name is unfortunate, as it does not remind us that IRBP is present elsewhere besides the IPM. IRBP is expressed by the pineal gland in man.6,7 Its function there is completely unknown. Equally unknown is the role of IRBP in the vitreous, one of the locations where IRBP was initially described. Interestingly, recent studies have shown decreased levels of IRBP in the vitreous in the early stages of diabetes.8 The source and turnover of vitreous IRBP is unknown although its mRNA has been detected in the ciliary epithelium.9 Finally, in the retina, IRBP is not restricted to the IPM as was commonly assumed, but is internalized avidly by the RPE (see below). Certainly, there will be much to be learned about IRBP as studies examine its function beyond the IPM.
IRBP is secreted into the IPM by the rods and cones, and interestingly by the RPE in some cases.10 The exclusion limit of the zonula adherens restricts it to the subretinal compartment. Despite restriction to the subretinal compartment, IRBP is rapidly turned over probably by RPE and photoreceptor endocytosis.11,12 The function of this interesting turnover is unknown, but may be linked to circadian changes in mRNA expression as part of a complex mechanism regulating IPM IRBP concentrations.13 A potential receptor mediated photoreceptor uptake mechanism needs to be revisited.14 IRBP turnover may be related to its function in transporting hydrophobic molecules, part of a degradation pathway, or both. These important and interesting questions remain largely unexplored.
The importance of IRBP to the retina has recently been underscored by its role in disease. Early studies noted reduced quantities of IRBP in the rod-cone degeneration in Abyssinian cats. Photoreceptor degeneration occurs in transgenic mice lacking IRBP. A mutation in IRBP has been associated with a form of autosomal recessive retinitis pigmentosa15 (Fig. 2B). Although the mechanism leading to the degeneration is largely unknown, these studies indicate that the presence of sufficient quantities of functional IRBP is critical to photoreceptor survival. Finally, IRBP is one of the most useful antigens in experimental autoimmune uveitis, and has been implicated in spontaneously occurring uveitis in animals and man.
Figure 2.
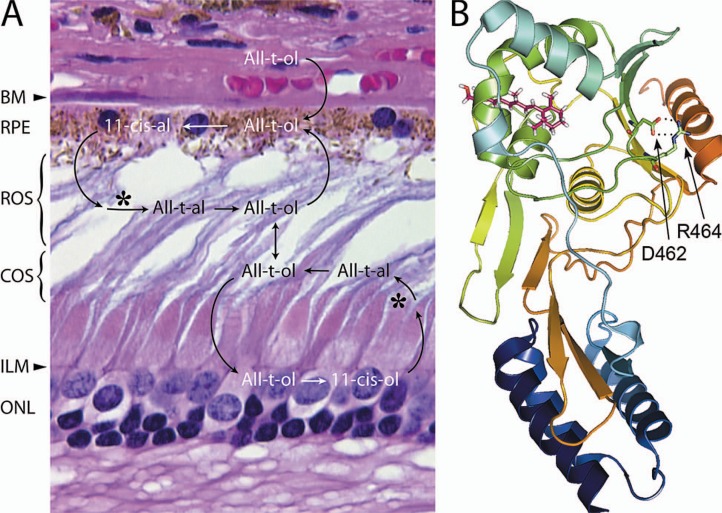
Overview of the visual cycle, and structure of an IRBP module. A) The diagram is overlaid on a hematoxylin & eosin stained paraffin section of human retina (near the fovea). Interphotoreceptor matrix (IPM) glyconjugates are largely responsible for the basophilic outer segment staining. The apparent spaces among the outer segments is a histological processing artifact. The larger cone nuclei are located near the external limiting membrane (ELM, arrowhead). Bruch’s membrane (BM, arrow head); outer nuclear layer (ONL). 11-cis retinal (11-cis-al) is photoisomerized (*) to its 11-cis isomer, which is reduced to all-trans retinol (All-t-ol). The All-t-ol is then released from rod and cone outer segments (ROS and COS respectively) into the IPM. B) Ribbon representation of the module II Xenopus IRBP structure docked with all-t-ol (magenta).The N- and C-terminal regions are shown in blue and red respectively. A salt bridge (dotted lines) extends between the carboxamide side group of D462, and guanidinium group of R464. These highly conserved residues are shown in stick representation (oxygen, red; nitrogen, blue). The corresponding aspartic acid of human IRBP is replaced by asparagine in a form of autosomal recessive retinitis pigmentosa. It is possible that this substitution (D1080N), by abolishing the conserved salt bridge, destabilizes the nearby retinoid-binding site. The structure shown in this panel is adapted from Hollander et al. Invest. Ophthal. Vis. Sci. 50:1864–72, 2009 with permission from the Association for Research in Vision and Ophthalmology.
IRBP was discovered three decades ago and shown to carry endogenous all-trans, 11-cis retinol and 11-cis retinal in a light dependent manner. However, its role in the visual cycle is still far from clear. The early prevailing view that IRBP simply solubilizes retinoids in the aqueous IPM milieu has never been completely satisfying, and not always born out experimentally as an absolute requirement for retinoid trafficking. At the same time, the true complexity of the visual cycle has become more apparent as briefly summarized in Fig. 2A. The remarkable complexity of the retinoid trafficking taking place across the IPM is certainly greater than was anticipated at the time of initial descriptions of IRBP. Following its photoisomerization, this trafficking begins with the release of all-trans retinol into the IPM. From there, it may enter the RPE or Muller cells to be enzymatically re-isomerized to the 11-cis isomer, which is returned through the IPM to the outer segments. A difference between the rod and cone visual cycles is that the RPE provides 11-cis retinal, while Muller cells release 11-cis retinol. It is thought that in this way, the cone visual cycle ensures a protected supply of the 11-cis isomer to the cones which are endowed with an 11-cis retinol dehydrogenase, allowing utilization of 11-cis retinol for pigment regeneration.16
How might IRBP function in this complex cycle? The visual cycle must accomplish the efficient delivery of three different retinoids (11-cis, all-trans retinol and 11-cis retinal) to four different cell types (rod, cone, RPE and Muller) at the correct time, protecting retinoids from isomeric and oxidative degradation. These tasks must be accomplished under large extremes of retinoid flux encountered in night and daytime vision. One possibility is that IRBP contains ligand-binding sites tailored for each visual cycle retinoid, and docking sites that can distinguish cell surface and IPM binding sites.17,18 Mechanisms must exist to regulate ligand affinities to accomplish the appropriate retinoid uptake or release. Some of the pieces of the puzzle are emerging.19–21 IRBP is known to promote outer segment release of all-trans retinol,22 and its delivery to the RPE. IRBP also enhances both the release of 11-cis retinal from the RPE, and its return to the outer segments. However, the molecular mechanism behind these functions remains unknown. Recent studies suggest an important role of IRBP in the cone visual cycle.23,24 Finally, a role of IRBP in protecting the oxidative and isomeric state of visual cycle retinoids24,25 is an exciting possibility that may reflect a thiol dependent antioxidant activity of the protein26. Uncovering the mechanisms of IRBP functions will require further detailed information on its structure.
IRBP is certainly unique among retinoid-binding proteins as it is composed of two to four homologous “modules”, each ∼300 amino acids in length.27 The significance of this structure, which makes IRBP the largest known retinoid-binding protein, is largely unknown. The individual modules, which are homologous with carboxyl-terminal processing proteases (CPTases) and crotonases, represent functional units of the protein. Photosystem II D1 CPTase (D1P), a prototypical CPTase, plays a role in the repair of oxidative damage to the reaction center by renewing the photosystem II D1P. The repair involves replacement of damaged D1P with new protein. During this replacement, D1P catalyzes the hydrophobic C-terminal cleavage of the new D1P. Each module of IRBP is structurally similar to the D1P domains A and C which correspond to the N- and C-terminal IRBP modules, respectively. Furthermore, the C-terminal domain B of the IRBP modules and domain C of D1P exhibit a structural homology with crotonases (enoyl Co-A hydratase/isomerases). The X-ray structure of the second module of Xenopus module 2 shares with these enzymes a structural core composed of three helices, and a five-stranded β-sheet in domain B forming a ββα-spiral fold.28 In view of IRBP’s homology with these two enzyme families, an enzymatic activity for IRBP should be kept in mind. The vitamin A binding domain of IRBP lies in a separate hydrophobic cavity. The shallow cleft formed by the fold was assumed to represent the retinol-binding site. However, a second hydrophobic site consisting of a highly restricted cavity was more recently appreciated during in silico ligand-docking studies. Site directed mutagenesis studies indicate that this hydrophobic site represents the retinoid-binding site29 (Fig. 2B).
Initially viewed as only providing a hydrophobic ligand-binding protein for retinoids crossing the IPM, IRBP appears to mediate a variety of specific roles particularly targeting the cellular delivery and release of visual cycle retinoids while protecting these molecules from isomeric and oxidative degradation. The complexity of the structure of IRBP and the visual cycles was not anticipated when IRBP was first described three decades ago. Emerging molecular and structural data taken together with increased understanding of the visual cycle, and ocular development promise to elucidate the function of this interesting protein. It is critical that potential roles for IRBP outside of the visual cycle and retina not be ignored, but addressed experimentally. Clearly, there is much more to be discovered compared to what has already been uncovered regarding the function of this protein and its role in disease.
Acknowledgments
The author would like to thank Dr. Debashis Ghosh (Syracuse, USA), and Dr. Francisco Javier Romero and Dr. Maria Miranda (Valencia, Spain) for helpful discussions. The work was supported by NIHRO1 EY09412 (D.G./F.G.-F), Merit Review Award I01BX007080 from the Biomedical Laboratory Research & Development Service of the Veterans Affairs Office of Research and Development (F.G.-F.), R24 EY 016662 core instrumentation grant, and an unrestricted research grant from Research to Prevent Blindness to the Department of Ophthalmology at SUNY at Buffalo. Dr. Gonzalez-Fernandez is the Ira Gile Ross & Elizabeth Pierce Olmsted Ross Endowed Chair in Ophthalmic Pathology.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Gonzalez-Fernandez F. Development of the Retina. In: Reynolds JD, Olitsky SE, editors. Pediatric Retina. New York: Springer; 2010. pp. 1–38. [Google Scholar]
- 2.Gonzalez-Fernandez F, Healy JI. Early expression of the gene for interphotoreceptor retinol-binding protein during photoreceptor differentiation suggests a critical role for the interphotoreceptor matrix in retinal development. J Cell Biol. 1990;111:2775–2784. doi: 10.1083/jcb.111.6.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liou GI, Wang M, Matragoon S. Precocious IRBP gene expression during mouse development. Invest Ophthalmol Vis Sci. 1994;35:1083–1088. [PubMed] [Google Scholar]
- 4.Wisard J, Faulkner A, Chrenek MA, Waxweiler T, Waxweiler W, Donmoyer C, et al. Exaggerated eye growth in IRBP-deficient mice in early development. Invest Ophthalmol Vis Sci. 2011;52:5804–5811. doi: 10.1167/iovs.10-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garlipp MA, Nowak KR, Gonzalez-Fernandez F. Cone outer segment extracellular matrix as binding domain for interphotoreceptor retinoid-binding protein. J Comp Neurol. 2012;520:756–769. doi: 10.1002/cne.22773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Fernandez F, Van Niel E, Edmonds C, Beaver H, Nickerson JM, Garcia-Fernandez JM, et al. Differential expression of interphotoreceptor retinoid-binding protein, opsin, cellular retinaldehyde-binding protein, and basic fibroblastic growth factor. Exp Eye Res. 1993;56:411–427. doi: 10.1006/exer.1993.1055. [DOI] [PubMed] [Google Scholar]
- 7.Lopes MB, Gonzalez-Fernandez F, Scheithauer BW, VandenBerg SR. Differential expression of retinal proteins in a pineal parenchymal tumor. J Neuropathol Exp Neurol. 1993;52:516–524. doi: 10.1097/00005072-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Ramírez M, Hernández C, Villarroel M, Canals F, Alonso MA, Fortuny R, et al. Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia. 2009;52:2633–2641. doi: 10.1007/s00125-009-1548-8. [DOI] [PubMed] [Google Scholar]
- 9.Salvador-Silva M, Ghosh S, Bertazolli-Filho R, Boatright JH, Nickerson JM, Garwin GG, et al. Retinoid processing proteins in the ocular ciliary epithelium. Mol Vis. 2005;11:356–365. [PubMed] [Google Scholar]
- 10.Stenkamp DL, Cunningham LL, Raymond PA, Gonzalez-Fernandez F. Novel expression pattern of interphotoreceptor retinoid-binding protein (IRBP) in the adult and developing zebrafish retina and RPE. Mol Vis. 1998;4:26. [PubMed] [Google Scholar]
- 11.Cunningham LL, Yang L, Gonzalez-Fernandez F. Interphotoreceptor retinoid-binding protein (IRBP) is rapidly cleared from the Xenopus interphotoreceptor matrix. Exp Eye Res. 1999;68:399–410. doi: 10.1006/exer.1998.0633. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham LL, Gonzalez-Fernandez F. Internalization of interphotoreceptor retinoid-binding protein by the Xenopus retinal pigment epithelium. J Comp Neurol. 2003;466:331–342. doi: 10.1002/cne.10861. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham LL, Gonzalez-Fernandez F. Coordination between production and turnover of interphotoreceptor retinoid-binding protein in zebrafish. Invest Ophthalmol Vis Sci. 2000;41:3590–3599. [PubMed] [Google Scholar]
- 14.Hollyfield JG, Varner HH, Rayborn ME, Liou GI, Bridges CD. Endocytosis and degradation of interstitial retinol-binding protein: differential capabilities of cells that border the interphotoreceptor matrix. J Cell Biol. 1985;100:1676–1681. doi: 10.1083/jcb.100.5.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.den Hollander AI, McGee TL, Ziviello C, Banfi S, Dryja TP, Gonzalez-Fernandez F, et al. A homozygous missense mutation in the IRBP gene (RBP3) associated with autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2009;50:1864–1872. doi: 10.1167/iovs.08-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Fernandez F, Baer CA, Ghosh D. Module structure of interphotoreceptor retinoid-binding protein (IRBP) may provide bases for its complex role in the visual cycle - structure/function study of Xenopus IRBP. BMC Biochem. 2007;8:15. doi: 10.1186/1471-2091-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin ZY, Li GR, Takizawa N, Si JS, Gross EA, Richardson K, et al. Structure-function relationships in interphotoreceptor retinoid-binding protein (IRBP) Mol Vis. 1997;3:17. [PubMed] [Google Scholar]
- 19.Gonzalez-Fernandez F, Ghosh D. Focus on Molecules: interphotoreceptor retinoid-binding protein (IRBP) Exp Eye Res. 2008;86:169–170. doi: 10.1016/j.exer.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Fernandez F. Interphotoreceptor retinoid-binding protein-an old gene for new eyes. Vision Res. 2003;43:3021–3036. doi: 10.1016/j.visres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 21.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Blakeley LR, Cornwall MC, Crouch RK, Wiggert BN, Koutalos Y. Interphotoreceptor retinoid-binding protein is the physiologically relevant carrier that removes retinol from rod photoreceptor outer segments. Biochemistry. 2007;46:8669–8679. doi: 10.1021/bi7004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin M, Li S, Nusinowitz S, Lloyd M, Hu J, Radu RA, et al. The role of interphotoreceptor retinoid-binding protein on the translocation of visual retinoids and function of cone photoreceptors. J Neurosci. 2009;29:1486–1495. doi: 10.1523/JNEUROSCI.3882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker R, Wang JS, Kefalov VJ, Crouch RK. Interphotoreceptor retinoid-binding protein as the physiologically relevant carrier of 11-cis-retinol in the cone visual cycle. J Neurosci. 2011;31:4714–4719. doi: 10.1523/JNEUROSCI.3722-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crouch RK, Hazard ES, Lind T, Wiggert B, Chader G, Corson DW. Interphotoreceptor retinoid-binding protein and alpha-tocopherol preserve the isomeric and oxidation state of retinol. Photochem Photobiol. 1992;56:251–255. doi: 10.1111/j.1751-1097.1992.tb02154.x. [DOI] [PubMed] [Google Scholar]
- 26.Lassman BJ, Griswold F, Gonzalez-Fernandez F, Ghosh D. Bovine IRBP functions in vitro as a thiol-based antioxidant. Invest Ophthalmol Vis Sci. 2009. p. 3531. (ARVO abstract, 2009).
- 27.Nickerson JM, Frey RA, Ciavatta VT, Stenkamp DL. Interphotoreceptor retinoid-binding protein gene structure in tetrapods and teleost fish. Mol Vis. 2006;12:1565–1585. [PMC free article] [PubMed] [Google Scholar]
- 28.Loew A, Gonzalez-Fernandez F. Crystal structure of the functional unit of interphotoreceptor retinoid binding protein. Structure. 2002;10:43–49. doi: 10.1016/s0969-2126(01)00698-0. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Fernandez F, Bevilacqua T, Lee KI, Chandrashekar R, Hsu L, Garlipp MA, et al. Retinol-binding site in interphotoreceptor retinoid-binding protein (IRBP): a novel hydrophobic cavity. Invest Ophthalmol Vis Sci. 2009;50:5577–5586. doi: 10.1167/iovs.08-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]