Background: One galectin-3 function is to bind glycoproteins and cross-link them.
Results: A glycoprotein engaged many more galectin-3 carbohydrate-binding sites than its number of relevant glycans.
Conclusion: The ligand induced binding of one galectin-3 to another galectin-3 to form oligomers in a previously unrecognized way.
Significance: This differs from previous models and provides a new framework to interpret biological effects of galectin-3.
Keywords: Carbohydrate-binding Protein, Endocytosis, Galectin, Glycoprotein, Protein Cross-linking, Fluorescence Polarization, Oligomerization
Abstract
Many functions of galectin-3 entail binding of its carbohydrate recognition site to glycans of a glycoprotein, resulting in cross-linking thought to be mediated by its N-terminal noncarbohydrate-binding domain. Here we studied interaction of galectin-3 with the model glycoprotein asialofetuin (ASF), using a fluorescence anisotropy assay to measure the concentration of free galectin carbohydrate recognition sites in solution. Surprisingly, in the presence of ASF, this remained low even at high galectin-3 concentrations, showing that many more galectin-3 molecules were engaged than expected due to the about nine known glycan-based binding sites per ASF molecule. This suggests that after ASF-induced nucleation, galectin-3 associates with itself by the carbohydrate recognition site binding to another galectin-3 molecule, possibly forming oligomers. We named this type-C self-association to distinguish it from the previously proposed models (type-N) where galectin-3 molecules bind to each other through the N-terminal domain, and all carbohydrate recognition sites are available for binding glycans. Both types of self-association can result in precipitates, as measured here by turbidimetry and dynamic light scattering. Type-C self-association and precipitation occurred even with a galectin-3 mutant (R186S) that bound poorly to ASF but required much higher concentration (∼50 μm) as compared with wild type (∼1 μm). ASF also induced weaker type-C self-association of galectin-3 lacking its N-terminal domains, but as expected, no precipitation. Neither a monovalent nor a divalent N-acetyl-d-lactosamine-containing glycan induced type-C self-association, even if the latter gave precipitates with high concentrations of galectin-3 (>∼50 μm) in agreement with published results and perhaps due to type-N self-association.
Introduction
Galectin-3 binds and cross-links β-galactoside-containing glycoconjugates (1–4). This leads to many of its cellular activities such as directing intracellular traffic of glycoproteins (5), induction of signaling at the cell surface (1), or modulation of cell adhesion (6), which in turn underlies many of the proposed roles of galectin-3 in inflammation, immunity, and cancer. The ligand binding is conferred by the C-terminal canonical galectin carbohydrate recognition domain (CRD)3 of about 135 amino acids, and the cross-linking in most cases requires the noncarbohydrate-binding N-terminal domains. The mechanism for this still remains unclear despite many studies. Galectin-3 occurs as a monomer in solution (7), and cross-linking only occurs upon encounter with certain ligands, which appear to have to be at least divalent (8–10). Most studies suggest that the cross-linking is mediated by interaction between the N-terminal domains of one galectin-3 molecule with those in another (Fig. 1A); in one case, the composition of its precipitates with a divalent saccharide suggested formation of galectin-3 pentamers (8), and this is how galectin-3 is depicted in many recent reviews (1–3). A few studies have also suggested interaction between CRDs of galectin-3 (11, 12).
FIGURE 1.
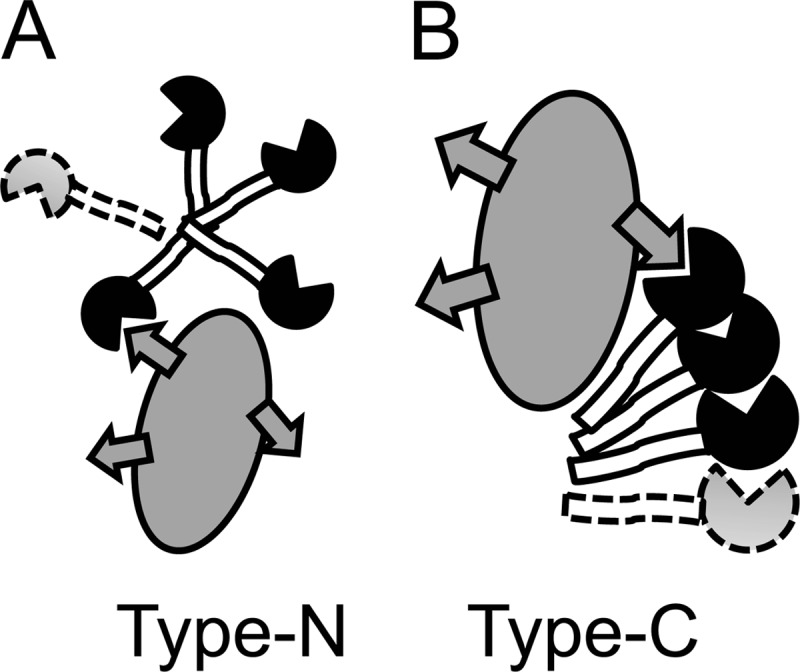
Two modes of galectin-3 self-association. The CRD is filled with a notch for the carbohydrate-binding site, the N-terminal domains are open, and a glycoprotein ligand is gray with glycans symbolized by arrows. In the common view, named type-N self-association here, the carbohydrate recognition sites remain free to interact with glycoconjugate ligands, and the N-domains of one galectin-3 bind the N-domains of another, forming dimers or higher oligomers, e.g. pentamers (8). In the view indicated by experiments here, named type-C self-association, the carbohydrate recognition site of one galectin-3 binds another site on the CRD of another galectin-3 molecule to form dimers or oligomers. The N-terminal domains are not required, but enhance the interaction. Efficient type-C self-association requires initiation (or nucleation) by interaction with e.g. a glycoprotein such as ASF.
In all cases, galectin-3 self-association has only been demonstrated at surfaces, artificial or natural, in precipitates, or by chemical cross-linking (8–15), which has made detailed quantitative analysis difficult. We have now analyzed galectin-3-ligand interaction in solution, using a fluorescence anisotropy assay, recently applied to interaction between galectin-1 with asialofetuin (ASF) (16). Surprisingly, the results with galectin-3 appear very different from the case with galectin-1 and are not consistent with the model in Fig. 1A. Instead they indicate that ASF binding nucleates self-association of galectin-3 to form oligomers mediated by its carbohydrate recognition site (Fig. 1B).
EXPERIMENTAL PROCEDURES
Materials
Chemicals including fluorescent probes, ASF, and galectin-1 and -3 and galectin-3 mutants were as described before (17), unless stated otherwise. Lacto-N-neohexaose (LNnH) was from Dextra Inc. Redding, UK (16, 17).
Assays
Fluorescence Anisotropy
The interaction of galectin-3 and mutants with the different ligands was analyzed by a fluorescence anisotropy assay with calculations as described before (16, 17). A new more sensitive plate reader, PHERAstar with software Mars version 2.10 R3 (BMG LABTECH, Offenburg, Germany), was also used.
Turbidity
Absorbance spectra (350–800 nm) were acquired on the same samples using the same instrument.
Dynamic Light Scattering
Dynamic light scattering was as described before for galectin-1 (16).
Fluorescence Microscopy
Fluorescence microscopy of galectin-3 cell binding and uptake was done as described in Ref. 18.
RESULTS
Galectin-3 interaction with ligands was analyzed by three methods. Fluorescence anisotropy of a galectin binding probe was used to measure occupancy of the galectin-3 carbohydrate-binding site, turbidity was used to measure the amount of galectin-3-ligand precipitates, and dynamic light scattering (DLS) was used to measure particle size. Galectin-3 itself is known to behave mainly as a monomer up to about 100 μm (7), and here this was confirmed by the instant observation of solution equilibrium by DLS (supplemental Fig. S1), which also revealed the possible formation of small amounts of dimer (∼4%) at the highest concentration tested (150 μm). Other larger aggregates were <0.1%. Galectin-3C also behaved as a monomer and showed no sign of dimerization by itself.
Occupancy of Galectin-3 Carbohydrate-binding Site
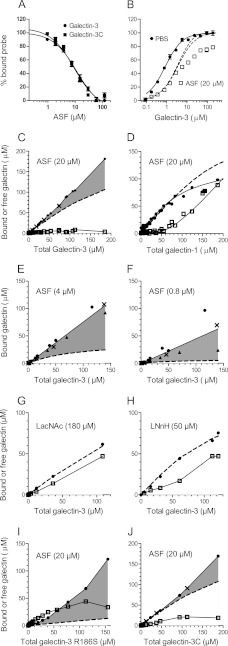
First, fixed low concentrations of galectin and fluorescein-tagged probe were mixed with a range of concentrations of ligand as inhibitor, fluorescence anisotropy was measured for each data point, and inhibition curves were calculated (Fig. 2A). This showed that ASF inhibited galectin-3 with a potency equivalent to a monovalent ligand with an affinity of Kd ∼6 μm or, for example, a nonavalent ligand (as suggested (19)) with an affinity of 54 μm for each binding site.
FIGURE 2.
Analysis of galectin-3 and -1 interaction with ligands (ASF and saccharides) by fluorescence anisotropy. A, decreasing binding (shown as percentage on y axis) to A-tetra probe (0.1 μm) by galectin-3 (1 μm) in the presence of increasing concentrations of ASF (x axis). B, binding (shown as percentage on y axis) of fluorescent A-tetra probe (0.1 μm) in the presence of increasing concentrations of galectin-3 (x axis) without (filled circles) or with 20 μm ASF as inhibitor (unfilled squares). Theoretically calculated binding curves are shown for no inhibitor (unbroken line) or 20 μm inhibitor of Kd 6 μm or 180 μm inhibitor of Kd 54 μm (broken lines). C–J, calculated concentrations of free (unfilled squares) and ligand-bound (filled symbols and unbroken lines) galectin (shown in μm on y axes). The concentration of free galectin was obtained from experiments as in Fig. 1B, using the probe binding curve without ligand as a standard and bound galectin = total − free galectin. To demonstrate reproducibility and consistency, the data of panels C–J are from multiple experiments with different probes of different affinities for galectin-3 (filled circles for A-tetra probe, X for Fucα1-2Galβ1-4Glc (FucLac) probe, and triangles for Lac-probe). The experimental data for galectin-1 (panel D) are from Salomonsson et al. (16). Theoretical binding curves are shown as broken lines, assuming nine binding sites per ASF-molecule of Kd 54 μm for galectin-3 and galectin-3C, 1600 μm for galectin-3 R186S, 36 μm for galectin-1, two binding sites per LNnH molecule of Kd 20 μm, and one binding site per LacNAc of Kd 100 μm for galectin-3. The shaded area is the excess bound galectin-3 as compared with the theoretically calculated curve.
Next, to determine the number of binding sites, we analyzed fixed concentrations of probe and ASF with increasing concentrations of galectin. With only the probe (0.1 μm), a curve is obtained going from the anisotropy of the free probe (∼30 mA normalized to 0% bound probe in Fig. 2B) to the anisotropy of the probe galectin complex as the excess galectin saturates all probe (165 mA normalized to 100% in Fig. 2B). Because the probe itself is present in only trace amounts, this curve can be used as a standard curve to estimate the concentration of free galectin. With inhibitor that binds some of the galectin, the concentration of free galectin will decrease, and the curve will shift to the right, but with enough added galectin, it will still reach 100% if binding sites on the inhibitor are approaching saturation, and therefore free galectin is increasing enough to saturate the probe as well. This is exemplified with the theoretically constructed curves in Fig. 2B that assume one or nine binding sites per ASF, respectively (broken lines). This was the case with galectin-1, and the curve was even steeper because the galectin-1-binding sites on ASF have a range of affinities, with the weakest binding last (16). With galectin-3 and ASF, however, after initially following the theoretical curve, data points (Fig. 2B, unfilled squares) followed a lower slope with increasing galectin, and 100% probe binding was not reached even at the highest galectin concentration. This showed that the concentration of free galectin remained low, in fact lower even than predicted if there were a large excess of saturable binding sites (90 per ASF molecule) (supplemental Fig. S2). This shows that more and more galectin-3, in excess of the theoretically predicted amount, bound ASF without sign of reaching saturation.
To analyze how much galectin-3 could be engaged by one ASF molecule, we calculated the concentration of bound galectin-3 (total minus free as determined by probe saturation) (Fig. 2C, filled symbols) and compared it with the theoretically calculated curves for nine binding sites per ASF molecule (broken line). The excess of bound galectin-3 could now be visualized directly (gray shaded) (Fig. 2C), and free galectin-3 (unfilled squares) remained low (∼5 μm) even with very high galectin-3 concentrations. This is in striking contrast to galectin-1 (Fig. 2D) where the binding is below the theoretical curve due to decreasing affinity of its binding sites on ASF (16), and free galectin-1 increases rapidly with higher galectin-1 concentration as ASF becomes saturated.
We also used lower concentrations of ASF to increase the ratio of galectin-3/ASF. Bound galectin-3 increased linearly, without sign of saturation, and at the highest galectin-3 concentration tested, it reached on average about 9 and 25 molecules per ASF molecule when analyzed with 20 and 4 μm ASF, respectively (Fig. 2, C and E) and even as high as on average 75 molecules with 0.8 μm ASF (Fig. 2F), although data varied more (range 25–125), as described in the legend for supplemental Fig. S2.
Apparently, ASF can engage the carbohydrate recognition site of far more galectin-3 molecules than its theoretical number of glycan-based binding sites. A possible explanation is that ASF induces nucleation of galectin-3 oligomers, where the carbohydrate recognition site of one molecule binds another site on the next (Fig. 1B). The data are consistent with the theory of protein multimers (20), where multimer formation is initiated at a certain threshold concentration (nucleation) and free monomer approaches a fixed value. This value is near the Kd of the monomer-monomer interaction, but also depends on the number of nucleation sites (20). We will refer to this mode of galectin-3 self-association as type-C, because it engages the carbohydrate recognition site (Fig. 1B), to distinguish it from the previously proposed modes that leave the carbohydrate-binding site free to interact with ligand and are mediated mainly by the N-terminal domains (8–10, 14), which we will refer to as type-N (Fig. 1A).
Formation of Precipitates
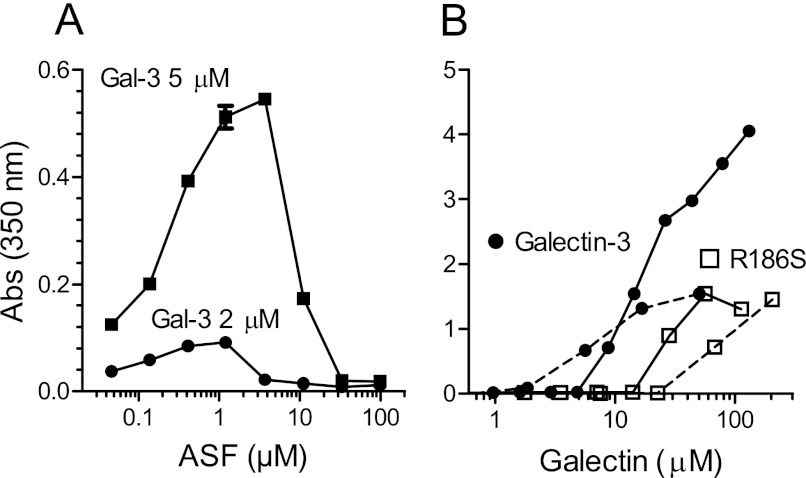
As expected, precipitates were formed within seconds at higher galectin-3 concentrations with ASF (8) and were quantitated by light absorption at 350 nm. With a fixed concentration of galectin and a range of ASF concentrations (Fig. 3A), precipitates were clearly detected already at <0.1 μm ASF in the presence of 2 or 5 μm galectin-3 and then increased in amount to reach a peak at about 1 and 4 μm ASF, respectively, and finally, precipitate formation was inhibited at higher ASF concentrations. With fixed ASF concentrations and increasing concentrations of galectin-3 (Fig. 3B), precipitates started to be detected at about 2 μm galectin (with 0.8 and 4 μm ASF) or about 8 μm (with 20 μm ASF) galectin and then continued to increase in amount without reaching a maximum. At the highest galectin-3 concentration tested, the molar ratio to ASF was about 60, 25, and 7 for the three concentrations used (0.8, 4, and 20 μm, respectively). These data are consistent with the data from the FA assay. Excess ASF will compete for the carbohydrate recognition site of galectin-3 and inhibit the type-C self-association, but with excess galectin-3, more and more aggregates are formed without sign of reaching saturation. The formation of precipitates was also detected at the same conditions as described above by dynamic light scattering, and their size increased over a small galectin concentration range and then remained a few μm in diameter (supplemental Fig. S3). The precipitates did not confound FA analysis by the experimental criteria shown before for galectin-1 (16), as described in the legend for supplemental Fig. S4.
FIGURE 3.
Detection of galectin-3-ASF precipitates. A, fixed concentrations of galectin-3 (2 and 5 μm) were mixed with a range of concentrations of ASF. B, fixed concentrations of ASF (0.8 μm (broken lines) or 20 μm (unbroken lines) were mixed with a range of concentrations of galectin-3. Absorbance (Abs) was measured at 350 nm (y axes), at room temperature within 10 min. Background (∼0.2), mainly due to the plate, was subtracted. Absorbance due to the components of the samples measured separately was <0.02.
Requirements for Type-C Galectin-3 Self-association
Analysis of galectin-3 with 180 μm LacNAc, corresponding to the maximum number of LacNAc residues in 20 μm ASF, gave no sign of self-association but had a binding curve consistent with a simple 1:1 interaction (Fig. 2G) and no precipitate. LNnH, a branched glycan with two terminal LacNAc residues, also did not induce any sign of type-C self-association in the FA assay (Fig. 2H), but galectin-3 bound it consistent with two saturable binding sites having Kd of about 20 μm in agreement with published data (21). This saccharide, however, formed precipitates with high galectin concentrations (over ∼100 μm galectin-3 and >∼25 μm of the saccharide, not shown) as reported for a similar branched saccharide (8) and proposed to be due to type-N self-association. Thus, some particular feature of ASF as compared with its component galectin-binding sites was required to induce the type-C self-association of galectin-3 detected by the FA assay.
To examine this further, we analyzed a galectin-3 mutant, R186S, that modifies the carbohydrate recognition site but does not destroy it. It has strongly reduced affinity for LacNAc (17) and >30-fold reduced affinity for ASF, calculated from the inhibition curve as for wild type galectin-3 (not shown), but can still be analyzed by the FA assay because it has retained affinity for the lactose-based probes. This showed that despite the lack of inhibition by ASF, a strong apparent type-C self-association was induced (Fig. 2I), and free galectin concentration appeared to approach a limit, as for galectin-3 WT but higher (about 30 μm). Surprisingly, this mutant also formed precipitates in the presence but not absence of ASF, but this required much higher galectin concentration as compared with galectin-3 WT (Fig. 3B, unfilled symbols). Precipitates started to be detected at ∼20–70 μm galectin, but in contrast to galectin-3 WT, lower ASF required more galectin for precipitation to start, possibly because now glycans on ASF are not competing with the self-association. At the highest galectin-3 R186S concentration tested, the molar ratio to ASF was about 250, 50, and 4 for the three concentrations used (0.8, 4, and 20 μm, respectively). Thus, ASF-induced nucleation of galectin-3 type-C self-association occurs even with low or no binding to glycans, as shown with R186S, but is much more efficient and occurs at much lower galectin concentration if higher affinity glycan binding also occurs, as with galectin-3 WT.
Galectin-3C showed no precipitate with ASF (supplemental Fig. S3), but the FA assay showed evidence for type-C self-association with excess binding over the level of theoretical glycan-binding sites and the concentration of free galectin approaching a constant level (∼20 μm)(Fig. 2J). Thus, galectin-3C also appears to be able to associate with itself, as has been proposed (11), but much less efficiently as compared with intact galectin-3, and as expected from previous studies (3, 9, 10), it shows no ability to cross-link the ligand.
Galectin-3 Self-association at Cell Surface
Analysis of galectin-3 binding and uptake by a macrophage cell line provided evidence for type-C self-association at the cell surface. Preincubation with low (1 μm (Ref. 18) or high (5 μm (supplemental Fig. S5)) galectin-3 strongly enhanced uptake of subsequently added 0.2 or 2 μm fluorescein-labeled galectin-3, respectively. Thus, galectin-3 enhances its own cell surface binding and uptake at physiological concentration and even up to 5 μm range without sign of saturation. If galectin-3 binding approached saturation of glycan ligands, as is likely to occur without self-association or with type-N self-association, one would expect that the preincubation with high galectin-3 would reduce the binding of subsequently added galectin-3.
DISCUSSION
The type-C self-association of galectin-3 proposed here (Fig. 1B) is consistent with most previously published data but not with many interpretations. The key feature, that one galectin-3 CRD can bind another by a mechanism that engages the carbohydrate-binding site, was experimentally demonstrated by Yang et al. (11), but their model of CRD-dimer formation, with the carbohydrate-binding sites facing each other, would prevent oligomer formation. However, their data also agree with the model in Fig. 1B, where the carbohydrate-binding site of one CRD binds at the other side of the next CRD, and the finding of CRD trimers by electron microscopy (13) supports the possibility of oligomer formation. The N-terminal domains of galectin-3 are not required for type-C self-association (Fig. 2J), but they clearly enhance it (Fig. 2C) (and are required for the concomitant aggregation with ligands as discussed below). Despite this enhancement, type-C self-association is not efficient for galectin-3 by itself in solution as it was captured only at low level by chemical cross-linking (Fig. 4 in Ref. 11) and not detected by DLS for <100 μm galectin (supplemental Fig. S1).
Efficient type-C self-association also needs a step of initiation or nucleation, here triggered by ASF in solution (Fig. 2, C, E, and F), but not by small saccharides (Fig. 2, G and H). How this makes galectin-3 more prone to self-association remains unknown, but different orientations of the N-terminal domains relative to the CRD have been proposed (8, 10–13). The nucleation can occur even without (or with very weak) initial binding to a β-galactoside-containing glycan, as seen, albeit less efficiently, with the R186S mutant (Fig. 2I). This may explain lactose-inhibitable binding of galectin-3 to some non-galactoside-containing ligands (22–24) if the lactose blocked the CRD-CRD interaction rather than the initial binding to the ligand.
Efficient nucleation followed by type-C self-association is consistent with the binding of galectin-3 to surface-immobilized laminin and other glycoproteins with apparent positive cooperativity and without saturation (9, 10, 12, 15), but contradicts the suggestion that galectin-3 binds to ASF in solution with negative cooperativity (19). It may agree, however, with the isothermal titration calorimetry in Ref. 19 if galectin-galectin interaction contributed to the heat release as ASF was titered into galectin-3 (34 μm), the most for the early injections when the galectin was in excess over ASF.
Different variants of type-N self-association (Fig. 1A) have been suggested to induce ligand cross-linking by galectin-3 because the N-terminal domains are required for glycoprotein precipitation, cell agglutination, the positive cooperative binding described above, and other effects (3, 6). These models are supported by the tendency of the N-terminal domains to aggregate by themselves, e.g. into filaments (13), but there is no other direct evidence for these models. The same aggregation tendency could in fact explain ligand cross-linking by type-C self-association, now between multiple N-domains exposed as part of galectin-3 oligomers. In either model, the details of the galectin-ligand complex structure remain unknown. Experimentally, the two models can be distinguished if fixed ligand concentration is titered with increasing galectin concentration; excess galectin is expected to saturate binding sites and inhibit complex formation for the type-N model as for classical antibody-antigen complexes, but not for the type-C model as found here with ASF in solution (Figs. 2, C, E, and F, and 3B) and with glycoprotein-coated surfaces (9, 10, 12, 15). The two models cannot be distinguished if fixed galectin concentration is titered with increasing ligand concentration (e.g. Figs. 3–4 in Ref. 8) because excess ligand would inhibit complex formation in the classical way for both models and also by interfering with the CRD-CRD interaction of type-C model (Fig. 3A).
It remains to be determined whether and when either model in Fig. 1 (or a mixture of them) occurs in cellular systems; the analysis of galectin-3 interaction with itself at the cell surface by fluorescence resonance energy transfer (FRET) (14) is consistent with both. The type-C model will be favored when there is a sufficiently potent nucleating ligand and the local concentration of galectin-3 exceeds the local concentration of competing galactosides, as shown in Fig. 3 and described above. The low concentrations of galectin-3 (μm) and the model glycoprotein ASF (<1 μm) required make it plausible that it will occur in cellular systems, also supported by the promotion of galectin-3 cell surface binding by itself (supplemental Fig. S5) (18). It may be particularly favored in the cytosol and nucleus where no competing galactosides have been found, provided that there is a potent enough nucleating ligand, and perhaps contribute to galectin-3 interaction with, for example, K-Ras as part of nanoclusters (24) or Bcl-2 (22). A special case may be the very rapid accumulation of cytosolic galectin-3 around phagosomes lysed by bacteria (25); the exposure to glycoproteins normally inside the phagosome may nucleate type-C self-association as a defense and/or membrane repair signal.
Type-C self-association provides an interesting new framework to interpret galectin-3 function and suggests new lines of investigation. One is more detailed molecular analysis of galectin-3 self-association, and in particular, identification of the binding site on the back of the CRD. Another is identification of new proteins with the potency to initiate type-C self-association of galectin-3 and more detailed analysis of the determinants required for this.
Acknowledgment
We thank Barbro Kahl-Knutson for excellent laboratory management and help in producing galectins.
This work was funded by the FP6 project “EuroPharm” Grant 043682 (to A. L.), the Swedish Research Council Grants 621-2009-5656 (to H. L.) and 621-2003-4265 (to U. N.), the program “Chemistry for Life Sciences” sponsored by the Swedish Strategic Research Foundation, and European Community Seventh Framework Programme (FP7-2007-2013) under Grant HEALTH-F2-2011-256986 (PANACREAS).

This article contains supplemental Figs. S1–S5.
- CRD
- carbohydrate recognition domain
- galectin-3C
- galectin-3 CRD
- FA assay
- fluorescence anisotropy assay
- LNnH
- lacto-N-neohexaose, LacNAcβ1-3(LacNAcβ1-6)Galβ1-4Glc
- A-tetra
- GalNAcα1-3(Fucα1-2)Galβ1-4Glc
- ASF
- asialofetuin
- DLS
- dynamic light scattering
- LacNAc
- N-acetyl-d-lactosamine.
REFERENCES
- 1. Boscher C., Dennis J. W., Nabi I. R. (2011) Glycosylation, galectins, and cellular signaling. Curr. Opin. Cell Biol. 23, 383–392 [DOI] [PubMed] [Google Scholar]
- 2. Dam T. K., Brewer C. F. (2010) Lectins as pattern recognition molecules: the effects of epitope density in innate immunity. Glycobiology 20, 270–279 [DOI] [PubMed] [Google Scholar]
- 3. Di Lella S., Sundblad V., Cerliani J. P., Guardia C. M., Estrin D. A., Vasta G. R., Rabinovich G. A. (2011) When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 50, 7842–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leffler H., Carlsson S., Hedlund M., Qian Y., Poirier F. (2004) Introduction to galectins. Glycoconj. J. 19, 433–440 [DOI] [PubMed] [Google Scholar]
- 5. Delacour D., Koch A., Jacob R. (2009) The role of galectins in protein trafficking. Traffic 10, 1405–1413 [DOI] [PubMed] [Google Scholar]
- 6. Newlaczyl A. U., Yu L. G. (2011) Galectin-3: a jack-of-all-trades in cancer. Cancer Lett. 313, 123–128 [DOI] [PubMed] [Google Scholar]
- 7. Morris S., Ahmad N., André S., Kaltner H., Gabius H. J., Brenowitz M., Brewer F. (2004) Quaternary solution structures of galectins-1, -3, and -7. Glycobiology 14, 293–300 [DOI] [PubMed] [Google Scholar]
- 8. Ahmad N., Gabius H. J., André S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C. F. (2004) Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279, 10841–10847 [DOI] [PubMed] [Google Scholar]
- 9. Hsu D. K., Zuberi R. I., Liu F. T. (1992) Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J. Biol. Chem. 267, 14167–14174 [PubMed] [Google Scholar]
- 10. Massa S. M., Cooper D. N., Leffler H., Barondes S. H. (1993) L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry 32, 260–267 [DOI] [PubMed] [Google Scholar]
- 11. Yang R. Y., Hill P. N., Hsu D. K., Liu F. T. (1998) Role of the carboxyl-terminal lectin domain in self-association of galectin-3. Biochemistry 37, 4086–4092 [DOI] [PubMed] [Google Scholar]
- 12. Kuklinski S., Probstmeier R. (1998) Homophilic binding properties of galectin-3: involvement of the carbohydrate recognition domain. J. Neurochem. 70, 814–823 [DOI] [PubMed] [Google Scholar]
- 13. Birdsall B., Feeney J., Burdett I. D., Bawumia S., Barboni E. A., Hughes R. C. (2001) NMR solution studies of hamster galectin-3 and electron microscopic visualization of surface-adsorbed complexes: evidence for interactions between the N- and C-terminal domains. Biochemistry 40, 4859–4866 [DOI] [PubMed] [Google Scholar]
- 14. Nieminen J., Kuno A., Hirabayashi J., Sato S. (2007) Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J. Biol. Chem. 282, 1374–1383 [DOI] [PubMed] [Google Scholar]
- 15. Barboni E. A., Bawumia S., Hughes R. C. (1999) Kinetic measurements of binding of galectin-3 to a laminin substratum. Glycoconj. J. 16, 365–373 [DOI] [PubMed] [Google Scholar]
- 16. Salomonsson E., Larumbe A., Tejler J., Tullberg E., Rydberg H., Sundin A., Khabut A., Frejd T., Lobsanov Y. D., Rini J. M., Nilsson U. J., Leffler H. (2010) Monovalent interactions of galectin-1. Biochemistry 49, 9518–9532 [DOI] [PubMed] [Google Scholar]
- 17. Salomonsson E., Carlsson M. C., Osla V., Hendus-Altenburger R., Kahl-Knutson B., Oberg C. T., Sundin A., Nilsson R., Nordberg-Karlsson E., Nilsson U. J., Karlsson A., Rini J. M., Leffler H. (2010) Mutational tuning of galectin-3 specificity and biological function. J. Biol. Chem. 285, 35079–35091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lepur A., Carlsson M., Novak R., Dumić J., Nilsson U., Leffler H. (2012) Galectin-3 endocytosis by carbohydrate-independent and -dependent pathways in different macrophage-like cell types. Biochim. Biophys. Acta 1820, 804–818 [DOI] [PubMed] [Google Scholar]
- 19. Dam T. K., Gabius H. J., André S., Kaltner H., Lensch M., Brewer C. F. (2005) Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 44, 12564–12571 [DOI] [PubMed] [Google Scholar]
- 20. Oosawa F. (1970) Size distribution of protein polymers. J. Theor. Biol. 27, 69–86 [DOI] [PubMed] [Google Scholar]
- 21. Ahmad N., Gabius H. J., Sabesan S., Oscarson S., Brewer C. F. (2004) Thermodynamic binding studies of bivalent oligosaccharides to galectin-1, galectin-3, and the carbohydrate recognition domain of galectin-3. Glycobiology 14, 817–825 [DOI] [PubMed] [Google Scholar]
- 22. Yang R. Y., Hsu D. K., Liu F. T. (1996) Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. U.S.A. 93, 6737–6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kohatsu L., Hsu D. K., Jegalian A. G., Liu F. T., Baum L. G. (2006) Galectin-3 induces death of Candida species expressing specific β-1,2-linked mannans. J. Immunol. 177, 4718–4726 [DOI] [PubMed] [Google Scholar]
- 24. Shalom-Feuerstein R., Plowman S. J., Rotblat B., Ariotti N., Tian T., Hancock J. F., Kloog Y. (2008) K-Ras nanoclustering is subverted by overexpression of the scaffold protein galectin-3. Cancer Res. 68, 6608–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paz I., Sachse M., Dupont N., Mounier J., Cederfur C., Enninga J., Leffler H., Poirier F., Prevost M. C., Lafont F., Sansonetti P. (2010) Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell. Microbiol. 12, 530–544 [DOI] [PubMed] [Google Scholar]