Background: Toxic components of lipofuscin in the retina are proposed to arise from all-trans-retinal, a by-product of light detection.
Results: Lipofuscin precursors form from 11-cis-retinal; lipofuscin accumulation is independent of light exposure.
Conclusion: 11-cis-Retinal is the primary source of lipofuscin components.
Significance: 11-cis-Retinal may play a major role in the pathogenesis of macular degenerations.
Keywords: ABC Transporter, Aging, Fluorescence, Retina, Retinal Degeneration, Retinoid, 11-cis-Retinal, A2E, Lipofuscin
Abstract
The age-dependent accumulation of lipofuscin in the retinal pigment epithelium (RPE) has been associated with the development of retinal diseases, particularly age-related macular degeneration and Stargardt disease. A major component of lipofuscin is the bis-retinoid N-retinylidene-N-retinylethanolamine (A2E). The current model for the formation of A2E requires photoactivation of rhodopsin and subsequent release of all-trans-retinal. To understand the role of light exposure in the accumulation of lipofuscin and A2E, we analyzed RPEs and isolated rod photoreceptors from mice of different ages and strains, reared either in darkness or cyclic light. Lipofuscin levels were determined by fluorescence imaging, whereas A2E levels were quantified by HPLC and UV-visible absorption spectroscopy. The identity of A2E was confirmed by tandem mass spectrometry. Lipofuscin and A2E levels in the RPE increased with age and more so in the Stargardt model Abca4−/− than in the wild type strains 129/sv and C57Bl/6. For each strain, the levels of lipofuscin precursor fluorophores in dark-adapted rods and the levels and rates of increase of RPE lipofuscin and A2E were not different between dark-reared and cyclic light-reared animals. Both 11-cis- and all-trans-retinal generated lipofuscin-like fluorophores when added to metabolically compromised rod outer segments; however, it was only 11-cis-retinal that generated such fluorophores when added to metabolically intact rods. The results suggest that lipofuscin originates from the free 11-cis-retinal that is continuously supplied to the rod for rhodopsin regeneration and outer segment renewal. The physiological role of Abca4 may include the translocation of 11-cis-retinal complexes across the disk membrane.
Introduction
Lipofuscin, a fluorescent pigment, has been shown to accumulate with age in granules in postmitotic cells (1, 2), including the retinal pigment epithelium (RPE)3 of the eye (3–6). Lipofuscin is a complex mixture of partially digested lipid and protein components, with recent analysis showing that protein contributes ∼2% by weight (7). The best characterized components are bis-retinoids (8), with A2E, the product of the condensation of two molecules of all-trans-retinal with ethanolamine, being one of the most abundant (9, 10). As lipofuscin and A2E have been shown to be cytotoxic in vitro (9, 11–13), it has been suggested that their accumulation contributes to retinal degenerative diseases such as age-related macular degeneration (14, 15) and Stargardt disease (16, 17). Stargardt disease has been associated with mutations in the multidrug resistance family transport protein ABCA4 (18, 19). Mice that lack Abca4 exhibit a large increase in RPE lipofuscin and bis-retinoid, including A2E, accumulation (17, 20, 21).
Previous studies have shown that the accumulation of lipofuscin has two prerequisites as follows: 1) the generation of 11-cis-retinal, as Rpe65−/− animals have strongly reduced levels of lipofuscin (22); and 2) the phagocytosis of photoreceptor outer segments by the RPE (23, 24). The mechanism of A2E and lipofuscin accumulation in the RPE is not well understood, but it is likely that components generated from retinal in the outer segment are introduced to the RPE through phagocytosis of photoreceptors. According to current models (1, 8, 22), A2E formation begins with the photoactivation of rhodopsin, the initial step in the detection of light by photoreceptor cells. Rhodopsin photoactivation involves the isomerization of its 11-cis retinyl chromophore to all-trans (25). Subsequently, all-trans-retinal is released into the photoreceptor disk membrane. Once released, all-trans-retinal can covalently bind to the amine group of phosphatidylethanolamine (PE), forming N-retinylidene-PE (NRPE). Then a second molecule of retinal binds to NRPE, and one 14-methyl group forms an aromatic ring at the 15C position of the other retinyl chain (26, 27) generating N-retinylidene-N-retinylphosphatidylethanolamine (A2PE). A2PE can be digested by phospholipase D (27), cleaving the phospholipid portion of PE and producing A2E.
In the context of this model, ABCA4 is proposed to function as a transporter of N-retinylidene-PE that has been formed from the all-trans-retinal released by photoactivated rhodopsin (17). The transporter is proposed to flip N-retinylidene-PE from the intradiscal to the cytosolic side of the disk (17, 28, 29), making all-trans-retinal available for reduction to all-trans-retinol by retinol dehydrogenase. As such, deficiencies in ABCA4 function would lead to an accumulation in N-retinylidene-PE, driving the formation of the precursors of A2E and lipofuscin after photoactivation of rhodopsin.
The proposed models for lipofuscin and A2E accumulation in normal and Abca4-deficient retinas posit free all-trans-retinal as a necessary precursor, the generation of which requires light. We have therefore measured the levels of lipofuscin fluorescence and A2E in mice of different ages, reared either in dark or in cyclic light. We found no significant differences of lipofuscin or A2E levels between animals reared in the dark and those reared in cyclic light. This observation held for wild type as well as Abca4−/− animals, although levels of both lipofuscin and A2E were much higher in the Abca4-deficient mice. Conversely, lipofuscin levels were considerably lower, and A2E was undetectable in Rpe65−/− animals. These results demonstrate that light activation of the visual pigment is not necessary for the formation of lipofuscin or A2E, suggesting that other forms of retinal may be involved. Pursuing the potential identity of these forms of retinal, we have detected lipofuscin-like fluorophores in the outer segments of dark-adapted isolated rod photoreceptors, including those from dark-reared animals. Addition of 11-cis-retinal to intact wild type dark-adapted rods led to formation of lipofuscin-like fluorophores.
The results suggest that 11-cis-retinal is a major source for the formation of lipofuscin. They further suggest that N-11-cis-retinylidene-PE (11-cis-NRPE) may be a physiologically important substrate for ABCA4. The results are consistent with ABCA4 working in the same direction as other ABCA transporters (30), translocating the substrate away from the cytoplasm, i.e. from the cytosolic to the intradiscal leaflet of the disk membrane.
EXPERIMENTAL PROCEDURES
Animals
Wild type 129/sv and C57Bl/6, as well as Abca4−/− and Rpe65−/−, transgenic mice originated from established colonies at the Medical University of South Carolina. 129/sv and C57Bl/6 mice were originally obtained from Harlan Laboratories (Indianapolis, IN); to establish the colonies, breeding pairs of Abca4−/− and Rpe65−/− animals were generous gifts of Drs. G. H. Travis and T. M. Redmond, respectively. The background strain of the Abca4−/− animals was 129/sv. Animals of all strains were reared in cyclic light with a 12-h light cycle (06:00–18:00); 129/sv and Abca4−/− animals were also born and reared in the dark, in ventilated cabinets, exposed to dim red light only when checking on their health and for cage changes. Animal ages were 1–12 months. All animal procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and were consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. For experiments, animals were dark-adapted overnight and sacrificed under dim red light.
Dissection of RPE and Retina
Eyes were enucleated, cleaned of fat and muscle, and then hemisected at the level of the ora serrata under a mammalian physiological solution (in mmol/liter: 130 NaCl, 5 KCl, 0.5 MgCl2, 2 CaCl2, 25 hemisodium-HEPES, 5 glucose, pH 7.40). The procedure was carried out under infrared light. The dark-adapted state of the animals and the dissection under dim red light allowed the separation of the retina from the RPE with minimal cross-contamination (supplemental Fig. 1).
Flat Mounting for RPE Imaging
The lens, vitreous, and retina were carefully removed, and four shallow incisions were then made in the eyecup, which was flattened on a glass slide with the RPE facing upward. The eyecup was then gently covered with a glass coverslip.
Color Photography
Color photographs were taken on a Zeiss Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) using a ×63 oil immersion objective (NA = 1.4) with a Nikon D200 (Nikon, Inc., Melville, NY) digital camera. Fluorescence was excited with 450–490 nm light, and the emission was collected >510 nm.
RPE Fluorescence Measurement
Eyecups on slides were imaged on an SP2 Leica laser scanning confocal microscope (Leica Microsystems, Inc., Buffalo Grove, IL) using a ×10 lens (NA = 0.3) with 488 nm excitation, with the pinhole fully open, and emission collected from 565 to 725 nm. Fluorescence intensity was measured over a 3.2 × 3.2-mm square centered at the optic nerve, covering >80% of the total eyecup area. Using the same size area allowed for fluorescence comparison across eyes of different sizes and did not include the eyecup areas around the rim that were damaged during dissection. These areas typically had low levels of lipofuscin fluorescence, and their exclusion does not affect the conclusions of this study. Eyecup fluorescence was corrected for background by subtracting the fluorescence intensity of an area of equal size adjacent to the eyecup that did not contain any tissue.
Fluorescence levels varied significantly across eyes from animals of different strains or ages, necessitating the use of different detector settings. Thus, to allow comparisons across eyes, the total fluorescence for each eyecup was converted to “bead units” (BU) using InSpeckOrange (540/560) microscope image calibration kit beads (Invitrogen). Lipofuscin fluorescence is reported as “bead units per megapixel” (BU/MP). The area of one megapixel is equal to 2.44 mm2. For each age, three animals providing six eyecups were used.
Lipofuscin granule spectra were obtained from the same eyes with a ×63 oil immersion lens (NA = 1.4) using 488 nm excitation and measuring the emission across the range from 515 to 739 nm (bandwidth 14 nm). The pinhole aperture was reduced to ∼1 Airy to eliminate as much as possible the inclusion of nongranule tissue fluorescence. Spectra were corrected for background by subtracting the fluorescence intensities of a spectrum of a tissue area from the same field that did not contain granules.
A2E Quantification
After enucleation, hemisection, and removal of the lens, vitreous, and retina, the RPE-choroids were collected by gently scraping them off the sclera in mammalian physiological buffer solution. For each experiment, two to six eyecups were used; higher numbers of eyecups were needed in cases of younger animals, which contain lower A2E amounts. Excess buffer was removed by centrifuging the solutions in a tabletop centrifuge (14,000 rpm; Eppendorf Centrifuge 5415C; Eppendorf, AG, Hamburg, Germany). The RPE-choroid tissue pellets were homogenized in 1.5 ml of phosphate buffered saline with 3 ml of 1:1 chloroform/methanol; 1 ml of chloroform, and 1 ml of methylene chloride were subsequently added to the homogenate. After mixing, the homogenate was centrifuged in a tabletop clinical centrifuge, and the organic phase was collected and dried under argon.
HPLC experiments were repeated three times. For each experiment, a dried extract from two to six eyecups was suspended in methanol with 0.1% trifluoroacetic acid (TFA) and analyzed with a Waters 1525 binary HPLC (Waters Corp., Milford, MA) using a reverse phase gradient from 85% acetonitrile with 0.1% TFA and 15% water with 0.1% TFA to 100% acetonitrile/TFA. Absorbance was monitored by a Waters 2998 PDA detector, and the A2E peak was determined by retention time and absorbance spectrum through comparison with a synthetic A2E standard (10). The peak was collected under dim red light and subjected to LC-MS/MS to confirm identification and for quantification of A2E by published methods (31). HPLC experiments with retinas separated from the RPE were carried out the same way.
Rod Outer Segment Membrane Preparation
Mouse rod outer segment membranes were prepared from isolated mouse retinas with a sucrose density flotation method (32). The supernatant containing the rod outer segment membranes and the pellet were collected separately and processed for HPLC analysis.
Fluorescence Imaging of Isolated Rods
Single, living isolated rod photoreceptors were obtained and imaged as described previously (33). Lipofuscin-like fluorescence was measured with 490 nm excitation and collecting emission >515 nm. Retinol fluorescence was measured with 360 nm excitation and collecting emission >420 nm. All-trans- and 11-cis-retinal were delivered using 1% bovine serum albumin (BSA) as carrier. Experiments were carried out at 37 °C. Fluorescence emission spectra of isolated rod outer segments were measured in the SP2 Leica laser scanning confocal microscope, at room temperature. Isolated cells were applied to poly-l-lysine-coated (0.01%; diluted with distilled water from 0.1% solution, Sigma) superfrost microscope slides (Fisherbrand, Fisher), allowed to settle for 2–5 min, and then covered with coverslips. Fluorescence spectra were measured using the same method as for lipofuscin granule spectra but with the pinhole fully open. All preparation and measurements were performed under dim red light. All reagents were of analytical grade; organic solvents were HPLC grade.
Statistical Analysis
Statistical significance was tested with analysis of variance. In the figures, statistically significant differences are indicated with an asterisk.
RESULTS
Lipofuscin Is Present in the RPE of Dark-reared Animals
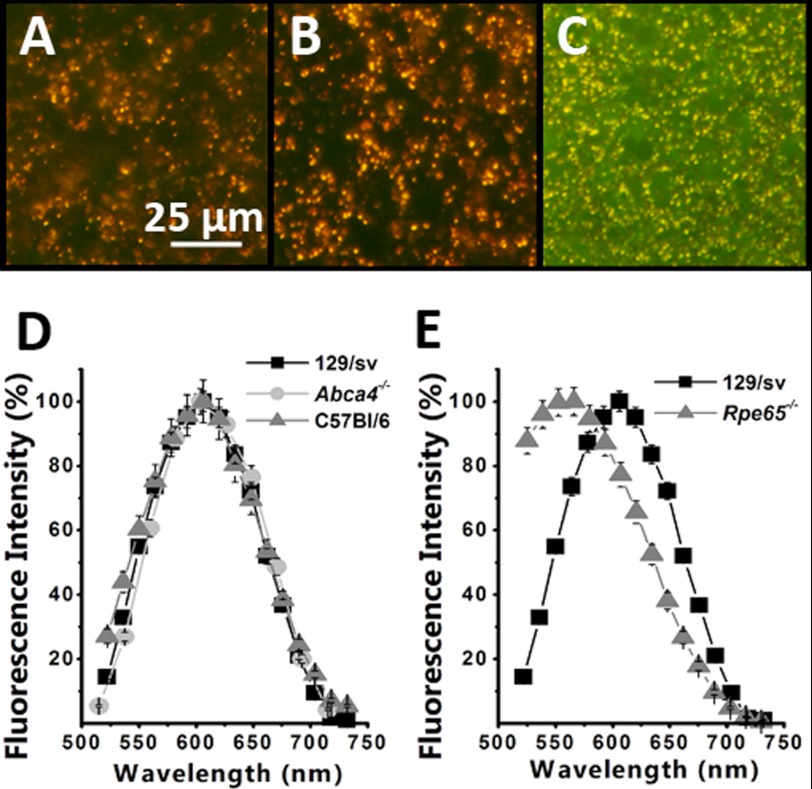
Fluorescence images (excitation 450–490 nm) of RPE from dark-reared wild type and Abca4−/− animals reveal the presence of the characteristic orange lipofuscin granules (Fig. 1, A and B). The fluorescence emission spectra of these granules collected with excitation 488 nm (Fig. 1D) peak at ∼610 nm. Although the number and size of granules increased with age, the color of the granules and their emission spectra were essentially the same for animals reared in cyclic light and continuous darkness and did not depend on age or the strain for 129/sv, C57BL/6, and Abca4−/− mice.
FIGURE 1.
Accumulation of fluorescent granules in the RPE of dark-reared and Rpe65−/− animals. Color images of the fluorescence (excitation 450–490 nm) emitted by flat-mounted RPEs from 3-month-old dark-reared 129/sv (A), 2-month-old dark-reared Abca4−/− (B), and 16-month-old cyclic light-reared Rpe65−/− (C). The orange-gold color of the granules in the wild type and Abca4−/− animals is characteristic of RPE lipofuscin, and the color of the granules in the Rpe65−/− is green-yellow. Emission spectra of the granule fluorescence (excitation 488 nm) were from dark-reared 12-month-old 129/sv (n = 130 granules), C57Bl/6 (n = 42 granules), and Abca4−/− (n = 187 granules) (D) and dark-reared 12-month-old 129/sv (data from D) and cyclic light-reared Rpe65−/− (n = 95 granules) (E). The emission spectra of the granules from wild type and Abca4−/− animals peak ∼610 nm and are characteristic of RPE lipofuscin, although those from Rpe65−/− animals are shifted to shorter wavelengths, peaking ∼550 nm. Error bars represent S.E.
Interestingly, the RPE from cyclic light-reared Rpe65−/− mice, which do not produce 11-cis-retinal and hence do not form visual pigments, also showed accumulation of fluorescent granules (Fig. 1C). However, the color of the fluorescence of these pigments was yellow-green and their emission spectra had a peak at ∼550 nm (Fig. 1E). The fluorescence from Rpe65−/− mouse eyecups was much weaker than that from wild type and Abca4−/−animals, requiring longer exposure times, which also revealed the background tissue fluorescence (green) in the image in Fig. 1C. The number of fluorescent granules increased with age, but their emission spectrum did not change appreciably.
RPE Lipofuscin Levels Increase with Age Regardless of Light Exposure
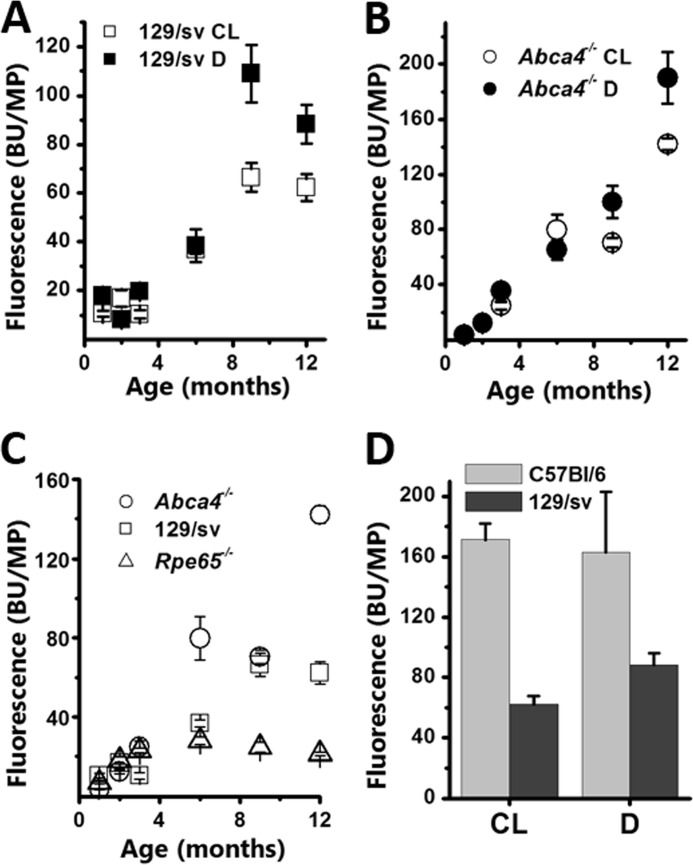
Because the distribution of lipofuscin fluorescence is not necessarily homogeneous across the RPE, we measured lipofuscin levels by collecting the fluorescence from a whole flat-mounted eyecup. Eyecup fluorescence increased approximately linearly with age in both cyclic light- and dark-reared wild type 129/sv mice (Fig. 2A). In 1-month-old cyclic light-reared animals, eyecup fluorescence was 10.7 ± 1.2 BU/MP. This fluorescence accumulated steadily with age at a rate of 5.6 ± 0.9 BU/MP/month to reach 62.2 ± 5.5 BU/MP in 12-month-old cyclic light-reared animals. In dark-reared animals, fluorescence increased at a rate of 8.7 ± 2.1 BU/MP/month, from 17.8 ± 0.2 BU/MP in 1-month-old to 88.3 ± 7.9 BU/MP in 12-month-old mice. Similarly, eyecup fluorescence increased linearly with age in both cyclic light- and dark-reared Abca4−/− mice (Fig. 2B), with rates of 11.6 ± 1.7 BU/MP/month and 15.7 ± 1.7 BU/MP/month, respectively. These rates were both substantially higher than those for wild type mice. The eyecup fluorescence levels in 1-month-old animals were 3.9 ± 0.3 and 3.6 ± 0.2 BU/MP for dark- and cyclic light-reared, respectively, both lower than those in wild type. By 12 months, the levels increased to 190.2 ± 18.5 and 142.1 ± 4.4 BU/MP, much higher than wild type. In Rpe65−/−animals, fluorescence levels showed only a modest increase with age, from 7.0 ± 0.6 at 1 month to 28.0 ± 1.9 BU/MP at 6 months (Fig. 2C). Then they declined slightly to 21.3 ± 1.0 BU/MP by 12 months. For most ages, the levels of fluorescence in Rpe65−/− mice were much lower than in wild type and in Abca4−/− animals (Fig. 2C).
FIGURE 2.
Total RPE fluorescence (excitation, 488 nm; emission, 565–725 nm) increases with age in both cyclic light-reared (CL) and dark-reared (D) wild type and Abca4−/− animals. A, similar increases in total RPE fluorescence in cyclic light- and dark-reared 129/sv animals. B, similar increases in total RPE fluorescence in cyclic light- and dark-reared Abca4−/− animals. C, total RPE fluorescence increases with age more in cyclic light-reared Abca4−/− than 129/sv and Rpe65−/− mice. D, total RPE fluorescence is similar in cyclic light- and dark-reared C57Bl/6 mice (12-month-old) and is higher than in 129/sv mice of the same age. Error bars represent S.E.
Substantial RPE fluorescence levels were also found in C57BL/6 mice, which have a much slower rate of rhodopsin regeneration than 129/sv due to the presence of a Rpe65 L450M variation (34). The levels were 171.3 ± 10.6 and 163.4 ± 39.4 BU/MP in 12-month-old cyclic light- and dark-reared mice, respectively (Fig. 2D). The data for the cyclic light-reared C57BL/6 mice have been re-plotted from Ref. 35 for comparison.
RPE A2E Content Increases with Age Regardless of Light Exposure
The intensity of lipofuscin fluorescence does not necessarily reflect the mass of accumulated material. Because the overall composition of lipofuscin is not known, we have used a major as well as the best characterized component, A2E, as an indicator of mass accumulation. In addition, the A2E fluorescence emission spectrum is similar to that of lipofuscin, and a specific model for its generation has been proposed.
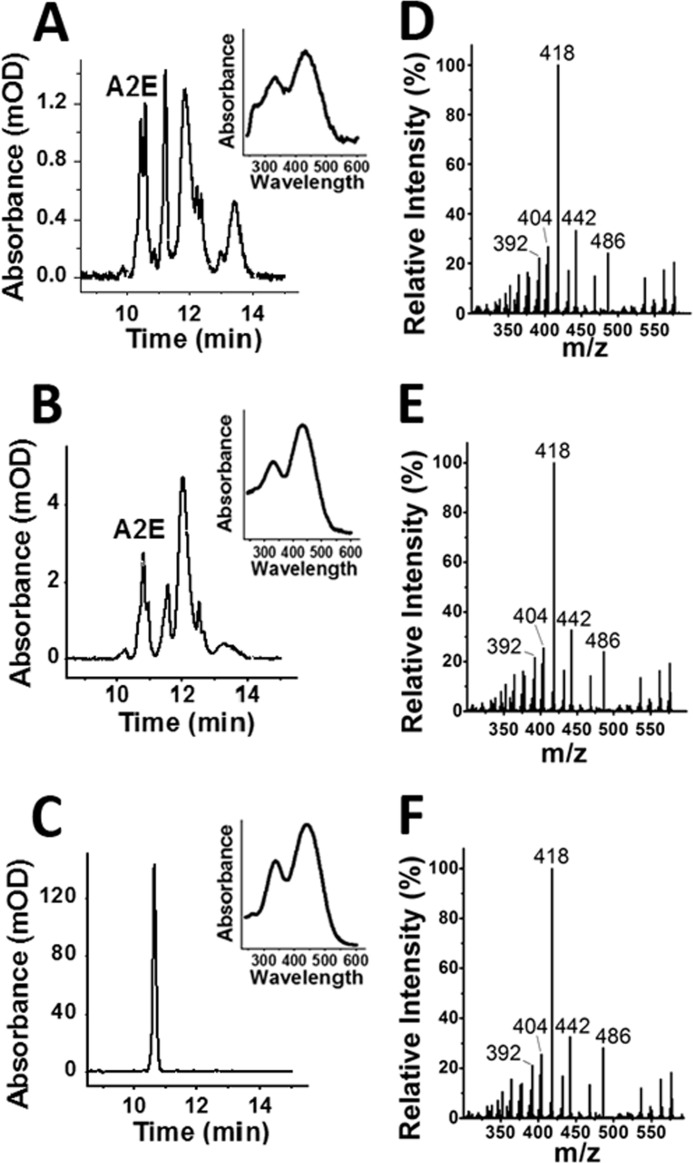
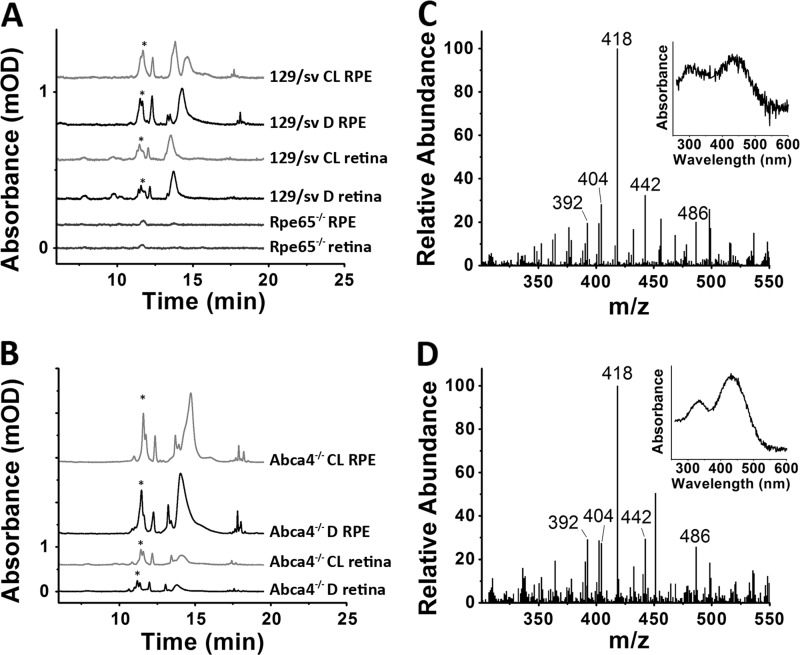
Chromatograms of organic extracts of RPE-choroid tissue from dark-reared 129/sv (Fig. 3A) and Abca4−/− (Fig. 3B) animals show the presence of large amounts of A2E, as judged by the absorption spectrum and comparison with the chromatogram of a synthetic standard (Fig. 3C). To confirm that the compound is A2E, the HPLC fraction eluting between 10 and 11 min was collected and analyzed with LC-MS/MS. A 592 m/z peak characteristic for A2E is present and upon fragmentation gives for both 129/sv (Fig. 3D) and Abca4−/− (Fig. 3E) patterns that are identical to that for synthetic A2E (Fig. 3F). The prominent 418 ion can also be used to accurately quantify A2E in the HPLC fraction (31). The total amount of A2E determined from the 418 ion was ∼40–50% of the amount determined from chromatographic absorption spectroscopy. This proportion was the same for extracts from dark-reared and cyclic light-reared animals and did not change significantly with age.
FIGURE 3.
Bis-retinoid A2E is present in the RPE of dark-reared wild type and Abca4−/− animals. Chromatograms at 430 nm of RPE-choroid extracts were from 6-month-old dark-reared 129/sv (A), 4-month-old dark-reared Abca4−/− (B), and a synthetic A2E standard (C). The insets show the absorbance spectra of the major peak with retention time at 10.4–10.8 min, characteristic of A2E. Nano-LC-MS/MS mass spectrometry fragmentation patterns of the HPLC fraction gathered from 10 to 11 min from 6-month-old dark-reared 129/sv (D), 4-month-old dark-reared Abca4−/− (E), and a synthetic A2E standard (F). Both samples demonstrate the characteristic fragmentation pattern for A2E, with major peaks at 392, 404, 418, 442, and 486 m/z.
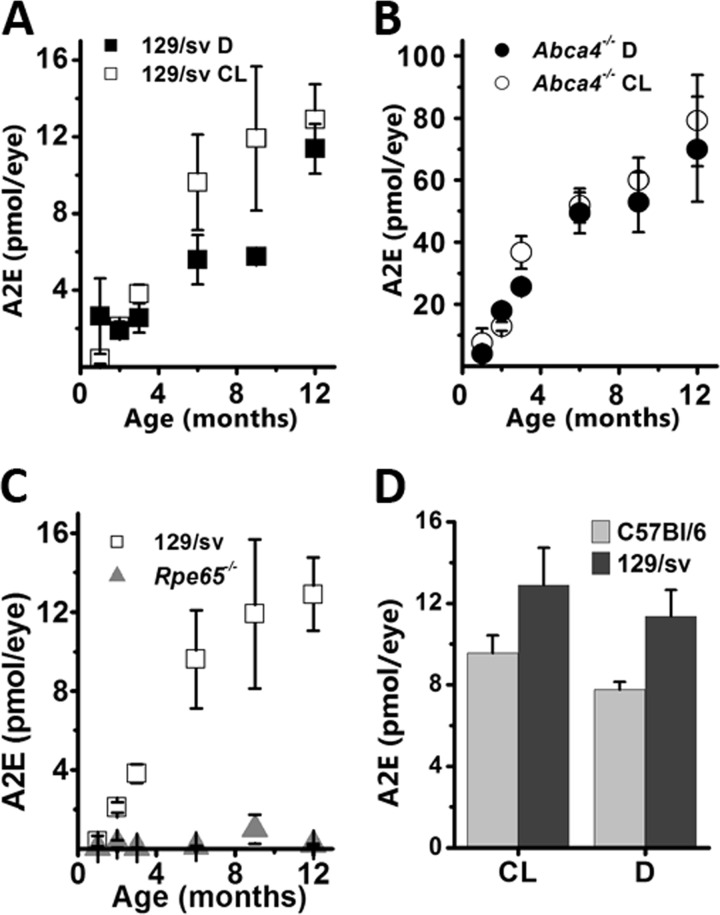
The amount of A2E increased in cyclic light- and dark-reared 129/sv mice from 0.4 ± 0.3 and 2.6 ± 2.0 pmol/eye at 1 month to 12.9 ± 1.9 and 11.4 ± 1.3 pmol/eye at 12 months. A2E accumulated in a linear fashion with rates of 1.2 ± 0.2 and 0.8 ± 0.1 pmol/eye/month in cyclic light- and dark-reared animals, respectively (Fig. 4A). In Abca4−/− mice, the levels of A2E were higher than in wild type at all ages, in both cyclic light- and dark-reared animals, beginning from 7.5 ± 4.6 and 4.0 ± 1.3 pmol/eye at 1 month, reaching 79.2 ± 14.7 and 70.0 ± 17.0 at 12 months, respectively. A2E accumulated with age at significantly higher rates in Abca4−/− than in wild type, 6.2 ± 0.9 (cyclic light) and 5.6 ± 0.7 (dark) pmol/eye/month (Fig. 4B).
FIGURE 4.
A2E levels increase with age in the RPE of cyclic light-reared (CL) and dark-reared (D) wild type and Abca4−/− animals. A, similar increases in total A2E levels in cyclic light- and dark-reared 129/sv animals. B, similar increases in total A2E levels in cyclic light- and dark-reared Abca4−/− animals. C, A2E is virtually undetectable in all ages of cyclic light-reared Rpe65−/− compared with cyclic light-reared 129/sv mice. D, total A2E RPE levels are similar in cyclic light- and dark-reared C57BL6 mice (12-month-old), and slightly lower than in 129/sv mice of the same age.
A2E was undetectable in RPE samples from cyclic light-reared Rpe65−/− mice of 1–12 months of age. For reporting purposes, a nominal level of A2E was measured by integrating the chromatogram intensity from 10 to 11 min, covering the retention time of the synthetic A2E standard (Fig. 4C). A2E in these samples was undetectable even with LC-MS/MS, which can detect femtomole levels.
Significant A2E accumulation takes place in cyclic light- and dark-reared C57Bl/6 mice as well, reaching 9.6 ± 0.9 and 7.8 ± 0.4 pmol/eye, respectively, at 12 months of age (Fig. 4D). The data for the cyclic light-reared C57BL/6 mice have been re-plotted from Ref. 35 for comparison.
Lipofuscin-like Fluorophores in Rod Outer Segments Originate from 11-cis-Retinal
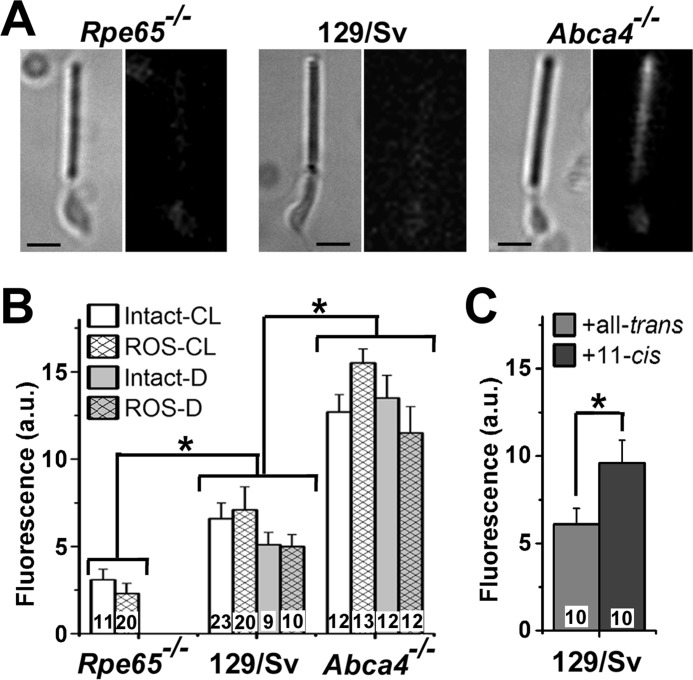
Because lipofuscin is expected to originate in the outer segments of rod photoreceptors, we examined isolated rods for the presence of lipofuscin-like fluorophores. Fluorescence images (excitation 490 nm; emission >515 nm) of dark-adapted rod photoreceptors show the presence of fluorophores in the outer segment (Fig. 5, A and B). The intensity of this fluorescence is the same in the outer segments of intact cells and broken off rod outer segments and independent of whether the animals were reared in cyclic light or in darkness. The fluorescence intensity was higher in Abca4−/− than wild type (p < 0.002), and higher in wild type than in Rpe65−/− (p < 0.05) rod outer segments (Fig. 5B). The emission spectra of this fluorescence signal are consistent with those of the lipofuscin granules found in the RPE of the respective strains (Fig. 6). Because the fluorophores responsible for this signal are present in the outer segments of dark-adapted cells from dark-reared animals, it is unlikely that their source is all-trans-retinal; 11-cis is a much more likely candidate. Indeed, addition of moderate (5 μm) concentrations of 11-cis-retinal to intact wild type rod photoreceptors resulted in an increase in outer segment fluorescence compared with the addition of all-trans (p < 0.04) (Fig. 5C). The addition of all-trans-retinal did not result in a significant increase in outer segment fluorescence (Fig. 5C).
FIGURE 5.
Lipofuscin-like fluorophores in rod outer segments. A, bright field (left) and fluorescence (right; excitation, 490 nm; emission, >515 nm) images of dark-adapted metabolically intact rod photoreceptors from 3-month-old Rpe65−/− (cyclic light-reared), 129/sv (dark-reared), and Abca4−/− (dark-reared) mice. Fluorescence images are shown at the same scaling. Bars, 5 μm. B, outer segment lipofuscin-like fluorescence levels in dark-adapted metabolically intact rods (Intact) and metabolically compromised broken off rod outer segments (ROS) from dark-reared (D) and cyclic light-reared (CL) mice. C, exposure of dark-adapted metabolically intact rods to 5 μm all-trans- or 11-cis-retinal for 5 min. All experiments were at 37 °C. Numbers of cells are shown within each column. Error bars represent standard errors.
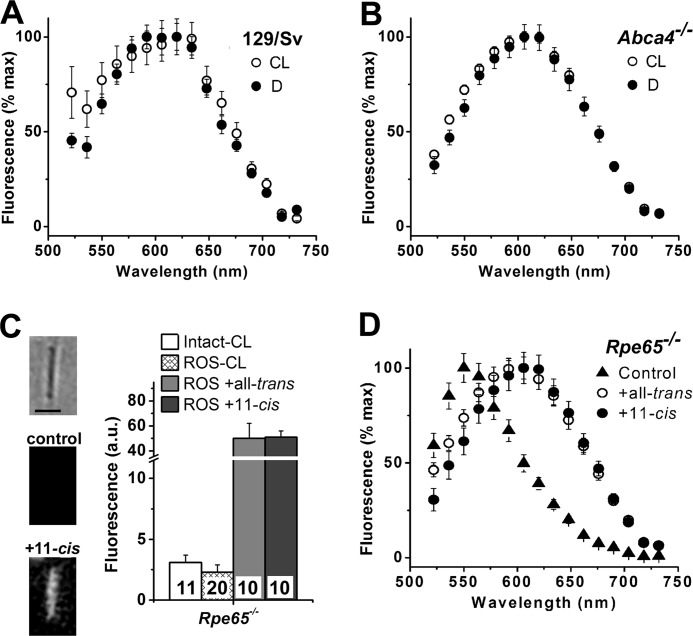
FIGURE 6.
Emission spectra of rod outer segment fluorescence. Dark-adapted metabolically compromised broken off rod outer segments from cyclic light-reared (CL) and dark-reared (D) mice were used in all experiments. Fluorescence was excited by 488 nm (A, B, and D) or 490 nm (C) light. A, 129/sv ROS (CL, n = 25; D, n = 99). B, Abca4−/− ROS (CL, n = 78; D, n = 51). C, addition of 50 μm all-trans- or 11-cis-retinal for 5 min to broken off ROS from Rpe65−/− mouse retinas results in a large increase in fluorescence (emission >515 nm). Experiments were done at 37 °C. Numbers of cells are shown within each column. Image insets show a bright field and fluorescence images of a broken off ROS before and after the addition of 11-cis-retinal. Fluorescence images are shown at the same scaling. Bar, 5 μm. Fluorescence levels for control “Intact” and “ROS” are from Fig. 5B and are re-plotted for comparison purposes. D, emission spectra of dark-adapted Rpe65−/− broken off ROS (control, n = 200) have a peak ∼550 nm. The spectra shift to longer wavelengths and have a peak ∼610 nm, 15 min after the addition of 7 μm all-trans-retinal (n = 82) or 11-cis-retinal (n = 47). Experiments were done at room temperature. In all panels, error bars denote standard errors.
The fluorescence emission spectra of dark-adapted rod outer segments from cyclic light- and dark-reared wild type and Abca4−/− animals were the same and peaked ∼610 nm (Fig. 6, A and B). Rod outer segments from Rpe65−/− mice had low levels of fluorescence (Fig. 5B), and their fluorescence emission spectrum was shifted to lower wavelengths, peaking ∼550 nm (▴, Fig. 6D). Addition of all-trans- or 11-cis-retinal to broken off Rpe65−/− rod outer segments resulted in a large increase in fluorescence (Fig. 6C), with a shift in the fluorescence emission spectrum to longer wavelengths, with a peak ∼610 nm (Fig. 6D).
The identity of the fluorophores with the lipofuscin-like emission spectra has not been established, but they are clearly by-products of retinal. HPLC analysis of organic extracts of retinas separated from the RPE show the presence of several species absorbing >400 nm, including A2E, in tissues from both cyclic light- and dark-reared animals (Fig. 7, A and B). The identity of A2E was confirmed from its absorbance spectrum and with tandem mass spectrometry (Fig. 7, C and D). Most of the A2E in the retina was present in the rod outer segments, as >85% (measured per mg of protein) of it was found in the rod outer segment membrane fraction of a sucrose density centrifugation of homogenates of isolated retinas. A detailed characterization of the levels of A2E in the retina and their change with age was not carried out. The 129/sv retina A2E levels measured with HPLC were lower than those found in the RPE, 1.7 ± 0.2 pmol/retina (3-month-old cyclic light-reared animals). Higher levels of A2E, 5.9 ± 1.0 pmol/retina (3-month-old cyclic light-reared animals), were found in retinas from Abca4−/− animals. Similar levels, 1.3 ± 0.1 pmol/retina (129/sv) and 4.7 ± 1.2 pmol/retina (Abca4−/−), were found in 3-month-old dark-reared animals.
FIGURE 7.
A2E is present in the retina of cyclic light-reared (CL) and dark-reared (D) wild type and Abca4−/− animals. HPLCs (430 nm absorbance) of extracts from 3-month-old 129/sv and Rpe65−/− (A) or Abca4−/− (B) animals. The A2E peak is indicated with an asterisk. Absorbance values have been scaled to correspond to one eyecup or retina. Nano-LC-MS/MS mass spectrometry fragmentation patterns of the HPLC fraction gathered from 10 to 11 min from the retina extracts of dark-reared 129/sv (C) and Abca4−/− (D) animals are consistent with A2E.
The substances absorbing >400 nm were present in the retinas of both cyclic light- and dark-reared animals and in both wild type and Abca4−/− animals. They were not present, however, in extracts from Rpe65−/− tissues, either retina or RPE, corroborating their retinoid origin (Fig. 7A); the peaks appearing in these chromatograms show absorbance peaks <350 nm. It is unlikely that the presence of these substances in retina extracts represents contamination from the RPE, as was most evident by comparing the HPLCs at 510 nm (supplemental Fig. 1); their relative amounts differ between the retina and the RPE, and there are peaks detected in one but not the other.
Lipofuscin-like Fluorophores in Rod Outer Segments Can Form from All-trans-retinal
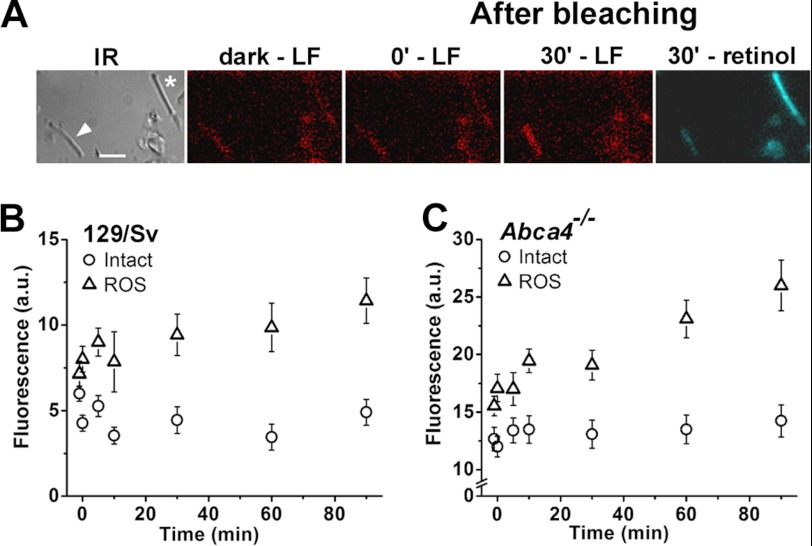
Fig. 6C demonstrates that lipofuscin-like fluorophores can form in rod outer segments upon the addition of exogenous all-trans-retinal. We examined whether these fluorophores can also form from the all-trans-retinal released from photoactivated rhodopsin after light excitation. In the outer segments of metabolically intact rod photoreceptors, most of the endogenously released all-trans-retinal is converted to all-trans retinol (33, 36); in the metabolically compromised broken off rod outer segments, however, it is not (37). The fluorescence images in Fig. 8A show that lipofuscin-like fluorophores form in a wild type broken off rod outer segment following exposure to light. By contrast, there is no detectable formation of these fluorophores in the outer segment of a metabolically intact rod, where retinol forms instead (Fig. 8A). The emission spectra of the fluorescence of bleached broken off rod outer segments are the same as those obtained with additions of exogenous all-trans-retinal shown in Fig. 6D (data not shown). Fig. 8B shows the kinetics of the increase in lipofuscin-like fluorescence in wild type broken off rod outer segments and the lack of any detectable increase in the outer segments of metabolically intact rods. Interestingly, the same results are obtained with rods from Abca4−/− mice (Fig. 8C), with no detectable fluorescence increase in metabolically intact rods. Compared with wild type, the Abca4−/− cells had higher overall levels of fluorescence (see also Fig. 5B). The rate of fluorescence increase in broken off rod outer segments after bleaching was 0.11 ± 0.02 units/min for Abca4−/−, almost three times as large as the rate of 0.04 ± 0.01 units/min for wild type.
FIGURE 8.
Formation of lipofuscin-like fluorophores in rod outer segments after light exposure. A, panels show images from an experiment with a metabolically intact rod (*) and a metabolically compromised broken off rod outer segment (arrowhead). IR, infrared image of dark-adapted cells; bar, 5 μm. Fluorescence images before (dark), immediately after, and 30 min after bleaching. LF, lipofuscin-like fluorescence (excitation, 490 nm; emission, >515 nm) in red pseudocolor. Retinol fluorescence (excitation 360 nm; emission >420 nm) in blue pseudocolor. Changes in level of the lipofuscin-like fluorescence with time after bleaching are shown in B for 129/sv metabolically intact (Intact, n = 11) and metabolically compromised (ROS, n = 7) and are shown in C for Abca4−/− metabolically intact (Intact, n = 12) and metabolically compromised (ROS, n = 13) rod outer segments. Bleaching was carried out between t = −1 and 0 min with >530 nm light from a 150-watt halogen lamp illuminator. All experiments at 37 °C with cells from cyclic light-reared animals. Error bars denote standard errors.
DISCUSSION
Lipofuscin and A2E Accumulate in RPE in the Absence of Light Exposure
The results of Figs. 1–4 demonstrate that lipofuscin and A2E accumulate in the RPE of wild type and Abca4-deficient mice in the absence of significant light exposure. The amounts of A2E (pmol/eye) measured in this study are in broad agreement with the amounts reported previously from wild type or Abca4−/− animals reared in cyclic light (20, 21, 38). Unfortunately, no direct comparison with previous studies is possible for total eyecup levels of lipofuscin, as it is difficult to compare fluorescence measurements across different studies utilizing different procedures and instruments. Nevertheless, the present results are in qualitative agreement with the previous observations of RPE lipofuscin fluorescence increasing with age (20, 23). The striking observation in this study is that absence of light exposure did not affect significantly the levels and rates of lipofuscin and A2E accumulation for a time period of up to 12 months (Figs. 2 and 4). This observation held for the 129/sv and C57BL/6 as well as for the Abca4−/− mice. This finding is inconsistent with the proposed models for lipofuscin and A2E formation (21, 22) and directly contradicts previous reports of a lack of A2E level increases in animals reared in darkness (21, 38).
A possible explanation for the discrepancy is the underestimation of the A2E levels in dark-reared animals previously, because the complexity of the chromatograms of RPE extracts can hamper the quantification of A2E from its absorbance. In this study, selected monitoring of a fragment (MS/MS) ion was used to confirm the identity of A2E and provide an independent quantification method with tandem mass spectra (Fig. 3). For estimating lipofuscin accumulation, previous studies relied on fluorescence measurements from selected representative areas. The distribution of lipofuscin fluorescence does not appear to be homogeneous over the whole eyecup (see images in Ref. 39), and observations under high magnification reveal variable granule accumulation even in neighboring cells (data not shown). Because of this variability, the total eyecup fluorescence is a better measure of overall lipofuscin accumulation. An important concern with the use of total eyecup fluorescence instead of a selected area is tissue damage during processing. Care was exercised during eyecup dissection and processing to limit any damage to the edge. The effects of any tissue damage are expected to contribute no more than 20% on the reported values of lipofuscin fluorescence (see “Experimental Procedures”).
Lipofuscin and A2E Accumulate in the RPE of Mice of Different Strains
In accordance with previous reports (20, 21, 23), this study found that lipofuscin (Fig. 2C) and A2E levels (Fig. 4, A and B) increased severalfold faster with age in the RPE of Abca4−/− mice than wild type. We have extended this observation to include measurement of lipofuscin and A2E accumulation in dark-reared animals. Specifically, in 129/sv wild type animals, eyecup fluorescence increased at a rate of 5.6–8.7 BU/MP/month, and in Abca4−/−with a rate about two times higher, 11.6–15.7 BU/MP/month. For A2E, the rate of increase was 0.8–1.2 pmol/eye/month in 129/sv, but almost six times higher, 5.6–6.2 pmol/eye/month in Abca4−/−. The lack of correspondence in the rates of increase of lipofuscin and A2E when comparing the two strains is not necessarily surprising. Although the emission spectrum of A2E is similar to that of lipofuscin, it represents only a single component. In addition, the intensity of lipofuscin fluorescence does not necessarily reflect the mass of accumulated material, and finally, it is likely that total eyecup fluorescence includes contributions from nonlipofuscin fluorophores.
This incongruence between lipofuscin and A2E levels is also seen when comparing the two wild type strains, 129/sv and C57BL/6 (Figs. 2D and 4D); although eyecup fluorescence is higher in C57BL/6 animals, the A2E levels are lower. The lower A2E levels in the C57BL/6 mice compared with 129/sv (Fig. 4D) are in some sense consistent with the slower rate of generation of 11-cis-retinal in these animals (34, 40). This difference, however, is not proportional to the severalfold difference in the Rpe65 enzyme activity (40).
Eyecup fluorescence increases modestly with age up to 6 months in Rpe65−/−animals. The fluorescence levels in this strain are much lower than those in wild type and Abca4−/− animals (Fig. 2C), in agreement with the undetectability of lipofuscin fluorescence reported previously (22). This fluorescence is clearly different from that observed in the other strains and is associated with background tissue fluorescence and with fluorescent granules with a blue-shifted emission spectrum (Fig. 1, C and E). It is not clear whether the granules observed in the Rpe65−/− eyecups are related to lysosomes in the same way as are those in the wild type and Abca4−/− animals. A2E is virtually undetectable in the RPE tissues of these cyclic light-reared Rpe65−/− animals at all ages, again pointing out the lack of correspondence between eyecup fluorescence and a chemically defined lipofuscin component.
The differences in lipofuscin and A2E levels between the different strains along with the lack of correspondence between lipofuscin and A2E changes make even more striking the agreement in those levels for cyclic light- and dark-reared animals of the same strain. These observations strongly reinforce the lack of a major effect on lipofuscin and A2E levels by exposure to light.
Effects of Light Exposure on Lipofuscin and A2E Levels
Exposure to light during cyclic light-rearing has no discernible effect on the emission spectrum of lipofuscin granules either. Interestingly, bright light exposure of all-trans-retinal dimer and A2E results in an increase of the intensity of their fluorescence (41). Such changes may be associated with the phototoxicity of lipofuscin and A2E, and with the generation of reactive photoproducts (42). However, it appears that they do not result in obvious changes in the RPE of the animal models examined here, for which the fluorescence intensity and spectrum of lipofuscin do not change appreciably with time of exposure to light. A possible explanation is that there are significant differences in the chemical composition of lipofuscin between cyclic light- and dark-reared animals, which are not reflected in the lipofuscin fluorescence intensity and spectrum and do not include A2E. The similarity in the levels of A2E between cyclic light- and dark-reared animals should also be interpreted with caution, as the overall metabolism of A2E (and the possibility of its clearance) is not well understood.
Retinoid Origins of Lipofuscin and A2E
Lipofuscin granule spectra from all strains that generate 11-cis-retinal are very similar, with a peak at ∼610 nm, regardless of age and rearing conditions. These spectra are essentially the same as those reported previously for lipofuscin and A2E (39). Absence of 11-cis-retinal results in a substantially shifted emission spectrum with a peak at ∼550 nm. In addition, no compounds with significant absorbance >400 nm, such as A2E, were detected in RPE extracts from Rpe65−/− mice. This suggests that the characteristic orange color and emission spectrum of RPE lipofuscin might be indicative of the contributions of retinoid metabolites. This is strongly supported by the results from the experiments with isolated rods and retinas. We followed previous work that has identified lipofuscin and A2E precursors in retinas (27, 43, 44). The fluorescence levels of dark-adapted Rpe65−/− rods were lower than those from strains with intact 11-cis-retinal generating machinery, and their fluorescence emission spectra were shifted to lower wavelengths, similar to the spectra of the RPE granules of the same strain. However, the fluorescence emission spectra of dark-adapted wild type and Abca4−/− rods were similar to those of the RPE granules of their strains, peaking at ∼610 nm. In addition, there were no compounds with significant absorbance of >400 nm detected in the extracts from Rpe65−/− retinas. Comparing the other two strains, the higher lipofuscin-like fluorescence intensity in Abca4−/− cells was commensurate with the higher levels of lipofuscin and A2E accumulating in their RPE. Overall, the results from the three strains lend further support to the present model for the origins of RPE lipofuscin from components that are generated from retinal in the outer segment, which are then introduced to the RPE through phagocytosis.
The presence of lipofuscin precursors in the retina, specifically in rod outer segments, is in agreement with previous reports (21, 26, 27). It has previously been shown that these precursors could form after bleaching, and A2E in particular was shown to form in retinas after the addition of exogenous all-trans-retinal (26). As pointed out previously (26), phospholipase D, which is present in rod outer segments (45), would cleave A2PE to generate A2E.
The amounts of A2E and other lipofuscin precursors found in the retina are fairly substantial when compared with the amounts found accumulated in the RPE. Keeping in mind that ∼10% of mouse rod outer segments is being phagocytosed daily by the RPE (46), comparison of the amounts of A2E found in the retina and the accumulated amounts in the RPE would indicate that A2E and probably other lipofuscin precursors be eliminated even in dark-reared animals. It is not clear whether such elimination reflects nonspecific degradation of the compounds or proceeds via a specialized lysosomal process.
All-trans-retinal Is Unlikely to be a Major Source of Lipofuscin and A2E
Chronic light exposure had no effect on the levels of RPE lipofuscin and A2E or on the levels of lipofuscin-like fluorescence in dark-adapted rod outer segments. This striking result indicates that all-trans-retinal is not a necessary intermediate for lipofuscin and A2E formation, as proposed in current models. The present results do not of course exclude the formation of lipofuscin and A2E from all-trans-retinal under conditions of cyclic light. However, the similarity in the A2E levels between cyclic light- and dark-rearing conditions suggests that any contribution of all-trans-retinal cannot be a major one. The lack of an effect of light exposure would be consistent with the clearance of all-trans-retinal generated by light through its reduction to all-trans retinol in the photoreceptor outer segments (33) and the lack of significant formation of lipofuscin-like fluorophores in metabolically intact rod outer segments (Fig. 8). The results with broken off rod outer segments after light exposure (Fig. 8) suggest that the conditions under which all-trans-retinal could make a significant contribution would involve limitations in the availability of NADPH. Such limitations might perhaps occur under continuous bright light that would generate large amounts of all-trans-retinal or in pathological conditions that involve defects in the NADPH-generating pathways.
11-cis-Retinal Is the Likely Source of Lipofuscin and A2E
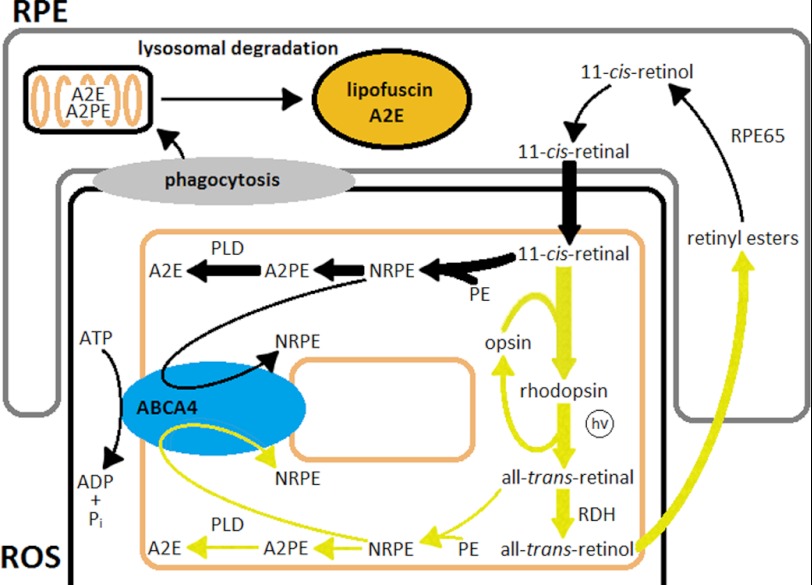
Because the continuous generation of 11-cis-retinal is required for lipofuscin and A2E formation, and the present data argue against an essential role for all-trans-retinal, this would leave 11-cis-retinal, free or as part of rhodopsin, as source. Free 11-cis-retinal, which flows into photoreceptors even under dark-rearing conditions for the purposes of outer segment renewal (47), is a likely source. This is especially so in view of the presence of lipofuscin-like fluorophores in the dark-adapted rods of dark-reared animals (Figs. 5 and 6), as well as of A2E along with other compounds of retinoid origin in retina extracts (Fig. 7). The possibility was demonstrated by the formation of lipofuscin-like fluorophores upon addition of moderate concentrations of exogenous 11-cis-retinal to metabolically intact dark-adapted rods (Fig. 5C). The result should not be too surprising, because 11-cis-retinal is not a good substrate for the rod photoreceptor retinol dehydrogenase enzyme, which is specific for the all-trans-isomer (48). This dehydrogenase activity and its specificity account for the lack of an effect by the same concentration of all-trans-retinal (Fig. 5C). The pathway of lipofuscin formation involving 11-cis-retinal would of course operate under light conditions as well, because fresh 11-cis-retinal would continuously flow into the rod outer segment for rhodopsin regeneration (Fig. 9).
FIGURE 9.
Pathways of lipofuscin and A2E formation. In this model, lipofuscin precursors, such as A2PE, are formed primarily by 11-cis-retinal in the photoreceptor outer segment membranes. Most of the all-trans-retinal generated by light exposure is reduced to retinol and is not a major source of lipofuscin and A2E. Lack of the Rpe65 protein stops the generation of 11-cis-retinal and eliminates formation of lipofuscin precursors regardless of light exposure. Abca4 translocates NRPEs from the cytosolic to the intradiscal side, thereby protecting the cytosolic enzymatic machinery from reactive retinaldehyde. RDH, retinol dehydrogenase; hv, light; PLD, phospholipase D.
The results do not exclude contributions to RPE lipofuscin and A2E by rhodopsin, which contains 11-cis-retinal. In this case, rhodopsin would enter the RPE lysosomal compartment through the daily phagocytosis of the rod outer segments (49); 11-cis-retinal would then be released during lysosomal digestion and would form bis-retinoid adducts with PE that would eventually end up as A2E through a pathway similar to the one that has been described for all-trans-retinal.
Reexamination of the Role of the Abca4 Transporter
None of the experiments presented here provide support for the current model for Abca4 function (17, 50), and some are directly inconsistent with it. These include the presence of lipofuscin precursors in the rod outer segments of dark-adapted dark-reared wild type and Abca4−/− mice, as well as the absence of a detectable increase in lipofuscin precursors in metabolically intact Abca4−/− rods after bleaching. Most important, chronic light exposure had no effect on the levels of RPE lipofuscin and A2E in Abca4−/− mice. Abca4 has been proposed to facilitate the clearance of the all-trans-retinal generated by light by translocating the Schiff base of the all-trans-retinal with PE from the intradiscal side to the cytoplasmic side; on the cytoplasmic side, all-trans-retinal can be converted to all-trans-retinol by retinol dehydrogenase and removed from the cell. In this model, absence of Abca4 would result in the accumulation of all-trans-retinal and formation of bis-retinoid adducts like A2E. The model would predict that in the absence of light exposure, the absence of Abca4 would not make a difference to the generation of A2E. The present results directly invalidate this model for Abca4 and are in line with other recent experiments showing that the formation of all-trans-retinol after light exposure is not affected by the lack of Abca4 (36). The higher levels of lipofuscin precursors in rods, along with the high levels of lipofuscin and A2E accumulation in the absence of Abca4 even in dark-reared animals, suggest that Abca4 has an important role in retinoid processing, albeit not one that involves specifically all-trans-retinal.
The results are consistent with Abca4 facilitating the sequestration of an 11-cis-retinal complex, which would form in both dark and light conditions from free 11-cis-retinal arriving from the RPE. The experiments described here provide no direct data on the direction in which Abca4 would be translocating its substrate. They are consistent with it operating in the same direction as other ABCA transporters (30), i.e. moving the substrate away from the cytoplasmic side (51, 52). The results, however, have invalidated the need for the translocation of NRPE from the intradiscal to the cytosolic side of the disk membrane. Such a direction of translocation of 11-cis-NRPE, from the cytosolic to the intradiscal side, would provide protection of the cytosolic enzymatic machinery from the reactive retinaldehyde. Such protection would be especially needed against 11-cis-retinal, which is not processed by the rod outer segment retinol dehydrogenase (Fig. 5C) (48). The results do not exclude all-trans-NRPE being an important substrate for Abca4 as well. Translocation of all-trans-NRPE to the intradiscal side would be important under conditions of excessive buildup of all-trans-retinal when reduction by Rdh8, the rod outer segment retinol dehydrogenase, cannot keep up. Such a function would be consistent with the observation that mice deficient in both Abca4 and Rdh8 show much more prominent retinal degeneration and are more susceptible to retinal toxicity than either of the strains deficient in only one (53, 54). Fig. 9 presents a scheme that incorporates this model for Abca4 along with the pathways proposed to generate lipofuscin.
The model of Fig. 9 does not provide an explanation for the higher levels of lipofuscin and bis-retinoids found in Abca4−/− animals compared with wild type. One possible explanation could be the higher levels of PE found in the rod outer segments of Abca4−/− animals (17), which would promote the formation of more bis-retinoid adducts. Such an explanation is supported by the formation of more lipofuscin-like fluorophores in Abca4−/− broken off rod outer segments after bleaching (Fig. 8). Another possibility, for which there is presently no evidence, would be a more complex processing of NRPE by Abca4.
In summary, the results presented demonstrate that the formation of lipofuscin and the lipofuscin component A2E in the RPE do not require the generation of all-trans-retinal by exposure to light. Instead they suggest that lipofuscin originates from free 11-cis-retinal, which is continuously supplied to the rod photoreceptor for rhodopsin regeneration and outer segment renewal. The results further suggest that Abca4 may well function in the same direction as other ABCA transporters, and that its physiological role involves the translocation of 11-cis- and perhaps all-trans-NRPE.
Acknowledgment
This work was conducted in a facility constructed with support from National Institutes of Health Grant C06 RR015455 from the Extramural Research Facilities Program of NCRR.
This work was supported, in whole or in part, by National Institutes of Health Grants EY020661 (to Z. A. and R. K. C.), EY004939 (to R. K. C.), EY014850 (to Y. K.), and EY014793 (to Medical University of South Carolina Vision Core). This work was also supported by the Foundation Fighting Blindness, Inc. (to R. K. C.) and unrestricted awards to the Departments of Ophthalmology at Medical University of South Carolina from Research to Prevent Blindness.

This article contains supplemental Fig. 1.
- RPE
- retinal pigment epithelium
- A2E
- N-retinylidene-N-retinylethanolamine
- PE
- phosphatidylethanolamine
- NRPE
- N-retinylidenephosphatidylethanolamine
- 11-cis-NRPE
- N-11-cis-retinylidenephosphatidylethanolamine
- A2PE
- N-retinylidene-N-retinylphosphatidylethanolamine
- BU
- bead unit
- MP
- megapixel.
REFERENCES
- 1. Katz M. L., Robison W. G., Jr. (2002) What is lipofuscin? Defining characteristics and differentiation from other autofluorescent lysosomal storage bodies. Arch. Gerontol. Geriatr. 34, 169–184 [DOI] [PubMed] [Google Scholar]
- 2. Terman A., Brunk U. T. (2004) Lipofuscin. Int. J. Biochem. Cell Biol. 36, 1400–1404 [DOI] [PubMed] [Google Scholar]
- 3. Feeney L. (1978) Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest. Ophthalmol. Vis. Sci. 17, 583–600 [PubMed] [Google Scholar]
- 4. Wing G. L., Blanchard G. C., Weiter J. J. (1978) The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 17, 601–607 [PubMed] [Google Scholar]
- 5. Delori F. C., Dorey C. K., Staurenghi G., Arend O., Goger D. G., Weiter J. J. (1995) In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest. Ophthalmol. Vis. Sci. 36, 718–729 [PubMed] [Google Scholar]
- 6. Delori F. C., Goger D. G., Dorey C. K. (2001) Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest. Ophthalmol. Vis. Sci. 42, 1855–1866 [PubMed] [Google Scholar]
- 7. Ng K. P., Gugiu B., Renganathan K., Davies M. W., Gu X., Crabb J. S., Kim S. R., Rózanowska M. B., Bonilha V. L., Rayborn M. E., Salomon R. G., Sparrow J. R., Boulton M. E., Hollyfield J. G., Crabb J. W. (2008) Retinal pigment epithelium lipofuscin proteomics. Mol. Cell. Proteomics 7, 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sparrow J. R., Wu Y., Kim C. Y., Zhou J. (2010) Phospholipid meets all-trans-retinal. The making of RPE bisretinoids. J. Lipid Res. 51, 247–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eldred G. E., Lasky M. R. (1993) Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature 361, 724–726 [DOI] [PubMed] [Google Scholar]
- 10. Parish C. A., Hashimoto M., Nakanishi K., Dillon J., Sparrow J. (1998) Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. U.S.A. 95, 14609–14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rózanowska M., Wessels J., Boulton M., Burke J. M., Rodgers M. A., Truscott T. G., Sarna T. (1998) Blue light-induced singlet oxygen generation by retinal lipofuscin in nonpolar media. Free Radic. Biol. Med. 24, 1107–1112 [DOI] [PubMed] [Google Scholar]
- 12. Sparrow J. R., Nakanishi K., Parish C. A. (2000) The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest. Ophthalmol. Vis. Sci. 41, 1981–1989 [PubMed] [Google Scholar]
- 13. Sparrow J. R., Parish C. A., Hashimoto M., Nakanishi K. (1999) A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest. Ophthalmol. Vis. Sci. 40, 2988–2995 [PubMed] [Google Scholar]
- 14. Sparrow J. R., Boulton M. (2005) RPE lipofuscin and its role in retinal pathobiology. Exp. Eye Res. 80, 595–606 [DOI] [PubMed] [Google Scholar]
- 15. Winkler B. S., Boulton M. E., Gottsch J. D., Sternberg P. (1999) Oxidative damage and age-related macular degeneration. Mol. Vis. 5, 32. [PMC free article] [PubMed] [Google Scholar]
- 16. Delori F. C., Staurenghi G., Arend O., Dorey C. K., Goger D. G., Weiter J. J. (1995) In vivo measurement of lipofuscin in Stargardt's disease, Fundus flavimaculatus. Invest. Ophthalmol. Vis. Sci. 36, 2327–2331 [PubMed] [Google Scholar]
- 17. Weng J., Mata N. L., Azarian S. M., Tzekov R. T., Birch D. G., Travis G. H. (1999) Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell 98, 13–23 [DOI] [PubMed] [Google Scholar]
- 18. Allikmets R., Singh N., Sun H., Shroyer N. F., Hutchinson A., Chidambaram A., Gerrard B., Baird L., Stauffer D., Peiffer A., Rattner A., Smallwood P., Li Y., Anderson K. L., Lewis R. A., Nathans J., Leppert M., Dean M., Lupski J. R. (1997) A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 15, 236–246 [DOI] [PubMed] [Google Scholar]
- 19. Stone E. M., Webster A. R., Vandenburgh K., Streb L. M., Hockey R. R., Lotery A. J., Sheffield V. C. (1998) Allelic variation in ABCR associated with Stargardt disease but not age-related macular degeneration. Nat. Genet. 20, 328–329 [DOI] [PubMed] [Google Scholar]
- 20. Kim S. R., Jang Y. P., Jockusch S., Fishkin N. E., Turro N. J., Sparrow J. R. (2007) The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc. Natl. Acad. Sci. U.S.A. 104, 19273–19278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mata N. L., Weng J., Travis G. H. (2000) Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 97, 7154–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katz M. L., Redmond T. M. (2001) Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 42, 3023–3030 [PubMed] [Google Scholar]
- 23. Katz M. L., Drea C. M., Eldred G. E., Hess H. H., Robison W. G., Jr. (1986) Influence of early photoreceptor degeneration on lipofuscin in the retinal pigment epithelium. Exp. Eye Res. 43, 561–573 [DOI] [PubMed] [Google Scholar]
- 24. Katz M. L., Eldred G. E. (1989) Retinal light damage reduces autofluorescent pigment deposition in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 30, 37–43 [PubMed] [Google Scholar]
- 25. Ebrey T., Koutalos Y. (2001) Vertebrate photoreceptors. Prog. Retin. Eye Res. 20, 49–94 [DOI] [PubMed] [Google Scholar]
- 26. Ben-Shabat S., Parish C. A., Vollmer H. R., Itagaki Y., Fishkin N., Nakanishi K., Sparrow J. R. (2002) Biosynthetic studies of A2E, a major fluorophore of retinal pigment epithelial lipofuscin. J. Biol. Chem. 277, 7183–7190 [DOI] [PubMed] [Google Scholar]
- 27. Liu J., Itagaki Y., Ben-Shabat S., Nakanishi K., Sparrow J. R. (2000) The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J. Biol. Chem. 275, 29354–29360 [DOI] [PubMed] [Google Scholar]
- 28. Beharry S., Zhong M., Molday R. S. (2004) N-Retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR). J. Biol. Chem. 279, 53972–53979 [DOI] [PubMed] [Google Scholar]
- 29. Sun H., Molday R. S., Nathans J. (1999) Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J. Biol. Chem. 274, 8269–8281 [DOI] [PubMed] [Google Scholar]
- 30. Dean M., Rzhetsky A., Allikmets R. (2001) The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 11, 1156–1166 [DOI] [PubMed] [Google Scholar]
- 31. Gutierrez D. B., Blakeley L., Goletz P. W., Schey K. L., Hanneken A., Koutalos Y., Crouch R. K., Ablonczy Z. (2010) Mass spectrometry provides accurate and sensitive quantitation of A2E. Photochem. Photobiol. Sci. 9, 1513–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwok-Keung Fung B., Stryer L. (1980) Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc. Natl. Acad. Sci. U.S.A. 77, 2500–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen C., Blakeley L. R., Koutalos Y. (2009) Formation of all-trans-retinol after visual pigment bleaching in mouse photoreceptors. Invest. Ophthalmol. Vis. Sci. 50, 3589–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wenzel A., Reme C. E., Williams T. P., Hafezi F., Grimm C. (2001) The Rpe65 L450M variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J. Neurosci. 21, 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyer N. P., Tang P. H., Higbee D., Ablonczy Z., Crouch R. K., Koutalos Y. (2012) Lipofuscin and A2E accumulate with age in the retinal pigment epithelium of Nrl−/− mice. Photochem. Photobiol. in press, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blakeley L. R., Chen C., Chen C. K., Chen J., Crouch R. K., Travis G. H., Koutalos Y. (2011) Rod outer segment retinol formation is independent of Abca4, arrestin, rhodopsin kinase, and rhodopsin palmitylation. Invest. Ophthalmol. Vis. Sci. 52, 3483–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen C., Koutalos Y. (2010) Rapid formation of all-trans-retinol after bleaching in frog and mouse rod photoreceptor outer segments. Photochem. Photobiol. Sci. 9, 1475–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mata N. L., Tzekov R. T., Liu X., Weng J., Birch D. G., Travis G. H. (2001) Delayed dark adaptation and lipofuscin accumulation in abcr+/− mice. Implications for involvement of ABCR in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 42, 1685–1690 [PubMed] [Google Scholar]
- 39. Grey A. C., Crouch R. K., Koutalos Y., Schey K. L., Ablonczy Z. (2011) Spatial localization of A2E in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 52, 3926–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lyubarsky A. L., Savchenko A. B., Morocco S. B., Daniele L. L., Redmond T. M., Pugh E. N., Jr. (2005) Mole quantity of RPE65 and its productivity in the generation of 11-cis-retinal from retinyl esters in the living mouse eye. Biochemistry 44, 9880–9888 [DOI] [PubMed] [Google Scholar]
- 41. Kim S. R., Jang Y. P., Sparrow J. R. (2010) Photooxidation of RPE lipofuscin bisretinoids enhances fluorescence intensity. Vision Res. 50, 729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu Y., Yanase E., Feng X., Siegel M. M., Sparrow J. R. (2010) Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 107, 7275–7280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bui T. V., Han Y., Radu R. A., Travis G. H., Mata N. L. (2006) Characterization of native retinal fluorophores involved in biosynthesis of A2E and lipofuscin-associated retinopathies. J. Biol. Chem. 281, 18112–18119 [DOI] [PubMed] [Google Scholar]
- 44. Katz M. L., Gao C. L., Rice L. M. (1996) Formation of lipofuscin-like fluorophores by reaction of retinal with photoreceptor outer segments and liposomes. Mech. Ageing Dev. 92, 159–174 [DOI] [PubMed] [Google Scholar]
- 45. Salvador G. A., Giusto N. M. (1998) Characterization of phospholipase D activity in bovine photoreceptor membranes. Lipids 33, 853–860 [DOI] [PubMed] [Google Scholar]
- 46. Young R. W. (1967) The renewal of photoreceptor cell outer segments. J. Cell Biol. 33, 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. LaVail M. M. (1980) Circadian nature of rod outer segment disc shedding in the rat. Invest. Ophthalmol. Vis. Sci. 19, 407–411 [PubMed] [Google Scholar]
- 48. Palczewski K., Jäger S., Buczyłko J., Crouch R. K., Bredberg D. L., Hofmann K. P., Asson-Batres M. A., Saari J. C. (1994) Rod outer segment retinol dehydrogenase. Substrate specificity and role in phototransduction. Biochemistry 33, 13741–13750 [DOI] [PubMed] [Google Scholar]
- 49. Young R. W., Bok D. (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol. 42, 392–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Molday R. S., Zhong M., Quazi F. (2009) The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim. Biophys. Acta 1791, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hamon Y., Broccardo C., Chambenoit O., Luciani M. F., Toti F., Chaslin S., Freyssinet J. M., Devaux P. F., McNeish J., Marguet D., Chimini G. (2000) ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2, 399–406 [DOI] [PubMed] [Google Scholar]
- 52. Wang N., Lan D., Gerbod-Giannone M., Linsel-Nitschke P., Jehle A. W., Chen W., Martinez L. O., Tall A. R. (2003) ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J. Biol. Chem. 278, 42906–42912 [DOI] [PubMed] [Google Scholar]
- 53. Chen Y., Okano K., Maeda T., Chauhan V., Golczak M., Maeda A., Palczewski K. (2012) Mechanism of all-trans-retinal toxicity with implications for Stargardt disease and age-related macular degeneration. J. Biol. Chem. 287, 5059–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maeda A., Golczak M., Maeda T., Palczewski K. (2009) Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina. Invest. Ophthalmol. Vis. Sci. 50, 5435–5443 [DOI] [PMC free article] [PubMed] [Google Scholar]