Background: Arthritis is characterized by bone and cartilage destruction. Many conventional drugs suppress inflammation but not bone damage. Hence, new therapeutic agents are sought.
Results: Celastrus and its bioactive component celastrol inhibit osteoclastogenesis by controlling its mediators and their inducers/effectors.
Conclusion: Celastrus/celastrol controls inflammation-driven bone resorption by regulating the osteoimmune cross-talk.
Significance: Celastrus and celastrol are promising adjuncts to conventional drugs for arthritis treatment.
Keywords: Animal Models, Arthritis, Autoimmunity, Cytokine, Inflammation, Natural Products, Adjuvant Arthritis, Bone Damage, Bone Remodeling, Celastrol, Celastrus, Chinese Medicine, Experimental Arthritis, Matrix Metalloproteinases, Osteoprotegerin, Osteoclasts, Osteoimmunology, RANKL, Rheumatoid Arthritis
Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by bone erosion and cartilage destruction in the joints. Many of the conventional antiarthritic drugs are effective in suppressing inflammation, but they do not offer protection against bone damage. Furthermore, the prolonged use of these drugs is associated with severe adverse reactions. Thus, new therapeutic agents that can control both inflammation and bone damage but with minimal side effects are sought. Celastrus is a Chinese herb that has been used for centuries in folk medicine for the treatment of various inflammatory diseases. However, its utility for protection against inflammation-induced bone damage in arthritis and the mechanisms involved therein have not been examined. We tested celastrus and its bioactive component celastrol for this attribute in the adjuvant-induced arthritis model of RA. The treatment of arthritic rats with celastrus/celastrol suppressed inflammatory arthritis and reduced bone and cartilage damage in the joints as demonstrated by histology and bone histomorphometry. The protective effects against bone damage are mediated primarily via the inhibition of defined mediators of osteoclastic bone remodeling (e.g. receptor activator of nuclear factor-κB ligand (RANKL)), the deviation of RANKL/osteoprotegerin ratio in favor of antiosteoclastic activity, and the reduction in osteoclast numbers. Furthermore, both the upstream inducers (proinflammatory cytokines) and the downstream effectors (MMP-9) of the osteoclastogenic mediators were altered. Thus, celastrus and celastrol controlled inflammation-induced bone damage by modulating the osteoimmune cross-talk. These natural products deserve further consideration and evaluation as adjuncts to conventional therapy for RA.
Introduction
Rheumatoid arthritis (RA)2 is a chronic inflammatory polyarthritis characterized by hyperproliferation of synovial cells, infiltration of mononuclear cells into the synovium, destruction of cartilage and bone, and disability (1–3). The synovial cellular infiltrate consists of activated macrophages, T cells, and B cells, which secrete proinflammatory cytokines and other mediators of inflammation (2, 4, 5). In addition, activated synovial fibroblasts, chondrocytes, and osteoclasts contribute to the bone and cartilage damage associated with inflammatory arthritis (1, 6). Over the past decade, it has increasingly been realized that inflammation and immune activation can lead to bone damage and that there is a cross-talk between the immune system and the bone at cellular and molecular levels (“osteoimmunology”) (7, 8). Various immunological and biochemical mediators, including the proinflammatory cytokines, are known to increase bone resorption (9–14). In RA, there is a link between inflammation and increased bone turnover (10, 13–15). Several conventional drugs are available for treating RA (1, 3, 16). However, the effect of different therapeutic agents on inflammation and bone damage may be uncoupled. Certain drugs can suppress inflammation effectively, but they fail to protect against bone erosion (17, 18), whereas under other conditions, bone erosion can halt, but inflammation may continue unabated (19–22). In this context, it is essential to search for novel antiarthritic agents that can inhibit both inflammation and bone damage in arthritis and to define their mechanisms of action.
Bone remodeling is a dynamic process involving a balance between the formation of new bone by osteoblasts and the resorption of the existing bone by osteoclasts (11, 23–25). This balance is disturbed in RA, with bone resorption dominating over bone formation (10, 13–15). IL-17 is a major cytokine driving osteoclastogenesis leading to bone resorption (8, 12, 26). IL-17 can induce the production of receptor activator of nuclear factor κ-B ligand (RANKL) and various proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and granulocyte macrophage colony-stimulating factor (GM-CSF) (8, 12, 26). Preosteoblastic/stromal cells produce RANKL that binds to RANK on monocytes/macrophages (preosteoclast) and promotes the differentiation of preosteoclasts into mature osteoclasts, which function as bone-resorptive cells (9, 15, 24). On the other hand, osteoblasts produce osteoprotegerin (OPG), a decoy receptor for RANKL, and function as bone-forming cells (6, 15, 24). In fact, RANKL/OPG ratio is elevated in the synovial tissue/fluid in patients with untreated RA (14). Other mediators, such as osteopontin (OPN), osteocalcin (OCN) and insulin-like growth factor (IGF)-I also influence bone remodeling. OPN is an extracellular matrix protein that is expressed in both synovial cells and chondrocytes in RA, and it can mediate bone resorption by osteoclasts (27). OCN is the most abundant non-collagenous protein of bone, and it is an indicator of osteoblastic activity (28). IGF-I plays an important role in modulating the metabolism of bone and cartilage tissue by controlling both osteoblast-osteoclast interaction and osteoclast formation (29). Examination of the above-mentioned immunological and biochemical mediators permits an assessment of the mechanistic aspects of protection against bone damage by different therapeutic agents.
Celastrus aculeatus Merr. (celastrus) is a traditional Chinese herbal medicine that has been used in China for centuries for the treatment of various inflammatory diseases (30, 32). Celastrus belongs to the family Celastraceae, and celastrol represents one of the bioactive components of celastrus (30–33). We have previously reported that both celastrus and celastrol have anti-inflammatory activity and can reduce the severity of clinical arthritis in the rat adjuvant-induced arthritis (AA) model of human RA (30, 32). In the present study, we investigated the influence of celastrus/celastrol on bone damage in arthritic joints of rats with AA and examined the mechanisms involved in the inhibition of inflammation-mediated bone remodeling. Our results indicate that celastrus/celastrol reduced bone destruction in the joints of arthritic rats. This outcome was mediated primarily via reduction of the key mediators of osteoclastogenesis, via altering their ratio in favor of antiosteoclastic activity, and via suppression of the key upstream inducers as well as downstream effectors of the osteoclastogenic mediators. Our results validate celastrus and celastrol as promising antiarthritic agents that should be further tested in RA patients for their utility as adjuncts to conventional drugs for the treatment of inflammation and bone damage.
EXPERIMENTAL PROCEDURES
Animals
Lewis (LEW/SsNHsd) (RT.1l) rats, male, 5–6 weeks old, were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and then maintained in the animal care facility of the University of Maryland (Baltimore, MD). All experimental procedures performed on these rats were in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Preparation of Celastrus and Celastrol
Celastrus
The ethanol extract of C. aculeatus Merr. (celastrus) was prepared as described previously (30, 32). Briefly, roots and stem of celastrus were dried, powdered, and then extracted with 75% ethanol. The resulting extract was concentrated and then subjected to reverse-phase high performance liquid chromatography as well as identification of the three major groups of compounds, namely triterpenes (celastrol), flavonoids (epiafzelechin), and sesquiterpenes (orbiculin F) (30, 32).
Celastrol
Celastrol is one of the bioactive components of celastrus (30). Purified celastrol ((9β,13α,14β,20α)-3-hydroxy-9,13-dimethyl-2-oxo-24,25,26-trinoroleana-1(10),3,5,7-tetraen-29-oic acid), Mr = 450.6) isolated from Celastrus scandens was purchased from Calbiochem. A stock solution of celastrol (20 mg in 0.6 ml of dimethyl sulfoxide (DMSO) (Sigma-Aldrich)) was prepared and frozen at −20 °C in small aliquots until needed (30). The dose of celastrol (1 mg/kg) used in vivo in this study was based on that used in our previous study (30). Celastrol stock was diluted in PBS, and PBS-DMSO (1.2%) served as its control. For simplicity, throughout this work we refer to celastrol-DMSO and PBS-DMSO as celastrol and PBS, respectively.
Induction and Evaluation of AA
Lewis rats were immunized subcutaneously at the base of the tail with 1 mg/rat heat-killed Mycobacterium tuberculosis H37Ra (Mtb) (Difco) in 200 μl of mineral oil (Sigma-Aldrich). These rats were assessed for the signs of arthritis from the onset (day 9) to the peak (day 18) phase of the disease, and the severity of arthritis was graded on the basis of erythema and swelling of the paws as described previously (34, 35). The maximum arthritic score per rat was obtained by adding the score of individual paws.
Treatment of Arthritic Rats with Celastrus Extract or Purified Celastrol
A cohort of Lewis rats was randomly divided into four groups following the onset of arthritis on day 9 after Mtb injection. One group of rats (experimental group) received daily 3 g/kg of celastrus extract (30, 32), which was finely powdered and suspended in 2 ml of water using a pestle and mortar and then fed to rats by gavage (FNC-16-3, Kant Scientific Corp., Torrington, CT) beginning on day 9 after Mtb injection and then continued up to the peak phase of arthritis, day 18. On the corresponding days, the control group received water (vehicle) by gavage. Another experimental group was treated with celastrol (1 mg/kg/day) administered intraperitoneally (30). For this, the stock solution of celastrol was diluted in PBS (6 μl of stock in 500 μl of PBS/rat) and injected into rats from day 9 to day 18 as described in our previous study (30). The corresponding control group received the vehicle, DMSO (1.2%) in PBS. Thereafter, all rats were evaluated regularly for the severity of arthritis.
Histological Examination of Hind Paws of Rats
The hind paws were harvested from rats on day 18 after Mtb immunization and immersed for 9 days in Cal-Ex decalcifying solution CS510-1D (Fisher). Thereafter, the paws were immersed in 70% ethanol for 5 days and then embedded in paraffin, sectioned serially using a microtome, and mounted on microscope slides. Then the sections were stained either with hematoxylin and eosin (H&E) (Histology Core, University of Maryland School of Medicine, Baltimore, MD) (36, 37) or with safranin-O; the latter staining was performed following minor modifications to the method described elsewhere (38). (Safranin-O stain is taken up by the intact cartilage.) Histopathological changes in the joints like synovial hyperplasia, pannus formation, and cartilage and bone damage were observed under a microscope (Nikon Eclipse E800 Microscope, Nikon Industries Inc. Melville, NY) using the Spot Imaging Software (Diagnostic Instruments Inc., Sterling Heights, MI), and digital images were obtained (36).
Tartrate-resistant Acid Phosphatase (TRAP) Staining
The unstained, mounted microtome sections of the hind paws (as described above) were dehydrated in graded concentrations of ethanol and xylene and fixed for 2 min using 3.7% formaldehyde. After washing with deionized water, the sections were incubated in the reaction mixture (acid phosphatase, leukocyte (TRAP) kit; Sigma-Aldrich) at 37 °C in a humid and light-protected incubator for 1 h as directed by the manufacturer. Thereafter, the sections were washed three times with distilled water, counterstained with hematoxylin, and observed under a microscope using the Spot Imaging Software, and digital images were obtained.
Bone Histomorphometry
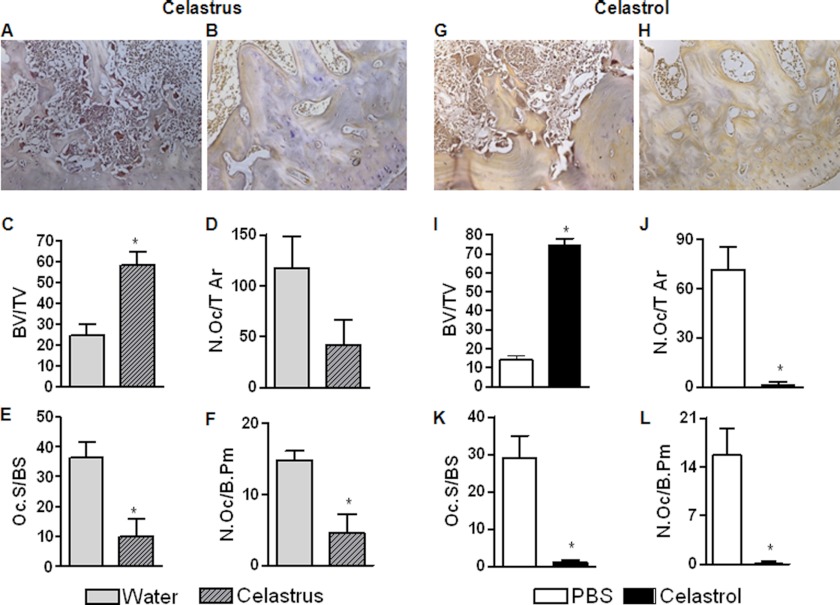
Bone histomorphometry was performed on the hind paw sections using the Osteomeasure bone histomorphometry system (Osteometrix, Atlanta, GA) linked to a Nikon Eclipse 50i inverted microscope and a Sony CCD video camera (39). Histomorphometric parameters follow the recommended nomenclature of the American Society for Bone and Mineral Research (40). The analyses were performed on serial transverse sections through the talus (n = 4). Bone volume versus total tissue volume (BV/TV), the number of osteoclasts per tissue area (N.Oc/T Ar), active resorption per bone surface area (Oc.S/BS), and the number of osteoclasts per bone perimeter (N.Oc/B.Pm) were assessed (see Fig. 2).
FIGURE 2.
Celastrus/celastrol reduces bone resorption and osteoclast numbers in arthritic rats. A group each (n = 8 per group) of Mtb-immunized Lewis rats was fed either water (vehicle; A) or celastrus (3 g/kg; B) (left), whereas another group of each was injected intraperitoneally with either PBS (control; G) or celastrol (1 mg/kg; H) (right) as described in the legend to Fig. 1. Animals were euthanized on day 18, and their hind limbs were harvested, followed by embedding and processing for TRAP staining and bone histomorphometry. A, B, G, and H, representative micrographs of the stained histological sections showing TRAP-positive cells (20×); C and I, bone volume versus total tissue volume (BV/TV); D and J, the number of osteoclasts per tissue area (N.Oc/T Ar); E and K, active resorption per bone surface area (Oc.S/BS); F and L, the number of osteoclasts per bone perimeter (N.Oc/B.Pm). *, p < 0.05, comparing experimental and control groups. Error bars, S.E.
Radiographic Assessment of Arthritis in Hind Paws of Rats
The severity of AA was assessed blindly on day 18 by radiography. High resolution digital radiographs (40 kV, 12 s) of hind limbs were performed on rats under ketamine-xylazine anesthesia with a Faxitron Digital x-ray system (Faxitron X-Ray, Lincolnshire, IL) (39).
Preparation of Synovium-infiltrating Cells (SIC), Their Restimulation with Mtb, and Testing for Mediators of Bone Damage
Hind paws of Mtb-immunized rats were harvested on day 18, and SIC (total SIC) were collected by opening the hind paw (ankle) joint using a sterile surgical blade (35). These SIC were washed 3–4 times with Hanks' Balanced Salt Solution and then were cultured for 90 min in a 12-well plate using DMEM supplemented with 5% fetal bovine serum (FBS), 2 mm l-glutamine, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate. The non-adherent cells were removed by washing the culture dish with Hanks' Balanced Salt Solution. The remaining cells (adherent SIC) were restimulated for 24 h with Mtb sonicate (10 μg/ml) in DMEM containing 5% FBS, and culture supernatant was collected and tested for cytokine protein expression by a multiplex assay in the Cytokine Core Facility (University of Maryland, Baltimore, MD) using the Luminex 100TM analyzer (Luminex Corp., Austin, TX).
Culture of MC3T3-E1 Cells, Their Treatment with Celastrol in Vitro, and Testing for Mediators of Bone Damage
MC3T3-E1 cells (mouse calvaria origin) (ATCC, Manassas, VA) were obtained from the laboratory of one of us (J.P.S.) and cultured in α-MEM containing 10% FBS, 2 mm glutamine, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate at 37 °C in a humidified atmosphere of 95% air and 5% CO2. These cells were seeded in a 12-well plate (1 × 105 cells/well) and grown to 80% confluence, and then the culture medium was replaced by fresh medium. Then the cells were stimulated for 24 h with IL-17 (100 ng/ml) in the presence or absence of different concentrations of celastrol. The concentration of celastrol used was determined after testing the cytotoxic action of celastrol on MC3T3-E1 cells (supplemental Fig. 5), and it matched that used for other cell types in our previous study (30). From one set of cells, the culture supernatant was collected and tested for cytokine protein levels using a multiplex assay. From another set of cells, total RNA was isolated using TRIzol reagent (Invitrogen), and then cDNA was prepared from RNA using the iScript cDNA synthesis kit (Bio-Rad). The cDNA was used for analysis of mRNA expression for M-CSF1. The appropriate primers were designed using the Primer Express 2.0 program (Applied Biosystems, Foster City, CA) and synthesized at the Genomics-Biopolymer Core Facility (University of Maryland, Baltimore, MD). The cDNA prepared above was amplified in an ABI Prism 7900HT cycler (Applied Biosystems) by quantitative RT-PCR using SYBR Green PCR Master Mix (Applied Biosystems). The mRNA level of the M-CSF1 gene was normalized to the hypoxanthine-guanine phosphoribosyltransferase gene, and the relative gene expression levels were determined and expressed as “relative message.”
Preparation of Fibroblast-like Synoviocytes (FLS), Their Restimulation with IL-1β in Presence/Absence of Celastrol in Vitro, and Testing for Matrix Metalloproteinase (MMP) Activity
SIC were collected from arthritic rats as described above and cultured in DMEM supplemented with 10% FBS, 2 mm l-glutamine, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate. After overnight incubation, the culture medium was replaced with fresh medium to remove non-adherent cells. Thereafter, the remaining adherent cells were split after they reached confluence, and cells in passage 2 were stimulated for 24 h with IL-1β (10 ng/ml) in serum-free medium in the presence or absence of different concentrations (0.1 and 0.3 μm) of celastrol based on our earlier study (30). Then, the supernatant was collected and analyzed for MMP activity as described below.
Culture of RAW 264.7 Cells and Their Treatment with Celastrol in Vitro
Murine macrophage RAW 264.7 cells (mouse leukemic monocyte macrophage cell line) (ATCC) were cultured in DMEM containing 10% FBS, 2 mm glutamine, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate at 37 °C in a humidified atmosphere of 95% air and 5% CO2. For osteoclast differentiation (41), cells were seeded in a 24-well plate and grown to 80% confluence in α-MEM containing 100 ng/ml RANKL for 5 days in the presence or absence of different concentrations of celastrol that was not cytotoxic for these cells (supplemental Fig. 5). Cell culture medium was changed every 2 days for 5 days. On day 5, culture supernatant was collected and analyzed for MMP activity.
Determination of MMP Activity
A zymogram assay was performed (34, 35) to determine the MMP activity in the serum and culture supernatants of cells (SIC, FLS, and RAW 264.7) collected after in vivo or in vitro treatment with celastrol as described above. Briefly, serum/supernatant sample was loaded onto a gelatin-coated, precast polyacrylamide gel (Bio-Rad). Standard MMP-9 and MMP-2 (Sigma) were used as positive controls. Electrophoresis was carried out under SDS-nonreducing conditions at constant voltage. Triton X-100 (2.5%) was added to the gel and incubated at room temperature for 1–2 h to remove SDS. The gel was washed three to four times with water to remove Triton X-100 and then incubated overnight at 37 °C in a developing buffer (Tris-HCl, pH 7.4) containing 5 mm CaCl2, 0.2 m NaCl, and 0.02% Brij 35. Thereafter, the gel was stained with Coomassie Brilliant Blue R-250. The MMP activity was visualized and scanned after destaining. The intensity of bands was quantitated by densitometry using ImageJ software (W.S. Rasband, National Institutes of Health, Bethesda, MD).
Statistics
The data were expressed as mean ± S.E. Student's t test and analysis of variance were used to assess the significance of differences using GraphPad Prism version 4.0. A p value of < 0.05 was considered significant.
RESULTS
Celastrus/Celastrol Suppresses Inflammation and Tissue Damage in Joints of Arthritic Rats
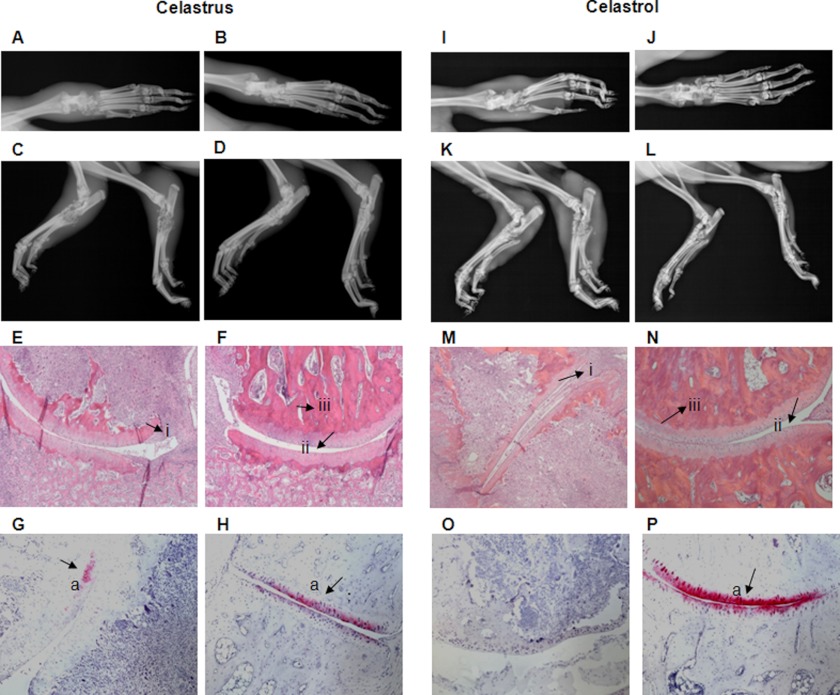
Arthritic Lewis rats were fed daily with celastrus (in water, by gavage) or injected intraperitoneally with celastrol (in 1.2% DMSO) beginning at the onset (day 9) of AA and then continued up to the peak phase (day 18) of the disease. The corresponding control rats received water by gavage or PBS-DMSO intraperitoneally, respectively. On day 18, the hind paws of rats were subjected to radiological examination. There was a significant reduction in the severity of clinical arthritis (supplemental Fig. 1), as well as both the inflamed soft tissue around the joints and bone damage in celastrus/celastrol-treated rats compared with control rats (Fig. 1, A–D and I–L). Further, histological examination revealed significant reduction of pannus formation, synovial mononuclear cell infiltration, and bone and cartilage destruction in celastrus/celastrol-treated rats compared with controls (Fig. 1, E–H and M–P).
FIGURE 1.
Celastrus/celastrol attenuates arthritic inflammation and joint damage in Lewis rats. One group each (n = 8 per group) of Mtb-immunized Lewis rats was fed either water (vehicle; A, C, E, and G) or celastrus (3 g/kg; B, D, F, and H) (left), whereas another group of each was injected intraperitoneally with either PBS (control; I, K, M, and O) or celastrol (1 mg/kg; J, L, N, and P) (right) beginning at the onset of AA and then continued through day 18 after Mtb injection. Animals were euthanized on day 18, and their hind limbs were harvested, followed by embedding and processing for bone histology. The top first (frontal view; A, B, I, and J) and second (lateral view; C, D, K, and L) panels show representative radiographs of limbs. The third panel (E, F, M, and N) depicts representative micrographs of H&E-stained histological sections of the hind paw joints showing the pannus containing the mononuclear cell infiltrate (i), the joint space (ii), and the bone mass (iii). The bottom panel (G, H, O, and P) depicts safranin-O-stained sections showing the extent of cartilage damage (a). (Celastrol was dissolved in 1.2% DMSO, and PBS-DMSO (1.2%) served as its control, but for simplicity, we have referred to these entities as celastrol and PBS, respectively.)
Celastrus/Celastrol Reduces Bone Loss and Osteoclast Number in Arthritic Lewis Rats
To further validate the protective effect of celastrus and celastrol on the inflammation mediated bone degradation in arthritic rats, we examined the subchondral bone loss and osteoclast numbers in the talus of the hind paw joints of the experimental and control rats (Fig. 2, A, B, G, and H, and supplemental Figs. 2 and 3). Treatment with celastrus/celastrol significantly reduced the subchondral bone loss and preserved the bone volume up to 58% (celastrus) and 81% (celastrol) compared with the respective controls (whose counts were adjusted to 100%) (Fig. 2, C and I). Histomorphometric analysis of TRAP-stained joint sections revealed that the number of osteoclasts was reduced to 36% or 2.2% following treatment with celastrus or celastrol, respectively, compared with that of controls (Fig. 2, D and J). The corresponding decrease in active resorption surfaces was 3.5- or 26.2-fold (Fig. 2, E and K), and the reduction in osteoclast number/bone perimeter was 69% or 98% after treatment with celastrus or celastrol, respectively, when compared with controls (Fig. 2, F and L).
Celastrus/Celastrol Regulates Critical Immune Mediators of Bone Remodeling in Arthritic Rats
To address the mechanisms underlying the observed effects of celastrus/celastrol on bone remodeling, we determined the effect of these natural products on the immune mediators of inflammation-induced bone damage (RANKL, OPG, OCN, GM-CSF, IGF, and OPN) in experimental and control rats. These mediators were measured in culture supernatants of SIC restimulated with sonicated, heat-killed M. tuberculosis H37Ra (Mtb sonicate) (Fig. 3) and in sera of the rats (Fig. 4 and supplemental Fig. 4).
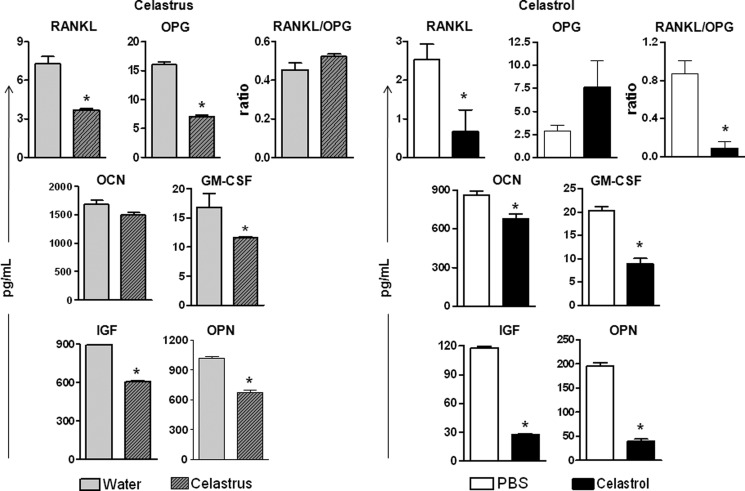
FIGURE 3.
Celastrus/celastrol regulates bone remodeling-related immune mediators in the joints of arthritic rats. SIC were harvested on day 18 from Mtb-immunized rats (n = 4 per group) treated with celastrus/water (left) or celastrol/PBS (right) as described in the legend to Fig. 1, and then these cells were restimulated for 24 h with Mtb sonicate (10 μg/ml). The levels of the indicated mediators were measured in culture supernatants of SIC using a multiplex assay, and the results were expressed as pg/ml. *, p < 0.05, comparing experimental and control samples. Error bars, S.E.
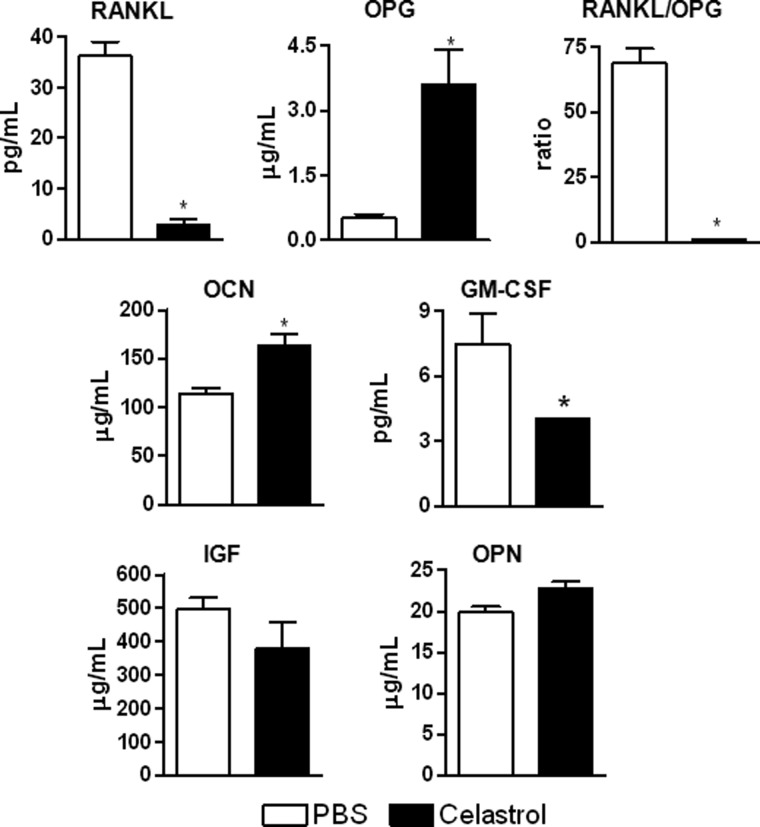
FIGURE 4.
Celastrol reduces the serum levels of the immune mediators of bone remodeling in arthritic rats. Serum was collected on day 18 from Mtb-immunized rats treated intraperitoneally with PBS (open bars) or celastrol (filled bars) as described in the legend to Fig. 1, and the levels of the indicated mediators were measured using a multiplex assay. The results were expressed as pg/ml. *, p < 0.05, comparing experimental and control samples. Error bars, S.E.
In SIC (Fig. 3), there was a significant (p < 0.05) decrease in all of the above-mentioned immune mediators tested in celastrus-treated rats compared with the control rats (Fig. 3, left) except for OCN, which was unchanged. Overall, similar results were obtained in celastrol-treated rats (Fig. 3, right), except that the level of OPG did not change significantly. Further, the RANKL/OPG ratio was significantly reduced following celastrol treatment but not after celastrus treatment. Nevertheless, RANKL was reduced in the celastrus-treated group. Taken together, celastrus/celastrol treatment altered the RANKL/OPG ratio in favor of antiosteoclastic activity and inhibited osteoclastogenic mediators of bone remodeling, namely RANKL, GM-CSF, M-CSF, OPN, and IGF-1 in the target organ, the joints.
In serum, celastrol-treated rats (Fig. 4) showed a significant decrease in serum levels of RANKL, RANKL/OPG ratio, and GM-CSF, coupled with a significant increase in OPG and OCN levels in comparison with the controls. In comparison, celastrus-treated rats (supplemental Fig. 4) showed a significant decrease in RANKL and RANKL/OPG ratio without any change in OPG level, but an increase in OCN level compared with the controls. However, there was no change in other mediators following celastrus/celastrol treatment. In summary, celastrus/celastrol treatment reduced serum levels of RANKL as well as the RANKL/OPG ratio but increased OCN levels.
Celastrol Regulates Mediators of Bone Turnover in Preosteoblastic (MC3T3-E1) Cells in Vitro
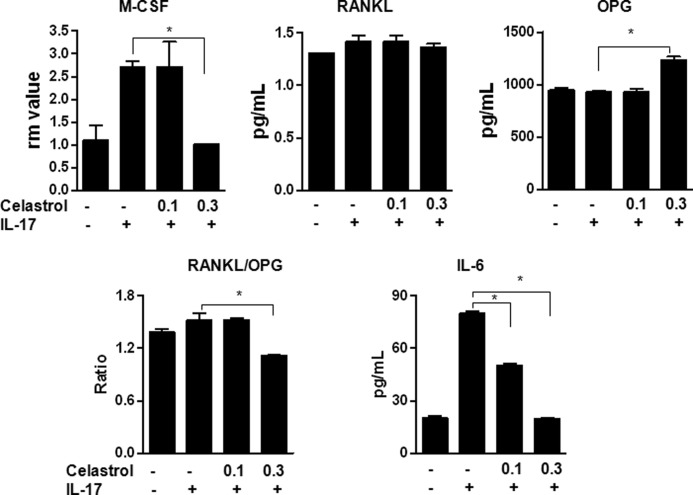
We studied the effect of celastrol in vitro on M-CSF1 production by preosteoblastic MC3T3-E1 cells treated with IL-17. Celastrol suppressed the IL-17-induced expression of M-CSF mRNA (Fig. 5). In regard to protein expression, there was an increase in OPG levels in the face of unchanged RANKL levels, resulting in a decrease in RANKL/OPG ratio in cells treated with celastrol (Fig. 5). In addition, there was a significant decrease in IL-6 levels. These results support the above-mentioned changes observed in vivo in celastrol-treated arthritic rats.
FIGURE 5.
Celastrol alters the expression of the mediators of bone remodeling in MC3T3 cells in vitro. MC3T3 cells were stimulated with IL-17 (100 ng/ml) after attaining 80% confluence. Prior to stimulation with IL-17, cells were treated with or without celastrol (0.1 and 0.3 μm). After 24 h, the cells were harvested, and the total RNA was prepared and tested by quantitative RT-PCR for M-CSF mRNA expression. Values were normalized to the respective hypoxanthine-guanine phosphoribosyltransferase mRNA levels, and the results are expressed as “relative message” (rm). In parallel, the culture supernatant was collected after 24 h from another set of cells, and the levels of RANKL, OPG, and IL-6 were measured using a multiplex assay. The results were expressed as pg/ml. *, p < 0.05, comparing experimental and control samples. Error bars, S.E.
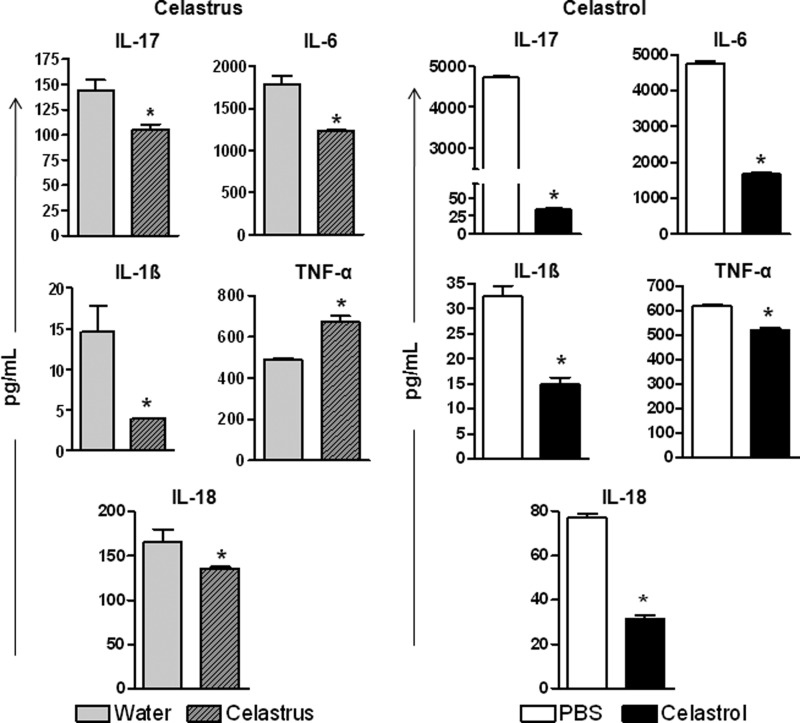
Celastrus/Celastrol Treatment of Arthritic Rats Inhibits Key Proinflammatory Cytokines Involved in Bone Damage
The osteoclastogenic proinflammatory cytokines (IL-17, IL-6, IL-1β, TNF-α, and IL-18) are critical mediators that stimulate the production of various immune mediators of bone remodeling mentioned above. To gain more insight into the cytokine mediators upstream of the bone-remodeling mediators, we examined the cytokine production in SIC that were harvested from celastrus/celastrol-treated or control rats and then restimulated in vitro for 24 h with Mtb sonicate. The celastrus-treated group showed a significant decrease in all cytokines tested except for TNF-α, which showed an unexpected increase (Fig. 6, left). Further, in the celastrol-treated group, all of the cytokines tested were significantly suppressed in SIC (Fig. 6, right). In contrast, there was no significant change in serum cytokine levels in either group compared with controls (data not shown).
FIGURE 6.
Celastrus/celastrol controls the production of proinflammatory cytokines in the joints of arthritic rats. SIC were harvested on day 18 from groups of Mtb-immunized rats (n = 4 per group) treated with celastrus/water (left) or celastrol/PBS (right) and then restimulated in vitro for 24 h with or without Mtb sonicate (10 μg/ml). The levels of different cytokines in culture supernatants were measured using a multiplex assay, and the results were expressed as pg/ml. *, p < 0.05, comparing experimental and control samples. Error bars, S.E.
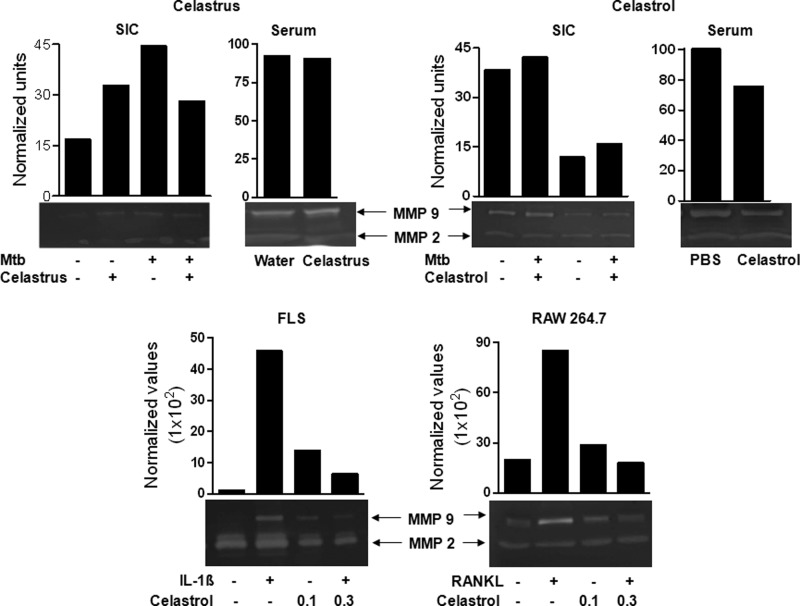
Celastrus/Celastrol Suppresses MMP-9 Enzyme Activity in SIC and Serum of Arthritic Rats and in FLS and RAW 264.7 Cells in Vitro
MMPs play an important role in cartilage and bone destruction in arthritic joints (42). Therefore, we evaluated MMP activity in SIC and serum collected from celastrus/celastrol-treated and control rats (Fig. 7, top) as well as in vitro using FLS stimulated with IL-1β (10 ng/ml), and RAW 264.7 cells stimulated with RANKL (100 ng/ml) (Fig. 7, bottom). SIC were restimulated with sonicated Mtb for 24 h, and their culture supernatants were tested for MMP activity using a gelatin zymogram assay. Treatment with celastrus caused a 1.6-fold decrease in MMP-9 activity in SIC but without much change in serum. In comparison, celastrol treatment caused a 2.6- and 1.3-fold suppression in the activity of MMP-9 in SIC and serum, respectively. In contrast, there was no consistent change in the activity of MMP-2. The in vivo suppression of MMP-9 activity by celastrol was supported by in vitro testing of FLS and RAW 264.7 cells. Celastrol suppressed both IL-1β-induced MMP-9 in FLS and RANKL-induced MMP-9 in RAW 264.7 cells in a dose-dependent manner.
FIGURE 7.
The effect of celastrus/celastrol treatment on MMP activity in defined cells and sera. SIC and sera were harvested on day 18 from Mtb-immunized rats treated with celastrus (top left) or celastrol (top right) (n = 4 each) as described in the legend to Fig. 1. Then the SIC were restimulated in vitro for 24 h with or without Mtb sonicate (10 μg/ml). The supernatants obtained from these cultured cells as well as the sera were analyzed for MMP-9 and MMP-2 activity using a gelatin zymogram assay. FLS (bottom left) were stimulated with IL-1β (10 ng/ml) in the presence or absence of celastrol for 48 h, whereas RAW 264.7 cells (bottom right) were stimulated with RANKL (100 ng/ml) for 5 days in the presence or absence of celastrol. Thereafter, culture supernatants of these cells were collected and analyzed for MMP activity. The results are representative of two independent experiments.
DISCUSSION
In this study, we have shown that celastrus/celastrol is effective in suppressing inflammation as well as bone erosion in rats with AA. In addition, we have elucidated the mechanisms underlying the antiresorptive effect of celastrus/celastrol on bones in the joints of arthritic rats. Compared with controls, celastrus/celastrol-treated arthritic rats showed marked reduction in the severity of clinical arthritis as well as pannus formation and mononuclear cell infiltration in the synovial tissue of the joints. Further analysis by bone histomorphometry showed that there was an increase in bone volume coupled with a reduction in osteoclast numbers in celastrus/celastrol-treated versus control rats. As discussed below, this protection against bone damage is mediated via modulation of the mediators of inflammation-induced bone damage.
Regulation of bone resorption by osteoclasts is essentially mediated via the RANKL/RANK/OPG system (8, 9, 11, 24, 43). We observed that celastrus/celastrol-treated rats had a decrease in serum RANKL levels along with a reduction in RANKL/OPG ratio compared with the control rats. Further, both celastrus and celastrol suppressed the expression of RANKL in SIC in vivo. However, in MC3T3 cells in vitro, OPG level was reduced without much change in RANKL level. Accordingly, the RANKL/OPG ratio was reduced following treatment with celastrus and celastrol. A reduction in RANKL production and the consequent decreased activation of RANK in turn inhibited osteoclastogenesis and bone resorption. Moreover, decreased RANKL/OPG ratio both locally and systemically shows that celastrol/celastrus restores the imbalance of RANKL/OPG production in AA. As far as other mediators of bone damage besides RANKL are concerned, we observed that celastrus/celastrol treatment caused a marked reduction in the three osteoclastic mediators produced by SIC, namely GM-CSF, IGF, and OPN. Furthermore, OCN, which is required for osteoblastic activity, was increased in sera but not SIC supernates. Thus, overall, celastrus and celastrol shifted the balance of mediators of bone remodeling in favor of antiosteoclastic activity.
Proinflammatory cytokines, such as IL-17, IL-6, TNF-α, IL-1, and IL-18, are modulators of osteoclastogenesis (8, 12, 26, 44, 45). These cytokines can induce the production of RANKL. Furthermore, the differentiation of osteoclasts is dependent on RANKL as well as M-CSF (46). In fact, M-CSF is the key molecule required for the survival of osteoclasts. IL-17 also can induce the expression of M-CSF in osteoblast-like cells and thus influence osteoclastogenesis (47). Our results show that celastrus/celastrol treatment leads to a marked reduction in the levels of proinflammatory cytokines in SIC (except for TNF-α in the celastrus-treated group) when compared with controls. The precise reasons for and impact of increased TNF-α in celastrus-treated rats are not yet clear. Celastrus extract has multiple components besides celastrol. In this regard, we propose that one or more of these other components might induce enhanced TNF-α production. Further, despite increased TNF-α, the severity of arthritis in these rats was reduced, apparently due to a significant reduction in IL-17. Overall, the inhibition of proinflammatory cytokine production by celastrus/celastrol parallels the reduction in RANKL and other osteoclastogenic mediators. On the basis of these results, we suggest that these natural products suppress RANKL production in part via targeting the proinflammatory cytokines that are upstream of RANKL in the osteoclastogenic pathway.
Another physiological modulator of RANKL activity is MMP-9, which has been shown to play an important role in bone remodeling (48). For example, MMP-9 activity is involved in the promotion of RANKL-induced osteoclastogenesis independent of NFATc1 signaling (48). Furthermore, IL-1β can stimulate FLS/osteoclasts and chondrocytes to produce MMPs that degrade cartilage (45). Our results show that celastrol blocks the production of both IL-1β and IL-1β-induced MMP-9. In addition, celastrol inhibited RANKL-induced MMP-9. Collectively, our results show that celastrol inhibited bone damage in part via down-regulating MMP-9, which represents an effector molecule downstream of RANKL in the osteoclastogenic pathway. Similar results have been reported using a different natural product, Genistein, an isoflavone (49). Genistein down-regulated RANKL production and MMP-9 activity and inhibited osteoclast formation, resulting in the inhibition of osteoclastic bone resorption and bone metastasis in cancer (49).
Taken together, the celastrus/celastrol-induced changes in the target organ (joints) in our study included the altered RANKL/OPG ratio in favor of OPG, the inhibition of osteoclastogenic mediators of bone remodeling (namely RANKL, GM-CSF, M-CSF, OPN, and IGF-1), and the suppression of proinflammatory cytokines (IL-1, IL-6, IL-17, and IL-18) and matrix-degrading enzyme (MMP-9) that promote bone damage. In comparison, changes in circulating (in serum) mediators of bone remodeling included the inhibition of RANKL as well as RANKL/OPG ratio but increased OCN levels. There was a slight reduction in MMP-9 activity following celastrol treatment but no effect after celastrus treatment. Further analysis of serum cytokines in celastrus/celastrol-treated rats versus controls showed no significant changes (data not shown). Thus, the study of joint-infiltrating cells and synovial fibroblasts revealed a much more comprehensive picture of the mechanism of action of celastrus/celastrol than that revealed by the circulating levels of the same mediators. These results highlight the significance of studying target organ biological processes in arthritis to unravel the mechanistic aspects of protection against bone damage by natural or synthetic products.
Other investigators have reported the beneficial effects of natural products against osteoclastogenesis and bone loss. For example, green tea has been shown to synergize with α-calcidol in affording protection against inflammation-induced bone loss in rats, and this effect was attributable to a reduction in TNF-α production as well as oxidative stress damage and inflammation (50, 51). Epigallocatechin gallate, a bioactive component of green tea, has been reported to inhibit IL-6, a critical proinflammatory cytokine involved in inflammation and bone damage in arthritis (52). Epigallocatechin gallate has also been shown to inhibit the formation and differentiation of osteoclasts via inhibition of MMPs (53). In another study, curcumin (from turmeric) was shown to inhibit overiectomy-induced bone loss by reducing osteoclastogenesis and impaired RANKL signaling (54). Other investigators have shown that silibinin, a flavonolignan, suppressed the induction of TRAP and cathepsin K as well as the activity of MMP-9 via perturbing the TNF receptor-associated factor 6 (TRAF6)-c-Src signaling pathways (41). While supporting the above studies, our report is the first to demonstrate the protective effect of celastrus/celastrol on bone damage in vivo in arthritic animals, and this effect is mediated via inhibition of RANKL and MMP-9.
Celastrol had mostly been credited for its anti-tumor activity (55–60) until the publication of our report describing that celastrus-derived celastrol had a beneficial effect against inflammatory autoimmune arthritis (as tested in the AA model) (30). Accordingly, most reports on the mechanism of action of celastrol were derived from studies performed in tumor models (55, 56, 58–60). In this context, our results showing the bone damage-protective effects of celastrus/celastrol in the AA model are supported by those of studies examining the effects of celastrol in tumor models. For example, celastrol was shown to suppress the osteolytic bone metastasis associated with experimentally induced breast cancer (61); celastrol suppressed trabecular bone loss and reduced the number and size of osteolytic bone lesions in rats (61). Furthermore, celastrol inhibited RANKL-induced signaling in osteoclasts, caused osteoclast apoptosis, and inhibited osteoclast formation (62). Taken together, the results of the studies performed in the AA model and the tumor models are complementary and supportive of each other. Considering that the pathophysiological milieu in an arthritic joint is quite different from that of a tumor site, it is remarkable that celastrol can afford protection against bone damage/bone loss in both of these settings. We suggest that the beneficial effect of celastrol in these two distinct yet related disease conditions is attributable in part to the fact that (a) inflammation is a component not only of autoimmune pathology but also of tumorigenesis (7, 63), and (b) the biochemical and immunological mediators of inflammation and bone damage are shared (the “osteoimmune cross-talk,” or “osteoimmunology”) (7, 8).
In summary, we demonstrate here that celastrus/celastrol inhibited inflammation-induced bone damage in arthritic rats by modulating the osteoimmune cross-talk. This modulation is composed primarily of (a) the inhibition of key proinflammatory cytokines (produced by the immune cells), which stimulate RANKL production and increase RANKL/OPG ratio, eventually leading to osteoclastogenesis; (b) the inhibition of the level/activity of matrix-degrading enzyme (MMP-9), which is the downstream effector biomolecule produced by proinflammatory cytokines as well as RANKL; and (c) the effect on the above two sets of mediators, resulting in the reduction of osteoclast numbers. We suggest that celastrus and celastrol are promising agents for use for the concurrent treatment of inflammation and bone damage associated with arthritis. Furthermore, these natural products should be evaluated in clinical studies for their use as adjuncts to conventional drugs for the treatment of RA.
Acknowledgments
We thankfully acknowledge the help of Lisa Hester for multiplex assays, David Jaffe for x-ray radiography, David Yoo for histomorphometric analysis, Carla Hebert for safranin-O staining, and Stefanie Vogel for providing the real-time PCR facility and RAW 264.7 cells. We also thank Rajesh Rajaiah and Brian Astry for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant R01AT004321.

This article contains supplemental Figs. 1–5.
- RA
- rheumatoid arthritis
- AA
- adjuvant-induced arthritis
- Mtb
- heat-killed M. tuberculosis H37Ra
- RANK
- receptor activator of nuclear factor-κB
- RANKL
- RANK ligand
- OPG
- osteoprotegerin
- OPN
- osteopontin
- OCN
- osteocalcin
- TRAP
- tartrate-resistant acid phosphatase
- SIC
- synovium-infiltrating cells
- MMP
- matrix metalloproteinase
- FLS
- fibroblast-like synoviocytes.
REFERENCES
- 1. Lipsky P. E. (2008) Rheumatoid arthritis. in Harrison's Principles of Intenrnal Medicine (Fauci A., Braunwald E., Kasper D. L., Hauser S. L., Longo D. L., Jameson J. L., Loscalzo J., eds) pp. 2083–2092, McGraw Hill, New York [Google Scholar]
- 2. Gorman C. L., Cope A. P. (2008) Immune-mediated pathways in chronic inflammatory arthritis. Best Pract. Res. Clin. Rheumatol. 22, 221–238 [DOI] [PubMed] [Google Scholar]
- 3. Hahn B. (2004) A pathophysiologic approach to the clinical management of arthritis and pain. Current and future implications. J. Clin. Rheumatol. 10, S3–S4 [DOI] [PubMed] [Google Scholar]
- 4. Steiner G., Tohidast-Akrad M., Witzmann G., Vesely M., Studnicka-Benke A., Gal A., Kunaver M., Zenz P., Smolen J. S. (1999) Cytokine production by synovial T cells in rheumatoid arthritis. Rheumatology 38, 202–213 [DOI] [PubMed] [Google Scholar]
- 5. Astry B., Harberts E., Moudgil K. D. (2011) A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J. Interferon Cytokine Res. 31, 927–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gravallese E. M., Manning C., Tsay A., Naito A., Pan C., Amento E., Goldring S. R. (2000) Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 43, 250–258 [DOI] [PubMed] [Google Scholar]
- 7. Jones D., Glimcher L. H., Aliprantis A. O. (2011) Osteoimmunology at the nexus of arthritis, osteoporosis, cancer, and infection. J. Clin. Invest. 121, 2534–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takayanagi H. (2007) Osteoimmunology. Shared mechanisms and cross-talk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304 [DOI] [PubMed] [Google Scholar]
- 9. Boyce B. F., Xing L. (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 473, 139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karmakar S., Kay J., Gravallese E. M. (2010) Bone damage in rheumatoid arthritis. Mechanistic insights and approaches to prevention. Rheum. Dis. Clin. North Am. 36, 385–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khosla S. (2001) Minireview. The OPG/RANKL/RANK system. Endocrinology 142, 5050–5055 [DOI] [PubMed] [Google Scholar]
- 12. Kotake S., Udagawa N., Takahashi N., Matsuzaki K., Itoh K., Ishiyama S., Saito S., Inoue K., Kamatani N., Gillespie M. T., Martin T. J., Suda T. (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schett G. (2006) Rheumatoid arthritis. Inflammation and bone loss. Wien. Med. Wochenschr. 156, 34–41 [DOI] [PubMed] [Google Scholar]
- 14. Xu S., Wang Y., Lu J., Xu J. (2011) Osteoprotegerin and RANKL in the pathogenesis of rheumatoid arthritis-induced osteoporosis. Rheumatol. Int., in press [DOI] [PubMed] [Google Scholar]
- 15. Maruotti N., Grano M., Colucci S., d'Onofrio F., Cantatore F. P. (2011) Osteoclastogenesis and arthritis. Clin. Exp. Med. 11, 137–145 [DOI] [PubMed] [Google Scholar]
- 16. Kremers H. M., Nicola P., Crowson C. S., O'Fallon W. M., Gabriel S. E. (2004) Therapeutic strategies in rheumatoid arthritis over a 40-year period. J. Rheumatol. 31, 2366–2373 [PubMed] [Google Scholar]
- 17. Fonseca J. E., Canhão H., Tavares N. J., Cruz M., Branco J., Queiroz M. V. (2009) Persistent low grade synovitis without erosive progression in magnetic resonance imaging of rheumatoid arthritis patients treated with infliximab over 1 year. Clin. Rheumatol. 28, 1213–1216 [DOI] [PubMed] [Google Scholar]
- 18. Joosten L. A., Helsen M. M., Saxne T., van De Loo F. A., Heinegard D., van Den Berg W. B. (1999) IL-1 αβ blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-α blockade only ameliorates joint inflammation. J. Immunol. 163, 5049–5055 [PubMed] [Google Scholar]
- 19. Gravallese E. M. (2002) Bone destruction in arthritis. Ann. Rheum. Dis. 61, ii84–ii86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lipsky P. E., van der Heijde D. M., St Clair E. W., Furst D. E., Breedveld F. C., Kalden J. R., Smolen J. S., Weisman M., Emery P., Feldmann M., Harriman G. R., Maini R. N. (2000) Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N. Engl. J. Med. 343, 1594–1602 [DOI] [PubMed] [Google Scholar]
- 21. Lubberts E., Joosten L. A., Chabaud M., van Den Bersselaar L., Oppers B., Coenen-De Roo C. J., Richards C. D., Miossec P., van Den Berg W. B. (2000) IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J. Clin. Invest. 105, 1697–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smolen J. S., Han C., Bala M., Maini R. N., Kalden J. R., van der Heijde D., Breedveld F. C., Furst D. E., Lipsky P. E. (2005) Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement. A detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum. 52, 1020–1030 [DOI] [PubMed] [Google Scholar]
- 23. Goltzman D. (2002) Discoveries, drugs, and skeletal disorders. Nat. Rev. Drug Discov. 1, 784–796 [DOI] [PubMed] [Google Scholar]
- 24. Hadjidakis D. J., Androulakis I. I. (2006) Bone remodeling. Ann. N.Y. Acad. Sci. 1092, 385–396 [DOI] [PubMed] [Google Scholar]
- 25. Zhao B., Ivashkiv L. B. (2011) Negative regulation of osteoclastogenesis and bone resorption by cytokines and transcriptional repressors. Arthritis Res. Ther. 13, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kolls J. K., Lindén A. (2004) Interleukin-17 family members and inflammation. Immunity 21, 467–476 [DOI] [PubMed] [Google Scholar]
- 27. Ohshima S., Kobayashi H., Yamaguchi N., Nishioka K., Umeshita-Sasai M., Mima T., Nomura S., Kon S., Inobe M., Uede T., Saeki Y. (2002) Expression of osteopontin at sites of bone erosion in a murine experimental arthritis model of collagen-induced arthritis. Possible involvement of osteopontin in bone destruction in arthritis. Arthritis Rheum. 46, 1094–1101 [DOI] [PubMed] [Google Scholar]
- 28. Franck H., Ittel T. H., Tasch O., Herborn G., Rau R. (1994) Osteocalcin in patients with rheumatoid arthritis. A one-year followup study. J. Rheumatol. 21, 1256–1259 [PubMed] [Google Scholar]
- 29. Hill P. A., Reynolds J. J., Meikle M. C. (1995) Osteoblasts mediate insulin-like growth factor-I and -II stimulation of osteoclast formation and function. Endocrinology 136, 124–131 [DOI] [PubMed] [Google Scholar]
- 30. Venkatesha S. H., Yu H., Rajaiah R., Tong L., Moudgil K. D. (2011) Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J. Biol. Chem. 286, 15138–15146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim D. H., Shin E. K., Kim Y. H., Lee B. W., Jun J. G., Park J. H., Kim J. K. (2009) Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur J. Clin. Invest. 39, 819–827 [DOI] [PubMed] [Google Scholar]
- 32. Tong L., Moudgil K. D. (2007) Celastrus aculeatus Merr. suppresses the induction and progression of autoimmune arthritis by modulating immune response to heat-shock protein 65. Arthritis Res. Ther. 9, R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu S., Sun C., Wang K., Pan Y. (2004) Preparative isolation and purification of celastrol from Celastrus orbiculatus Thunb. by a new counter-current chromatography method with an upright coil planet centrifuge. J. Chromatogr. A 1028, 171–174 [DOI] [PubMed] [Google Scholar]
- 34. Komeh-Nkrumah S. A., Nanjundaiah S. M., Rajaiah R., Yu H., Moudgil K. D. (2012) Topical dermal application of essential oils attenuates the severity of adjuvant arthritis in Lewis rats. Phytother. Res. 26, 54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rajaiah R., Puttabyatappa M., Polumuri S. K., Moudgil K. D. (2011) Interleukin-27 and interferon-γ are involved in regulation of autoimmune arthritis. J. Biol. Chem. 286, 2817–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang Y. H., Rajaiah R., Lee D. Y., Ma Z., Yu H., Fong H. H., Lao L., Berman B. M., Moudgil K. D. (2011) Suppression of ongoing experimental arthritis by a chinese herbal formula (huo-luo-xiao-ling dan) involves changes in antigen-induced immunological and biochemical mediators of inflammation. Evid. Based Complement Alternat. Med. 2011, 642027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu H., Yang Y. H., Rajaiah R., Moudgil K. D. (2011) Nicotine-induced differential modulation of autoimmune arthritis in the Lewis rat involves changes in interleukin-17 and anti-cyclic citrullinated peptide antibodies. Arthritis Rheum. 63, 981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sellers R. S., Peluso D., Morris E. A. (1997) The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J. Bone Joint Surg. Am. 79, 1452–1463 [DOI] [PubMed] [Google Scholar]
- 39. Chung D. J., Castro C. H., Watkins M., Stains J. P., Chung M. Y., Szejnfeld V. L., Willecke K., Theis M., Civitelli R. (2006) Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J. Cell Sci. 119, 4187–4198 [DOI] [PubMed] [Google Scholar]
- 40. Parfitt A. M., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R. (1987) Bone histomorphometry. Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2, 595–610 [DOI] [PubMed] [Google Scholar]
- 41. Kim J. L., Kang S. W., Kang M. K., Gong J. H., Lee E. S., Han S. J., Kang Y. H. (2012) Osteoblastogenesis and osteoprotection enhanced by flavonolignan silibinin in osteoblasts and osteoclasts. J. Cell Biochem. 113, 247–259 [DOI] [PubMed] [Google Scholar]
- 42. Grassi F., Cristino S., Toneguzzi S., Piacentini A., Facchini A., Lisignoli G. (2004) CXCL12 chemokine up-regulates bone resorption and MMP-9 release by human osteoclasts. CXCL12 levels are increased in synovial and bone tissue of rheumatoid arthritis patients. J. Cell Physiol. 199, 244–251 [DOI] [PubMed] [Google Scholar]
- 43. Shahrara S., Proudfoot A. E., Woods J. M., Ruth J. H., Amin M. A., Park C. C., Haas C. S., Pope R. M., Haines G. K., Zha Y. Y., Koch A. E. (2005) Amelioration of rat adjuvant-induced arthritis by Met-RANTES. Arthritis Rheum. 52, 1907–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dai S. M., Nishioka K., Yudoh K. (2004) Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis. Comparison with IL1 β and tumor necrosis factor α. Ann. Rheum. Dis. 63, 1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strand V., Kavanaugh A. F. (2004) The role of interleukin-1 in bone resorption in rheumatoid arthritis. Rheumatology 43, iii10–iii16 [DOI] [PubMed] [Google Scholar]
- 46. Asagiri M., Takayanagi H. (2007) The molecular understanding of osteoclast differentiation. Bone 40, 251–264 [DOI] [PubMed] [Google Scholar]
- 47. Zhang F., Wang C. L., Koyama Y., Mitsui N., Shionome C., Sanuki R., Suzuki N., Mayahara K., Shimizu N., Maeno M. (2010) Compressive force stimulates the gene expression of IL-17s and their receptors in MC3T3-E1 cells. Connect. Tissue Res. 51, 359–369 [DOI] [PubMed] [Google Scholar]
- 48. Franco G. C., Kajiya M., Nakanishi T., Ohta K., Rosalen P. L., Groppo F. C., Ernst C. W., Boyesen J. L., Bartlett J. D., Stashenko P., Taubman M. A., Kawai T. (2011) Inhibition of matrix metalloproteinase-9 activity by doxycycline ameliorates RANK ligand-induced osteoclast differentiation in vitro and in vivo. Exp. Cell Res. 317, 1454–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li Y., Kucuk O., Hussain M., Abrams J., Cher M. L., Sarkar F. H. (2006) Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-κB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 66, 4816–4825 [DOI] [PubMed] [Google Scholar]
- 50. Shen C. L., Yeh J. K., Cao J. J., Tatum O. L., Dagda R. Y., Wang J. S. (2010) Synergistic effects of green tea polyphenols and alphacalcidol on chronic inflammation-induced bone loss in female rats. Osteoporos. Int. 21, 1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shen C. L., Yeh J. K., Samathanam C., Cao J. J., Stoecker B. J., Dagda R. Y., Chyu M. C., Wang J. S. (2011) Protective actions of green tea polyphenols and alfacalcidol on bone microstructure in female rats with chronic inflammation. J. Nutr. Biochem. 22, 673–680 [DOI] [PubMed] [Google Scholar]
- 52. Ahmed S., Marotte H., Kwan K., Ruth J. H., Campbell P. L., Rabquer B. J., Pakozdi A., Koch A. E. (2008) Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production. Proc. Natl. Acad. Sci. U.S.A. 105, 14692–14697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oka Y., Iwai S., Amano H., Irie Y., Yatomi K., Ryu K., Yamada S., Inagaki K., Oguchi K. (2012) Tea polyphenols inhibit rat osteoclast formation and differentiation. J. Pharmacol. Sci. 118, 55–64 [DOI] [PubMed] [Google Scholar]
- 54. Kim W. K., Ke K., Sul O. J., Kim H. J., Kim S. H., Lee M. H., Kim H. J., Kim S. Y., Chung H. T., Choi H. S. (2011) Curcumin protects against ovariectomy-induced bone loss and decreases osteoclastogenesis. J. Cell Biochem. 112, 3159–3166 [DOI] [PubMed] [Google Scholar]
- 55. Kannaiyan R., Manu K. A., Chen L., Li F., Rajendran P., Subramaniam A., Lam P., Kumar A. P., Sethi G. (2011) Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3K/Akt signaling pathways. Apoptosis 16, 1028–1041 [DOI] [PubMed] [Google Scholar]
- 56. Kim Y., Kang H., Jang S. W., Ko J. (2011) Celastrol inhibits breast cancer cell invasion via suppression of NF-κB-mediated matrix metalloproteinase-9 expression. Cell Physiol. Biochem. 28, 175–184 [DOI] [PubMed] [Google Scholar]
- 57. Liu Z., Ma L., Zhou G. B. (2011) The main anticancer bullets of the Chinese medicinal herb, thunder god vine. Molecules 16, 5283–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mou H., Zheng Y., Zhao P., Bao H., Fang W., Xu N. (2011) Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria- and Fas/FasL-mediated pathways. Toxicol. In Vitro 25, 1027–1032 [DOI] [PubMed] [Google Scholar]
- 59. Tozawa K., Sagawa M., Kizaki M. (2011) Quinone methide tripterine, celastrol, induces apoptosis in human myeloma cells via NF-κB pathway. Int. J. Oncol. 39, 1117–1122 [DOI] [PubMed] [Google Scholar]
- 60. Yadav V. R., Prasad S., Sung B., Kannappan R., Aggarwal B. B. (2010) Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2, 2428–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Idris A. I., Libouban H., Nyangoga H., Landao-Bassonga E., Chappard D., Ralston S. H. (2009) Pharmacologic inhibitors of IκB kinase suppress growth and migration of mammary carcinosarcoma cells in vitro and prevent osteolytic bone metastasis in vivo. Mol. Cancer Ther. 8, 2339–2347 [DOI] [PubMed] [Google Scholar]
- 62. Idris A. I., Krishnan M., Simic P., Landao-Bassonga E., Mollat P., Vukicevic S., Ralston S. H. (2010) Small molecule inhibitors of IκB kinase signaling inhibit osteoclast formation in vitro and prevent ovariectomy-induced bone loss in vivo. FASEB J. 24, 4545–4555 [DOI] [PubMed] [Google Scholar]
- 63. Coussens L. M., Werb Z. (2002) Inflammation and cancer. Nature 420, 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]