Background: Although the active site mTOR inhibitor pp242 overcomes feedback activation of AKT, it may still be complicated by feedback ERK activation.
Results: In myeloma cell models, pp242 was more potent than rapamycin for activating ERK, causing resistance.
Conclusion: Activation of ERK is a complication of pp242.
Significance: PP242 would be more effective if used in combination with inhibitors of the ERK pathway.
Keywords: Apoptosis, Eif4e, ERK, mTOR, Multiple Myeloma, Rapamycin
Abstract
Activation of PI3-K-AKT and ERK pathways is a complication of mTOR inhibitor therapy. Newer mTOR inhibitors (like pp242) can overcome feedback activation of AKT in multiple myeloma (MM) cells. We, thus, studied if feedback activation of ERK is still a complication of therapy with such drugs in this tumor model. PP242 induced ERK activation in MM cell lines as well as primary cells. Surprisingly, equimolar concentrations of rapamycin were relatively ineffective at ERK activation. Activation was not correlated with P70S6kinase inhibition nor was it prevented by PI3-kinase inhibition. ERK activation was prevented by MEK inhibitors and was associated with concurrent stimulation of RAF kinase activity but not RAS activation. RAF activation correlated with decreased phosphorylation of RAF at Ser-289, Ser-296, and Ser-301 inhibitory residues. Knockdown studies confirmed TORC1 inhibition was the key proximal event that resulted in ERK activation. Furthermore, ectopic expression of eIF-4E blunted pp242-induced ERK phosphorylation. Since pp242 was more potent than rapamycin in causing sequestering of eIF-4E, a TORC1/4E-BP1/eIF-4E-mediated mechanism of ERK activation could explain the greater effectiveness of pp242. Use of MEK inhibitors confirmed ERK activation served as a mechanism of resistance to the lethal effects of pp242. Thus, although active site mTOR inhibitors overcome AKT activation often seen with rapalog therapy, feedback ERK activation is still a problem of resistance, is more severe than that seen with use of first generation rapalogs and is mediated by a TORC1- and eIF-4E-dependent mechanism ultimately signaling to RAF.
Introduction
The mammalian target of rapamycin (mTOR)2 is critical for tumor growth and, thus, has become a popular target for new therapeutics (reviewed in Ref. 1). However, targeting mTOR therapeutically with rapamycin or related rapalogs, can be complicated by several feedback loops whereby compensatory pathways are activated. These drugs primarily target the TORC1 complex and inhibit by an allosteric mechanism. TORC1 inhibition prevents the ability of mTOR or its p70S6kinase substrate to inhibit insulin receptor substrate-1 (IRS-1). The de-repression of IRS-1 hyperactivates the insulin growth factor-1(IGF-1) receptor/PI3-K/AKT signaling pathway and up-regulation of AKT activity limits anti-tumor effects of rapalog mTOR inhibitors. This occurs in multiple myeloma (MM) cells (2) as well as in other tumor models (3, 4).
MTOR also participates in a second kinase complex called TORC2. AKT is a TORC2 substrate with TORC2-mediated phosphorylation occurring on serine 473 of AKT. As Ser-473 phosphorylation is required for full activation of AKT, newer 2nd generation mTOR inhibitors, which competitively inhibit at the ATP-binding site of mTOR (termed active site mTOR inhibitors), have been developed that can inhibit TORC2 as well as TORC1. PP242 is an example of an active site inhibitor whose activity has been previously described (5). In a prior study (5), we demonstrated the ability of pp242 to inhibit TORC1 and TORC2 in MM cells and induce a significantly greater anti-MM effect when compared with rapamycin.
Although newer 2nd generation TORC1/TORC2 inhibitors may overcome the PI3-K/AKT feedback loop by inhibiting AKT phosphorylation, a second potentially complicating compensatory pathway is the ERK MAPK cascade, which can also be activated subsequent to mTOR inhibition. Previous work (6) with rapalogs, like rapamycin and everolimus, have demonstrated such feedback activation. However, it is unknown whether ERK activation occurs with use of 2nd generation active site mTOR inhibitors. We, thus, initiated the current study to test if ERK activation also occurred in MM cells exposed to pp242 and to identify if this was a mechanism of resistance. Our results surprisingly demonstrate pp242 is considerably more effective than rapamycin on a molar basis as an ERK activator, that ERK activation was regulated by effects on 4E-BP1/eIF4E and RAF and that such activation restricts pp242's anti-MM efficacy.
EXPERIMENTAL PROCEDURES
Cell Lines, Reagents, Plasmids, Transfections
All MM lines were obtained from ATCC (Manassas, VA) except for ANBL-6 which was a kind gift from Dr. Brian Van Ness (University of Minnesota, Minneapolis, MN). PP242 was purchased from Chemdea Pharmaceuticals (Ridgewood, NJ) and was dissolved in DMSO. All antibodies were purchased from Cell Signaling Technology unless otherwise specified. The short hairpin RNAs (shRNA)/pLKO.1 targeting rictor and raptor and the control scrambled sequence were obtained from Addgene and previously described (7). Lentiviral shRNA production and infection was performed as previously described (8). After infection, clones were selected in puromycin. The HA-tagged eif4e full-length coding sequence was isolated from pHA-eif4e (obtained from Addgene, plasmid 17343) by PCR, using primers Spe1HA-eif4e (5-AAAGACTAGTATGTACCCATACGACGTCCC-3) and Xho1HA-eif4e (5-GATGCATGCTCGAGTTAAACA-3) to produce 5′-Spe1HA-eif4eXho1–3′ PCR product. Insert from pLenti6/gfp-lc3 vector was removed by Spe1/Xho1 digestion and replaced with Spe1/Xho1-digested HA-eif4e to yield pLenti6HA-eif4e. The pLenti6-ev (empty vector) plasmid was generated by removing the insert from pLenti6/gfp-lc3 with Spe1/Xho1. Lentivirus was produced by the UCLA Vector Core facility. To make HA-eif4e expressing stable lines, cells (1 × 106) were infected with empty vector (EV) or HA-eif4e lentivirus at multiplicity of infection (MOI) of 10 with polybrene (8 μg/ml) overnight. After virus was removed, cells were selected with 2.5 μg/ml blasticidin (Invitrogen) to generate stable cell lines. For transient transfection, cells were similarly infected and assayed 72 h later without selection in blasticidin.
Primary Myeloma Specimens
Primary MM cells were purified from bone marrow of patients by negative selection as described (2, 8) using the RosettesSep antibody mixture method (Stem Cell Technologies). The purity was >99% plasma cells. The project was approved by the IRB of the VA and all participants gave written informed consent.
Apoptosis Assays
Apoptosis was identified (as described in Ref. 5) by flow cytometric staining for expression of activated caspase 3 (BD Biosciences, San Jose, CA) or membrane expression of annexin-V in propidium iodide (PI)-negative cells. The data is presented as % apoptosis above control (no drug exposure). The control apoptosis for 8226 and MM1.S cells was very low (always <10%). The caspase 3-PE and annexin-V-FITC antibodies were purchased from BD Pharmingen.
eIF-4E Pull-down Assay
Cell extracts were incubated with m7GTP-Sepharose to precipitate eIF-4E as previously described (9). The precipitate was then immunoblotted for eIF-4E, 4E-BP1, and eIF-4G.
In Vitro Kinase Assays
The ERK in vitro kinase assay (Cell Signaling) was performed as previously described (10). Phospho-ERK was immunoprecipitated and tested for its ability to phosphorylate ELK-1 in vitro. ELK-1 phosphorylation was detected by immunoblotting with a phospho-ELK-1 (Ser-383) antibody. The RAF in vitro kinase assay was performed as previously described (11). Briefly, cells were treated, RAF-1 was immunoprecipitated with anti-RAF-1 antibody (Transduction Laboratories, Lexington, KY) or nonspecific IgG and the immunoprecipitates were tested for their ability to phosphorylate recombinant MEK-1 (Santa Cruz Biotechnology) determined by immunoblot with a polyclonal phospho-MEK antibody (Cell Signaling, Danvers, Mass). Kinase activity was calculated by densitometric analysis of the ratio of phospho-MEK to the immunoprecipitated RAF. This ratio was arbitrarily made 1 in control cells (no pp242 treatment).
GST-RAF Pull-down Assay
To assay GTP loading of RAS, we used a GST-RAF pull-down assay kit from Millipore (Temecula, CA). As described (12), the GST-RAF fusion protein contains the RAS-binding domain of RAF which specifically binds to RAS-GTP (activated RAS) but not RAS-GDP (inactivated RAS). Cell lysates are incubated with beads coated with the GST-RAF fusion protein. Beads are washed, and bound protein was eluted with 2× Laemmli buffer and analyzed by immunoblot with a pan-RAS antibody.
Cell Survival Assays and Statistics
Quantitative increases in protein phosphorylation on Western blots were evaluated by densitometric analysis of ratio of phosphorylated-protein/total protein signal of treated MM cells. All Western blots were repeated 3–4 times and the mean fold increase (n = 3) in drug-treated groups versus non-treated cells is shown under the gels in the figures. The t test was used to determine significance of differences between groups. The viable recovery and apoptosis data shown in Fig. 7 are means (n = 4). The SDs of all groups were <5% of the means. Percent viable recovery is determined by enumeration of trypan blue-negative viable cells with comparison to that of cells not exposed to any drugs. Percent apoptosis is determined by FACs enumeration of activated caspase 3-positive cells or annexin-V-positive cells and presented as % apoptosis above control (cells not exposed to any drugs). Apoptosis of control cells was always <10%. The effect of combining pp242 with MEK inhibitors on induction of apoptosis was assessed by the median effect method using Calcusyn Software Version 1.1.1 (Biosoft). Combination indices (CI) values were calculated using the most conservative assumption of mutually nonexclusive drug interactions. CI values were calculated from median results of apoptosis assays.
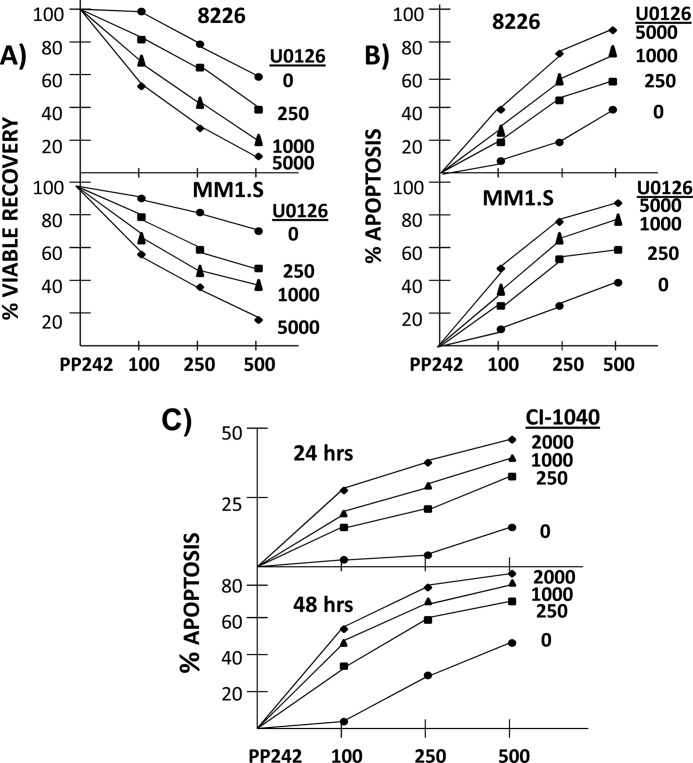
FIGURE 7.
ERK activation is a mechanism of resistance to pp242. In A (viable recovery) and B (apoptosis), 8226 or MM1.S cells treated ± U0126 (at 0 (circles), 250 (squares), 1000 (triangles), or 5000 (diamonds) nm) ± pp242 (at 100, 250, or 500 nm). Viable recovery (A) and % apoptosis (B) then assayed after 72 h for 8226 cells and 48 h for MM1.S cells, Viable recovery reported as percent of control (no drug treatment), mean ± S.D. (n = 4). Apoptosis reported as mean percent cells positive for activated caspase 3 expression over control by FACs analysis (n = 3). The SDs were all <5% of the means. In C, MM1.S cells similarly treated with pp242 at 100, 250, or 500 nm ± CI1040 at 250, 1000, or 2000 nm and apoptosis (mean of three separate experiments) evaluated by flow cytometry for annexin-V-positive cells. The SDs were all <5% of the means.
RESULTS
PP242 Activates ERK More Potently Than Rapamycin
Initially, 8226 and MM1.S MM cell lines were exposed to increasing concentrations of either rapamycin (rap) or the TORC1/TORC2 active kinase inhibitor pp242 for 30 min. Fig. 1A is a representative experiment (n = 4) that demonstrates both mTOR inhibitors enhanced phosphorylation of ERK but that pp242 was much more effective on a molar basis. This dose response experiment was repeated three additional times with identical results. By densitometry, the mean increase (n = 4) in phosphorylated ERK expression (phospho-ERK/total ERK ratio) in these experiments is shown below the gels as “fold increase.” For 8226 cells, a significant increase in phosphorylation of ERK (p < 0.05 shown by * in the figures) is not seen until 500 nm of Rap is used while a significant increase is identified at 100 nm of pp242. For the MM1.S cell line, similar results are seen with an increase in phosphorylated ERK only present at 1000 nm rap but obvious at the lowest concentration (100 nm) of pp242. In our previous work (2), much lower concentrations of rapamycin effectively activated the PI3K-AKT feedback pathway in these MM cell lines. Thus, ERK activation appears to be relatively resistant to rapamycin at least in MM cell lines. Additional experiments in two more MM cell lines (U266 and ANBL-6) demonstrated identical results (supplemental Fig. S1) with significant ERK phosphorylation present at the lowest concentration of pp242 (100 nm) while rapamycin was ineffective.
FIGURE 1.
PP242 activates ERK. In A, 8226 or MM1.S cells were exposed to increasing concentrations (in nm) of rapamycin (rap) or pp242 for 30 min, after which immunoblot assay was performed. Fold increase, determined as described under “Experimental Procedures” is mean of four independent experiments. *, significantly greater than control, non-treated cells, p < 0.05; In B, MM cells treated with DMSO (control), rapamycin (250 nm), pp242 (250 nm) or IL-6 (100 units/ml) for 30 min, after which phos-ERK was immunoprecipitated and tested for its ability to phosphorylate ELK-1 shown by immunoblotting with anti-phospho-ELK-1 antibody. In C, 3 separate primary MM samples treated as shown ± pp242 (in nm) with immunoblot for phospho-ERK (P-ERK) and total ERK (T-ERK). Fold increase in ERK phosphorylation is from a single experiment from each of the 3 patient samples. Patient 2 specimen treated ± 500 nm pp242 for 1 or 3 h.
Prior work (3) demonstrates that rapalog-induced activation of PI3K-AKT can be secondary to inhibition of p70S6K, preventing P70's ability to negatively regulate IRS-1 signaling to PI3K. If a similar mechanism was involved in ERK activation, a plausible explanation for pp242's more potent activation could be that it causes a more profound inhibition of p70S6K. However, rapamycin was comparable to pp242 in preventing p70S6K phosphorylation on Thr-389. As shown in Fig. 1A (lowest gels), p70 phosphorylation was ablated by concentrations of 100 nm of both rapamycin and pp242. However, only pp242 activated ERK at these low concentrations.
To more directly ascertain if induction of ERK phosphorylation results in activation of ERK activity, we performed an ERK in vitro kinase assay. As shown in Fig. 1B, pp242 was considerably more effective than an equimolar concentration of rapamycin in increasing the ability of immunoprecipitated ERK to phosphorylate ELK-1. The increase in ERK kinase activity was comparable to that achieved with interleukin-6 (IL-6), a well established MM growth factor (13). Fig. 1C also demonstrates that pp242 can enhance ERK phosphorylation in primary MM cells.
Enhanced ERK Phosphorylation Is Not Mediated by PI3-K
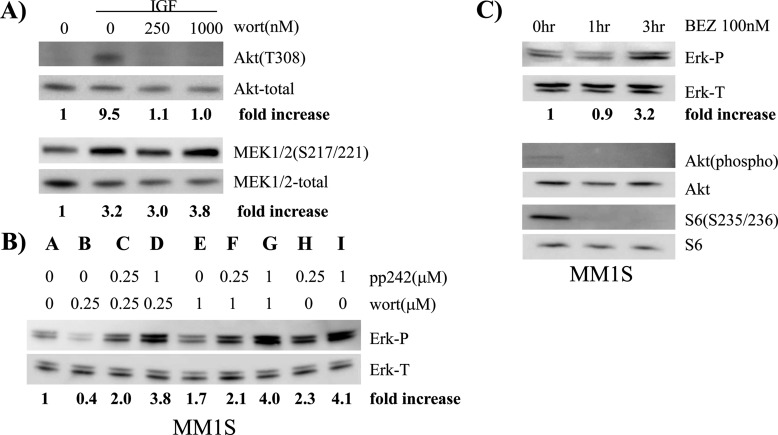
A previous study (6) demonstrated that rapamycin's ability to induce ERK phosphorylation was ablated by PI3-K inhibitors and p70S6K knock down. These data pointed to the well known p70S6K/PI3-K feedback loop as mediating ERK activation. However, as mentioned above, pp242-induced ERK activation did not correlate with p70S6K inhibition. To further test if feedback activation of PI3-K was involved, we co-incubated cell lines with pp242 ± the PI3-K inhibitor, wortmannin. Wortmannin, in the concentrations used, was very effective in preventing IGF-1-stimulated PI3-K-mediated AKT phosphorylation in these same cells (Fig 2A). However, wortmannin had little effect on IGF-1's ability to enhance MEK phosphorylation (Fig. 2A) suggesting no connection between PI3-K and the MEK/ERK pathway at least for MM cells stimulated by IGF-1. More importantly, wortmannin also had little effect on pp242-induced ERK phosphorylation in MM1.S MM cells (Fig. 2B). PP242 effectively activated ERK phosphorylation (compare control lane A to lanes H & I). Addition of wortmannin at 0.25 μm, a concentration that prevented PI3K activity in IGF-stimulated cells (Fig. 2A), had no effect against 0.25 μm pp242 (compare lanes C–H) and little effect against 1 μm pp242 (compare lanes D and I) in MM1S cells. Similar data were obtained in 8226 cells (supplemental Fig. S2) where the higher concentration of wortmannin, 1 μm, had no inhibitory effect against pp242-activated ERK, although a lower concentration (0.25 μm) had a modest unexplained effect. Collectively, these data indicated that PI3K activity was not involved in pp242-induced ERK activation. To independently gain further support for the notion that the PI3K pathway is not involved in ERK activation in MM cells exposed to pp242, we tested effects of the dual PI3K/mTOR inhibitor NEV-BEZ 235 (Fig. 2C). NEV-BEZ is an active site mTOR inhibitor (i.e. competitive inhibition at the ATP-binding site) with additional negative effects on PI3-K activity when used in MM cells (14) Treatment of MM1.S cells with BEZ at 100 nm inhibited TORC1 activity, shown as inhibited S6 phosphorylation, and inhibited PI3K-AKT activation shown as inhibited AKT phosphorylation (Fig. 2C). However, even with inhibition of PI3K-AKT, ERK activation was identified in NEV-BEZ-treated cells albeit after a longer incubation duration (3.2× fold increase at 3 h). Collectively, these data indicate that feedback activation of ERK subsequent to inhibition of mTOR with active-site inhibitors is not mediated by stimulation of the PI3K pathway.
FIGURE 2.
PP242-induced ERK activation is not mediated by feedback activation of PI3-K. In A, 8226 cells treated with IGF-1 (200 ng/ml) ± wortmannin (wort) for 30 min with immunoblot assay for phospho-AKT (T308), total AKT, phospho-MEK 1/2 (S217/221), or total MEK; In B, MM1.S cells treated with pp242 ± wortmannin at shown concentrations followed by immunoblot assay for phospho- or total ERK. In C, MM1.S cells treated for 0 (control), 1, or 3 h with NEV-BEZ 235 at 100 nm followed by immunoblot assay for phospho-ERK, total ERK, phospho AKT (Thr-308), total AKT, phospho S6 (Ser-235/236), or total S6.
ERK MAPK Cascade Activation Occurs Proximally at the Level of RAF
To initially test if ERK activation required input from MEK, we used the U0126 MEK inhibitor at 0.25, 1, and 5 μm. In both MM cell lines, U0126 caused a concentration-dependent decrease in pp242-induced ERK phosphorylation (Fig. 3A). PP242 was used at 500 nm in these experiments to induce a robust activation of ERK. ERK phosphorylation was inhibited by >70% when both pp242-treated cell lines were co-cultured with 250 nm U0126, inhibited by 90% when 1 μm U0126 was used, and completely ablated when 5 μm was used. As U0126 at these concentrations can inhibit both MEK 5 as well as MEK 1/2, we also tested a MEK inhibitor, CI1040 (15) with relative specificity for MEK 1/2 when used at concentrations of 250 nm to 2 μm (i.e. CI1040-induced MEK 5 inhibition requires 100× fold higher concentrations (16)). CI1040 is also more clinically relevant and is currently in clinical trials. As shown in supplemental Fig. S3, CI1040 at 250 or 1000 nm abrogated pp242-ERK activation.
FIGURE 3.
PP242-induces activation of RAF and phosphorylation of MEK. In A, MM1.S or 8226 cells treated for 30 min with pp242 (500 nm) ± U0126 used at 250 nm, 1 μm or 5μm. Immunoblot assay for phospho- and total ERK then performed. In B, MM1.S cells treated with 0, 250, or 1000 nm pp242 or 200 ng/ml IGF-1 followed by immunoblot assay for phospho-ERK, total ERK phospho-MEK, or total MEK. In C, RAF in vitro kinase assay performed by immunoprecipitating RAF-1 with anti-Raf antibody or nonspecific IgG from MM1.S cells, treated with 0, 250, or 1000 nm pp242 or IGF-1 (200 ng/ml). Immunoprecipitates tested for ability to phosphorylate MEK 1/2 shown by immunoblot for phospho-MEK. RAF-1 immunoblot shows equal amounts of RAF in the immunoprecipitates. Whole cell lysate (WCL) from the experiment shows concurrent pp242-induced ERK phosphorylation. In D, MM1.S cells pre-treated with sorafenib, AZ628 or L779445 at different concentrations for 1 h ± pp242 for 30 min, followed by immunoblot assay. In E, MM1.S cells were treated with pp242 or IGF-1 and Ras activation evaluated by pulling down active GTP-loaded Ras with a GST-fusion protein containing the RBD of RAF-1 and immunoblotting with anti-Ras antibody. Equal amounts of Ras in the extracts was confirmed by immunoblotting a fraction of lysates taken before the GST-Raf-RBD pull-down. Activation of ERK also shown in these cell lysates.
The finding that the MEK inhibitors prevented pp242-enhanced ERK phosphorylation suggested that MEK 1/2 was activated immediately upstream of ERK. As shown in Fig. 3B, pp242 exposure results in enhanced MEK phosphorylation (6.2 and 7.1× fold increase at 250 and 1000 nm pp242) which was comparable to that resulting from IGF-1 stimulation. To test for RAF activation upstream of MEK, we performed an in vitro kinase assay where immunoprecipitated RAF was tested for its ability to phosphorylate MEK 1/2. As shown in Fig. 3C, RAF kinase activity was significantly increased by pp242 at 250 and 1000 nm, with even greater effects than seen with IGF-1, used as a positive control. In addition, pretreatment with several raf inhibitors for 1 h prevented ERK phosphorylation by pp242 (Fig. 3D). These include sorafenib (17), AZ628 (18), and L779445 (19). The latter two RAF inhibitors are of particular interest because of their relative RAF specificity. These results further support a pp242-induced Raf activation paradigm.
To test if RAF/MEK activation was downstream of RAS stimulation, we assayed the ability of a GST-RAF fusion protein to pull-down RAS from cell lysates (Fig. 3E). GST-RAF contains the Ras binding domain (RBD) of Raf and, only if RAS is activated and GTP-bound, will it be capable of binding to GST-RAF. MM cells were treated with or without pp242 or with IGF-1 as a positive control. Cell lysates were then incubated with GST-RAF and associated GTP-bound RAS assessed by immunoblot assay with a pan-RAS antibody. GST-RAF pull down from lysates of MM cells treated with IGF demonstrates a modest but significant increase in associated RAS versus control non-treated cells. However, exposure of cells to pp242, which activated ERK in whole cell lysates, demonstrates no increase in RAS bound to GST-RAF. Immunoblot of whole cell lysates also confirm equal quantities of Ras were present in lysates before GST-RAF pull-down. Thus, although pp242 activates RAF, MEK, and ERK, it does not stimulate GTP-loading of RAS.
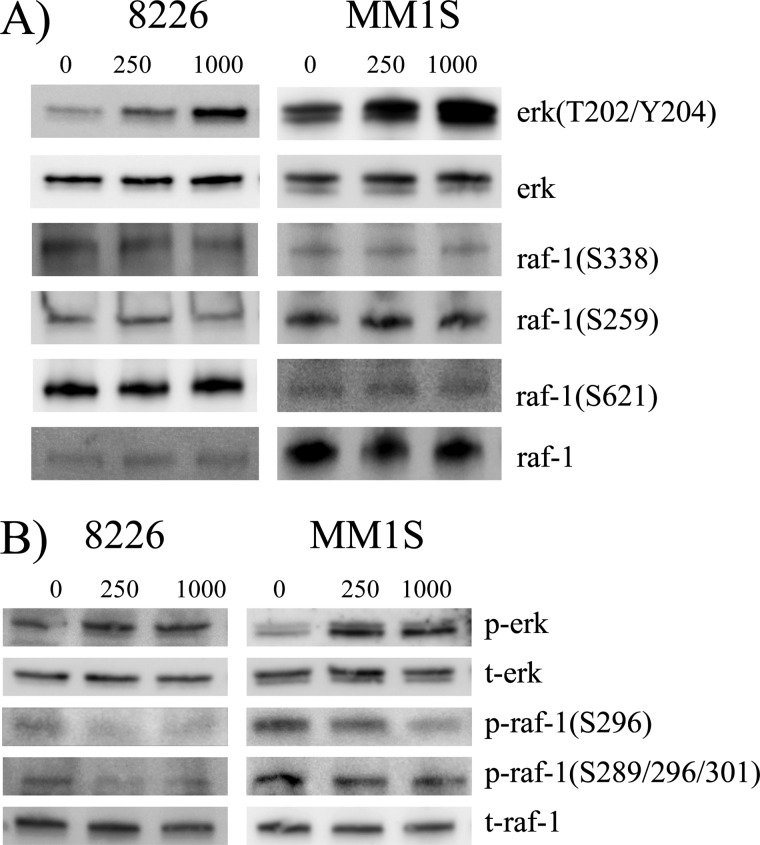
Since the phosphorylated state of specific residues on RAF can regulate the activation status of RAF (20), we assessed the phosphorylation profile on pp242-treated cells by immunoblot (fig 4). Ser-338 is a RAS-inducible activating phosphorylation site and is critical for RAF activation. Its phosphorylation was not significantly affected by pp242 treatment (Fig. 4A) in line with the previous data indicating absence of associated RAS activation (Fig. 3E). Ser-259 and Ser-621 are 14-3-3 interacting sites on RAF which facilitate binding to 14-3-3 which maintains RAF in an auto-inhibited state. In addition, Ser-259 is an AKT substrate and, since pp242 rapidly inhibits TORC2-induced AKT activation, we strongly considered the possibility that Ser-259 phosphorylation could be dampened, resulting in RAF activation. However, the 14-3-3 sites, Ser-259 and Ser-621, were not affected in their phosphorylation status (Fig. 4A). Additional experiments that demonstrated no significant effect of pp242 on the ability of 14-3-3 to bind RAF (supplemental Fig. S4) further support that RAF activation does not occur by alteration of these phosphorylation sites.
FIGURE 4.
Effects of pp242 on RAF phosphorylation. In A or B, 8226 and MM1.S cells treated ± pp242 at 250 or 1000 nm for 30 min, followed by immunoblot assay with phospho-specific antibodies and antibodies to total RAF-1.
We next analyzed inhibitory RAF residues that are involved in negative feedback regulation (21). Ser-29, Ser-43, Ser-289, Ser-296, Ser-301, and Ser-642 are phosphorylated by ERK with resulting inhibition of activity. These phosphorylation events are also regulated by the PP2A phosphatase (21). Using two independent antibodies, phosphorylation of these residues was significantly decreased by pp242 in both cell lines (Fig. 4B). This is most clearly seen with the Ser-296 phosphospecific antibody but also with the second phospho-specific antibody, especially in 8226 cells. Presumably, this effect releases RAF from an inhibitory influence of basal ERK phosphorylation. Because we never observed an ERK inhibition preceding pp242-induced activation, a good possibility is that pp242 is activating the PP2A phosphatase.
We also considered the possibility that the mTOR kinase inhibitor pp242 was inducing RAF activation in a similar mechanism to what occurs when B-RAF kinase inhibitors are used which induces paradoxical C-RAF activation via enhanced B-RAF:C-RAF dimerization (22). However, co-IP experiments did not demonstrate any enhanced B-RAF:C-RAF binding in pp242-treated cells (supplemental Fig. S4B).
PP242-induced ERK Activation Is Mediated by Effects on TORC1
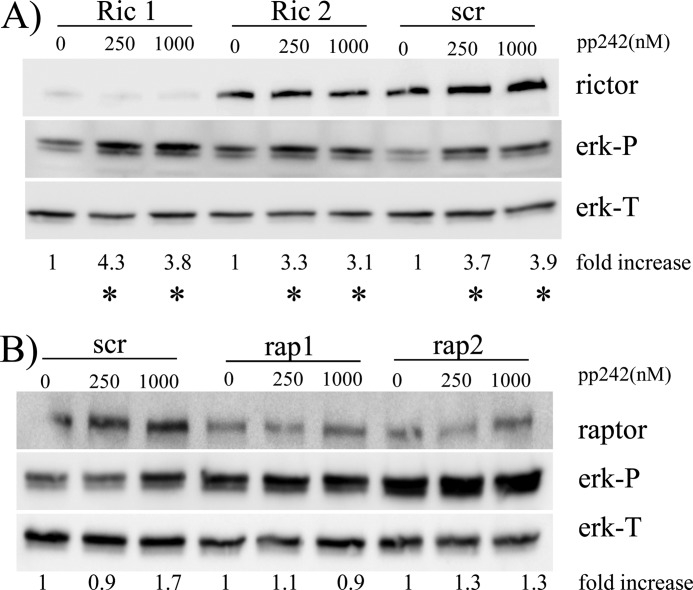
To test if ERK activation was mediated by effects on TORC1 or TORC2, we knocked down either raptor or rictor by shRNA. We targeted two separate sequences of RAPTOR or RICTOR that have successfully resulted in effective knockdowns in a previous publication (7). Rictor knockdown in these MM cells is associated with inhibited TORC2 activity as shown by decreased phosphorylation of the SGK1 substrate NDRG1 (supplemental Fig. S5). Supplemental Fig. S5 also demonstrates that raptor knockdown was associated with inhibition of TORC1 activity (p70S6K phosphorylation). As shown in Fig. 5A, RICTOR knockdown in clone 1, targeting sequence 1853, was considerably more effective than in clone 2, targeting sequence 1854. Nevertheless, knockdown had no effect on basal ERK phosphorylation nor the ability of pp242 to enhance ERK activation. In contrast, RAPTOR knockdown in both clones (Fig. 5B) resulted in a significant increase in basal ERK phosphorylation when compared with scrambled sequence control-transfected cells (2.4 ± 0.4 and 2.7 ± 0.6× fold increase versus control for rap 1 and rap 2, respectively, mean ± S.D. of three experiments). Furthermore, these RAPTOR-knocked down cells demonstrated little increase in ERK phosphorylation when exposed to 250 or 1000 nm of pp242. These data clearly demonstrate that pp242-induced ERK activation is due to inhibitory effects on TORC1.
FIGURE 5.
ERK activation is mediated by effects on TORC1- 8226 cells were transfected with shRNA targeting scrambled sequences (control;scr), or shRNA targeting two separate sequences in rictor (ric 1 and ric 2) or raptor (rap 1 and rap 2). In A, control or rictor knocked down clones treated ± pp242 for 30 min and, in B, control or raptor knocked down clones treated. Following treatment, immunoblot assay performed for phospho-ERK, total ERK, rictor, or raptor. Fold increase is ERK-P/ERK-T densitometry ratio compared with control, non-pp242 treated cells for each of the 6 separate clones. * denotes significant increase (p < 0.05) in ERK-phosphorylation induced by pp242.
PP242-induced ERK Activation Is Mediated by Effects on eIF-4E
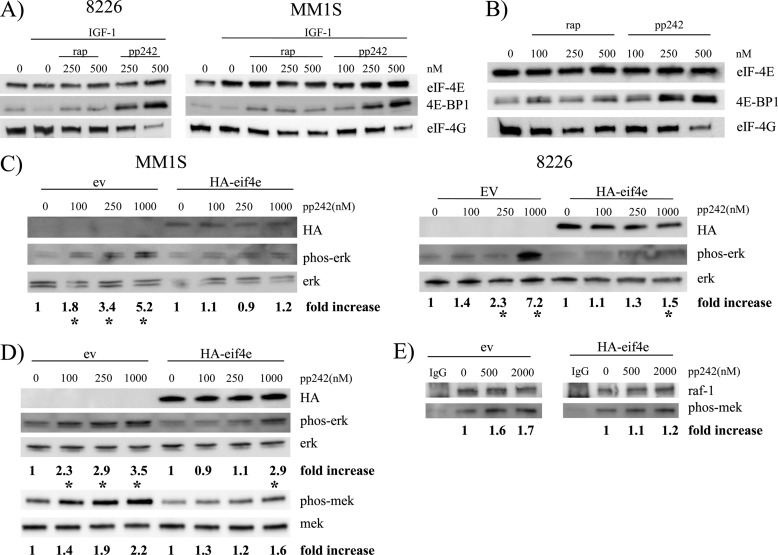
Because ERK activation was mediated by effects on TORC1, another possible explanation for the greater potency of pp242 versus rapamycin is that activation is induced by effects on 4E-BP1/eIF-4E. Previous studies (23, 24) have demonstrated that 4E-BP1 phosphorylation events are relatively resistant to rapamycin and more effectively prevented by active site mTOR inhibitors. The same is true in the MM cell model as shown in Fig. 6, A and B. In Fig. 6A, MM cells were stimulated with IGF-1 ± rapamycin or pp242 and their eIF-4E precipitated by m7GTP-Sepharose. In Fig. 6B, 8226 cells were similarly treated with rapamycin or pp242 but without IGF-1. The precipitated eIF-4E was then immunoblotted for bound 4E-BP1 or eIF-4G. As expected, treatment with IGF-1 decreased the binding of eIF-4E to 4E-BP1 (Fig. 6A), presumably due to TORC1-mediated phosphorylation of the latter translation repressor. When rapamycin was used at 100–500 nm in IGF-treated cells, binding to 4E-BP1 was increased although with little effect on eIF-4E binding to 4G. However, pp242 induced much greater binding to 4E-BP1 when used at 250 or 500 nm (versus equimolar concentrations of rapamycin) and had a significant inhibitory effect on eIF-4E binding to 4G (especially noted at 500 nm). A greater efficacy of pp242>rapamycin is also seen in the absence of IGF stimulation (Fig. 6B). As shown, 100–500 nm of rapamycin had little effect while pp242 greatly enhanced the binding of eIF-4E to 4E-BP1 when used at 250 or 500 nm and significantly decreased binding of eIF-4E to eIF-4G at 500 nm. Thus, pp242 was considerably more effective than rapamycin at sequestering eIF-4E through its enhanced binding to 4E-BP1 and, in turn, inhibiting eIF-4E binding to eIF-4G. To test if this greater sequestering of eIF-4E explained pp242's more potent activation of ERK, we stably transfected eIF-4E into MM1.S and 8226 MM cells as shown in Fig. 6C (immunoblot for HA of the HA-tagged eIF-4E). These eIF-4E overexpressing cell lines showed a significant decrease in pp242-induced ERK phosphorylation. Since compensatory pathways could have been altered during in vitro selection of these stable eIF-4E-expressing clones, we repeated the experiment with a transient transfection of 8226 cells although our lentiviral transfection efficiency in 8226 cells is only 60–80%. Nevertheless, Fig. 6D demonstrates that transient transfection of eIF-4E likewise blunted the pp242-induced activation of ERK. A significant activation of ERK was present in empty vector (EV)-transfected cells at all concentrations of pp242 while significant activation in eIF-4E transiently transfected cells was only present at the 1000 nm concentration of pp242. To test if eIF-4E overexpression attenuated pp242-induced ERK phosphorylation is occurring proximally at the level of RAF, we also compared MEK phosphorylation between MM1S(EV) and MM1S (eIF-4E) cells treated with pp242. Fig. 6D (lower panel) also shows a reduction in pp242-induced MEK phosphorylation in MM1S (eIF-4E) versus MM1S (EV) cells at 250 and 1000 nm. When these transfected MM cells are assayed for RAF activity by a RAF in vitro kinase assay (Fig. 6E), it is clear that eIF-4E over-expression blunts the response to pp242. In EV-transfected cells, 500 or 2000 nm of pp242 induced a significant increase in RAF kinase activity of 1.6 and 1.7× fold. In contrast, the eIF-4E-transfected cells did not demonstrate any significant pp242-induced increase in RAF activity. These data indicate that a decrease in free eIF-4E subsequent to pp242 exposure mediates RAF and ERK activation.
FIGURE 6.
Effects on 4E-BP1/eIF-4E mediate ERK activation. In A, 8226 or MM1.S cells treated with IGF-1 (200 ng/ml) ± rapamycin or pp242 for 30 min followed by eIF-4E precipitation and subsequent immunoblot of the precipitate for eIF-4E, 4E-BP1, and eIF-4G presence. In B, similar experiment performed in 8226 cells without presence of IGF-1. In C, empty vector (EV) or HA-eIF-4E- stably transfected MM1.S cells (left panel) or 8226 cells (right panel) treated with increasing concentrations of pp242 followed by immunoblot assay for phospho-ERK, total ERK, and HA tag. In D, empty vector or HA-eIF-4E transiently transfected 8226 cells treated with increasing concentrations of pp242 followed by immunoblot assay for phosphorylated and total ERK or phosphorylated and total MEK. In E, empty vector or eIF-4E-transfected cells treated with increasing concentrations of pp242 followed by immunoprecipitation of c-RAF and assay for raf in vitro kinase activity against MEK substrate. Fold increase in kinase activity calculated as described under “Experimental Procedures.”
ERK Activation Is a Mechanism of Resistance to pp242
In a previous study (5), we demonstrated how the TORC1/TORC2 inhibitor pp242 was a potent agent against MM cells, preventing tumor cell expansion and inducing apoptosis. To test whether the observed ERK activation could impact such therapy, we co-incubated 8226 and MM1.S MM cell lines with pp242 alone or with the addition of the MEK inhibitors with concentrations that were previously shown (in Fig. 3A) to significantly inhibit ERK activation in these cells exposed to pp242. In both cell lines, the MEK inhibitor U0126 had little effect on viable recovery when used alone. A 72 h exposure of 8226 to 250, 1000, or 5000 nm resulted in viable recoveries of 96–102% (versus control untreated cells). Similar lack of toxicity was seen after 48 h incubations in MM1.S cells (95–97% of control). In contrast, assay of viable recovery demonstrated that pp242 exhibited its dose-dependent anti-MM effects when used alone at 100, 250, or 500 nm (circles in Fig. 7A). When the non-toxic concentrations of U0126 were added to pp242, a significant increase in this inhibitory effect was seen (Fig. 7A). Even 100 nm pp242, a concentration which is insufficient to induce any death in several MM cell lines ((5) and this report), becomes effective when added to U0126. Similar results were seen when we assayed apoptosis by FACs analysis for expression of activated caspase 3 (Fig. 7B). Exposure of 8226 to U0126 alone for 72 h or MM1.S for 48 h resulted in no significant apoptosis (<5% above controls untreated cells). PP242 alone induced a modest dose-dependent apoptosis as shown previously (5). A significant increase in pp242-induced apoptosis was seen when the non-apoptotic concentrations of U0126 were added to pp242. The enhanced apoptotic effect is again noted at all concentrations of U0126. Using median effect combination indices (CI) analysis (supplemental Fig. S6) for apoptosis induction, co-exposure to pp242 and U0126 resulted in synergistic drug interactions with CI values less than 1 across several concentrations tested.
We also tested possible enhanced anti-MM effects with the more clinically relevant MEK inhibitor, CI1040, when the latter was used in the concentrations previously shown to inhibit pp242-induced ERK activation. Fig. 7C shows the results of CI1040 combined with pp242 when apoptosis was assayed by annexin-V staining. Similar to results with U0126, CI1040, used alone at 250, 1000, or 2000 nm, had no effect on annexin V staining. However, a significant increase in apoptosis was present when CI1040 was added to all concentrations of pp242 (Fig. 7C). CI values were consistently <1 (supplemental Fig. S6). Thus, these results with MEK inhibitors support the notion that the ERK activation accompanying pp242-induced mTOR inhibition provides a mechanism of resistance against MM cell death.
Because mTOR inhibitors activate autophagy in MM cells (25) and autophagy may protect cells against apoptosis, we considered the possibility that, by inhibiting pp242-induced autophagy, the MEK inhibitors were disarming this protective influence, thus inducing greater pp242-induced apoptosis. However, Western blot analysis for expression of LC3-I versus II, used as an assay for autophagy induction (supplemental Fig. S7) demonstrated a modest increase in autophagy when a MEK inhibitor was added to pp242 rather than a decrease. PP242 used alone at 0.5 μm significantly increased the ratio of LC3-II/LC3-I in both cell lines by 2–3× fold. In contrast, 0.25 μm pp242 had little effect. The U0126 MEK inhibitor also had little effect when used alone at 0.25 or 1 μm. However, the addition of U0126 at these concentrations significantly increased the induction of autophagy (i.e. LC3-II/LC3-I ratios) by the lower concentration of pp242 (0.25 μm). Thus, the synergistic anti-MM effects seen when MEK inhibitors are added to pp242 are not due to an inhibition of possible protective effects of autophagy.
DISCUSSION
The results of this study support the notion that feedback ERK activation in MM cells is a significant complication of therapy with active site mTOR inhibitors. A previous report (5) demonstrated greater anti-myeloma efficacy of such drugs when compared with rapamycin and active site inhibitors are now in clinical trials. Although these newer 2nd generation mTOR inhibitors are improvements over rapalogs because they can overcome feedback activation of PI3K-AKT, they stimulate feedback activation of ERK to a greater extent. The activation of ERK was associated with enhanced phosphorylation of eIF-4E (data not shown). EIF-4E phosphorylation is a pro-tumoral signal (26, 27) presumably via its promotion of cap-dependent translation. Thus, the ERK feedback activation pathway attempts to restore cellular homeostasis over protein translation. When TORC1 inhibition sequesters eIF-4E and initiates a restriction of cap-dependent translation, the cell responds by increasing eIF-4E phosphorylation through an ERK-dependent pathway which would promote translation initiation and counter the negative effects on protein expression.
Although the mechanism by which pp242 mediates ERK activation is not completely clear, it is evident that it differs from that described for rapalogs (4, 6) where ERK activation is mediated by a p70S6K- and PI3-K-dependent activation of upstream kinases of the MAPK ERK cascade. In contrast, the protection from eIF-4E overexpression suggests the most proximal signal of pp242 exposure that results in ERK activation is its dephosphorylation of 4E-BP1 and sequestering of eIF-4E. This explains why pp242 is so much more effective than rapamycin in ERK activation as 4E-BP1/eIF-4E function is relatively resistant to rapamycin but quite sensitive to pp242. This is also true in MM cells (Fig. 6). Furthermore, pp242-induced ERK activation is independent of PI3K and the proximal stimulating signal in the ERK MAPK cascade occurs at the level of RAF-1. The activation of RAF correlated with a decrease in RAF phosphorylation at sites that normally inhibit RAF activation (Ser-289, Ser-296, and Ser-301). These residues are ERK-dependent kinase targets and are dephosphorylated by PP2A. Prior work (21) demonstrates a negative feedback circuit whereby activation of ERK induces phosphorylation at these inhibitory residues resulting in dampening of activation of the MAPK cascade. How sequestering of eIF-4E results in decreased phosphorylation of the inhibitory residues is currently under study. One possibility is that pp242 treatment induces activation of the PP2A phosphatase with subsequent decreased phosphorylation at these RAF sites.
The U0126 and CI1040 MEK inhibitors, when used alone in concentrations that clearly down-regulated ERK, did not induce any anti-MM effects in 8226 or MM1.S cells. This is in contrast to a recent report (28) which demonstrated the ability of a MEK inhibitor, by itself, to cause significant cytoreduction in MAF overexpressing MM cell lines like 8226 and MM1.S. One possible reason for this inconsistency is that cell survival assays reported previously (28) were performed after 8 days of exposure and with much higher concentrations of MEK inhibitors. Nevertheless, while these inhibitors had no significant effect on MM cell survival over 72 h, they markedly enhanced the apoptotic effects of pp242 clearly demonstrating that activation of ERK induces resistance. The mechanism of this resistance is unknown. We ruled out the possibility that ERK-mediated resistance involves maintenance or further activation of pp242-induced autophagy. It is also unknown which downstream molecular effects of the ERK MAPK pathway are critical. Although the ERK activation results in hyperphosphorylation of eIF-4E, it is unknown if this latter effect mediates resistance. ERK activation has a number of other downstream consequences (29–31), many of which could protect tumor cells from loss of viability.
In summary, our results document a TORC1 and eIF-4E-dependent pathway by which active site mTOR inhibitors induce activation of ERK. More importantly, the results indicate a potential mechanism of resistance to the use of active site inhibitors in MM cells and also suggest combination therapy with mTOR and MEK/ERK inhibitors will be highly successful.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA109312 and R01 CA111448, research funds of the Veteran's Administration and Dept. of Defense and the Multiple Myeloma Research Foundation. The UCLA Vector Core is supported by JCCC/P30 CA016042 and CURE/P30 DK041301.

This article contains supplemental Figs. S1–S7.
- mTOR
- mammalian target of rapamycin
- TORC
- target of rapamycin complex
- eIF
- eukaryotic translation initiation factor
- MM
- multiple myeloma
- RBD
- Ras-binding domain.
REFERENCES
- 1. Alessi D. R., Pearce L. R., Garcia-Martinez J. M. (2009) Early events of B cell activation by antigen. Science Signaling 2, 1–419318623 [Google Scholar]
- 2. Shi Y., Yan H., Frost P., Gera J., Lichtenstein A. (2005) Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol. Cancer Ther. 4, 1533–1540 [DOI] [PubMed] [Google Scholar]
- 3. O'Reilly K. E., Rojo F., She Q. B., Solit D., Mills G. B., Smith D., Lane H., Hofmann F., Hicklin D. J., Ludwig D. L., Baselga J., Rosen N. (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66, 1500–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun S. Y., Rosenberg L. M., Wang X., Zhou Z., Yue P., Fu H., Khuri F. R. (2005) Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 65, 7052–7058 [DOI] [PubMed] [Google Scholar]
- 5. Hoang B., Frost P., Shi Y., Belanger E., Benavides A., Pezeshkpour G., Cappia S., Guglielmelli T., Gera J., Lichtenstein A. (2010) Targeting TORC2 in multiple myeloma with a new mTOR kinase inhibitor. Blood 116, 4560–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carracedo A., Ma L., Teruya-Feldstein J., Rojo F., Salmena L., Alimonti A., Egia A., Sasaki A. T., Thomas G., Kozma S. C., Papa A., Nardella C., Cantley L. C., Baselga J., Pandolfi P. P. (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest. 118, 3065–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 8. Sharma S., Nemeth E., Chen Y. H., Goodnough J., Huston A., Roodman G. D., Ganz T., Lichtenstein A. (2008) Involvement of hepcidin in the anemia of multiple myeloma. Clin. Cancer Res. 14, 3262–3267 [DOI] [PubMed] [Google Scholar]
- 9. Shi Y., Gera J., Hu L., Hsu J. H., Bookstein R., Li W., Lichtenstein A. (2002) Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 62, 5027–5034 [PubMed] [Google Scholar]
- 10. Shi Y., Sharma A., Wu H., Lichtenstein A., Gera J. (2005) Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. J. Biol. Chem. 280, 10964–10973 [DOI] [PubMed] [Google Scholar]
- 11. Bondzi C., Grant S., Krystal G. W. (2000) A novel assay for the measurement of Raf-1 kinase activity. Oncogene. 19, 5030–5033 [DOI] [PubMed] [Google Scholar]
- 12. Reynolds L. F., de Bettignies C., Norton T., Beeser A., Chernoff J., Tybulewicz V. L. (2004) Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J. Biol. Chem. 279, 18239–18246 [DOI] [PubMed] [Google Scholar]
- 13. Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H., Kuramoto A., Kishimoto T. (1988) Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 332, 83–85 [DOI] [PubMed] [Google Scholar]
- 14. McMillin D. W., Ooi M., Delmore J., Negri J., Hayden P., Mitsiades N., Jakubikova J., Maira S. M., Garcia-Echeverria C., Schlossman R., Munshi N. C., Richardson P. G., Anderson K. C., Mitsiades C. S. (2009) Antimyeloma activity of the orally bioavailable dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Cancer Res. 69, 5835–5842 [DOI] [PubMed] [Google Scholar]
- 15. Solit D. B., Garraway L. A., Pratilas C. A., Sawai A., Getz G., Basso A., Ye Q., Lobo J. M., She Y., Osman I., Golub T. R., Sebolt-Leopold J., Sellers W. R., Rosen N. (2006) BRAF mutation predicts sensitivity to MEK inhibition. Nature 439, 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mody N., Leitch J., Armstrong C., Dixon J., Cohen P. (2001) Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 502, 21–24 [DOI] [PubMed] [Google Scholar]
- 17. Wilhelm S. M., Carter C., Tang L., Wilkie D., McNabola A., Rong H., Chen C., Zhang X., Vincent P., McHugh M., Cao Y., Shujath J., Gawlak S., Eveleigh D., Rowley B., Liu L., Adnane L., Lynch M., Auclair D., Taylor I., Gedrich R., Voznesensky A., Riedl B., Post L. E., Bollag G., Trail P. A. (2004) BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109 [DOI] [PubMed] [Google Scholar]
- 18. Montagut C., Sharma S. V., Shioda T., McDermott U., Ulman M., Ulkus L. E., Dias-Santagata D., Stubbs H., Lee D. Y., Singh A., Drew L., Haber D. A., Settleman J. (2008) Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 68, 4853–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shelton J. G., Moye P. W., Steelman L. S., Blalock W. L., Lee J. T., Franklin R. A., McMahon M., McCubrey J. A. (2003) Differential effects of kinase cascade inhibitors on neoplastic and cytokine-mediated cell proliferation. Leukemia 17, 1765–1782 [DOI] [PubMed] [Google Scholar]
- 20. Chong H., Vikis H. G., Guan K. L. (2003) Mechanisms of regulating the Raf kinase family. Cellular Signalling 15, 463–469 [DOI] [PubMed] [Google Scholar]
- 21. Dougherty M. K., Müller J., Ritt D. A., Zhou M., Zhou X. Z., Copeland T. D., Conrads T. P., Veenstra T. D., Lu K. P., Morrison D. K. (2005) Regulation of Raf-1 by direct feedback phosphorylation. Mol. Cell 17, 215–224 [DOI] [PubMed] [Google Scholar]
- 22. Heidorn S. J., Milagre C., Whittaker S., Nourry A., Niculescu-Duvas I., Dhomen N., Hussain J., Reis-Filho J. S., Springer C. J., Pritchard C., Marais R. (2010) Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140, 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thoreen C. C., Kang S. A., Chang J. W., Liu Q., Zhang J., Gao Y., Reichling L. J., Sim T., Sabatini D. M., Gray N. S. (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284, 8023–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feldman M. E., Apsel B., Uotila A., Loewith R., Knight Z. A., Ruggero D., Shokat K. M. (2009) Active site inhibitors of mTOR target rapamycin-resistant outputs of TORC1 and TORC2. PLoS Biol. 7, 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoang B., Benavides A., Shi Y., Frost P., Lichtenstein A. (2009) Effect of autophagy on multiple myeloma cell viability. Mol. Cancer Ther. 8, 1974–1984 [DOI] [PubMed] [Google Scholar]
- 26. Hay N. (2010) Mnk earmarks eIF4E for cancer therapy. Proc. Natl. Acad. Sci. U.S.A. 107, 13975–13976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wendel H. G., Silva R. L., Malina A., Mills J. R., Zhu H., Ueda T., Watanabe-Fukunaga R., Fukunaga R., Teruya-Feldstein J., Pelletier J., Lowe S. W. (2007) Dissecting eIF4E action in tumorigenesis. Genes Dev. 21, 3232–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Annunziata C. M., Hernandez L., Davis R. E., Zingone A., Lamy L., Lam L. T., Hurt E. M., Shaffer A. L., Kuehl W. M., Staudt L. M. (2011) A mechanistic rationale for MEK inhibitor therapy in myeloma based on blockade of MAF oncogene expression. Blood 117, 2396–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xia Z., Dickens M., Raingeaud J., Davis R. J., Greenberg M. E. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270, 1326–1331 [DOI] [PubMed] [Google Scholar]
- 30. Scheid M. P., Schubert K. M., Duronio V. (1999) Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J. Biol. Chem. 274, 31108–31113 [DOI] [PubMed] [Google Scholar]
- 31. Chang F., Steelman L. S., Shelton J. G., Lee J. T., Navolanic P. M., Blalock W. L., Franklin R., McCubrey J. A. (2003) Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review). Int. J. Oncol. 22, 469–480 [PubMed] [Google Scholar]