Background: RTP1S facilitates the translocation of odorant receptors (ORs).
Results: Different domains in RTP1S are important for different stages of OR trafficking, odorant-mediated responses, and interaction with ORs.
Conclusion: RTP1S mediates the trafficking and ligand-induced response of ORs by acting through multiple steps.
Significance: Probing the structure-function of RTP1S is crucial for understanding the mechanism of OR trafficking and activation.
Keywords: G-protein-coupled Receptors (GPCRs), Lipid Raft, Olfaction, Receptor Regulation, Trafficking, Odorant Receptors
Abstract
Odorant receptor (OR) proteins are retained in the endoplasmic reticulum when heterologously expressed in cultured cells of non-olfactory origins. RTP1S is an accessory protein to mammalian ORs and facilitates their trafficking to the cell-surface membrane and ligand-induced responses in heterologous cells. The mechanism by which RTP1S promotes the functional expression of ORs remains poorly understood. To obtain a better understanding of the role(s) of RTP1S, we performed a series of structure-function analyses of RTP1S in HEK293T cells. By constructing RTP1S deletion and chimera series and subsequently introducing single-site mutations into the protein, we found the N terminus of RTP1S is important for the endoplasmic reticulum exit of ORs and that a middle region of RTP1S is important for OR trafficking from the Golgi to the membrane. Using sucrose gradient centrifugation, we found that the localization of RTP1S to the lipid raft microdomain is critical to the activation of ORs. Finally, in a protein-protein interaction analysis, we determined that the C terminus of RTP1S may be interacting with ORs. These findings provide new insights into the distinct roles of RTP1S in OR translocation and activation.
Introduction
The mammalian olfactory system is capable of detecting and discriminating a large number of odorants. The binding of odorant molecules to odorant receptors (ORs)2 located on the cell surface of olfactory sensory neurons (1) leads to the production of second messengers and the subsequent neuronal depolarization (2). The OR family contains as many as 1200 intact genes in mice and 400 in human, which are G-protein-coupled receptors with seven transmembrane (TM) domains (3–5). Understanding the fundamental properties of the olfactory system requires investigation of diverse OR proteins and a large number of odorant molecules (6), making heterologous OR expression systems, where ORs are robustly expressed to mimic their native states in olfactory sensory neurons, a compelling model to study OR ligand specificity and selectivity.
It is known that OR proteins are usually retained in the endoplasmic reticulum (ER) and subsequently degraded in cultured cell lines of non-olfactory origins (7, 8). Extensive efforts have been made to enhance the cell-surface expression of ORs in heterologous cells. It was first discovered that appending the first 20 amino acids of rhodopsin to the N terminus of ORs facilitates the surface expression of some ORs (9). Coexpression with other G-protein-coupled receptors is known to enhance the expression and function of certain ORs: the β2-adrenergic receptor dramatically increases the expression of a mouse OR (M71) in HEK293 cells (10), whereas the M3 muscarinic receptor modulates the signaling transduction of ORs (11). The use of accessory factors was also shown to be effective for the expression of a subset of ORs. For example, ODR-4, a protein that promotes the trafficking of a chemosensory receptor in nematodes, has a small effect on the cell-surface expression of rat olfactory receptor U131 (12). On the other hand, Ric-8B, a putative guanine nucleotide exchange factor, can amplify signaling in the Golf signaling cascade (13, 14). Given the limited effect of these accessory factors, it is likely that there are other conserved mechanisms for OR trafficking that are absent in heterologous cells. Saito et al. (15) first cloned receptor-transporting protein (RTP) and receptor expression-enhancing protein (REEP) family members, of which RTP1, RTP2, and, to a lesser degree, REEP1 promote the functional expression of a large number of ORs in HEK293T cells. Subsequently, a shorter form of RTP1 (RTP1S) was discovered to promote the cell-surface expression of ORs even more efficiently than the original RTP1 (16). These findings provided the basis for a high-throughput screening platform of the chemical selectivity of the mammalian OR repertoire (16–18).
As members of the putative chaperone protein families, RTPs and REEPs induce the functional expression of ORs; selected members also play important roles in other chemosensory organs. It has been reported that coexpression of RTP3 and RTP4 enhances the function of the human bitter taste receptor TAS2R (19), whereas REEP2 promotes the function of the sweet taste receptors TAS1R2 and TAS1R3 by recruiting them to the lipid raft microdomains on the plasma membrane (20). In addition, RTP4 increases the cell-surface expression of a heterodimer of two non-chemosensory G-protein-coupled receptors, the μ and δ opioid receptors (21). Finally, RTP1 forms a complex with Homer to increase the surface expression and to promote the signal transduction of TRPC2 (transient receptor potential channel type 2) through interaction with TRPC2 (22).
It has been hypothesized that the trafficking of ORs from the ER to the plasma membrane involves at least two steps (12); however, the exact mechanism underlying the promotion of OR functional expression by RTP1S remains unknown, and the functional domains of RTP1S are unidentified. Here, we employed a structure-function analysis of RTP1S to examine its role as an OR chaperone. We show a multifaceted mode of function for RTP1S, which regulates the functional expression of ORs in multiple steps. We identified specific domains that are crucial for these steps and for interacting with ORs. These findings may provide clues to the function of RTP family members.
EXPERIMENTAL PROCEDURES
Chemicals
The odorant compounds octanoic acid and 2-coumaranone were purchased from Sigma. Odorant solutions were diluted to 1 m stock solutions and kept at −20 °C until used.
Plasmid Construction
Rho (MNGTEGPNFYVPFSNATGVVR), FLAG (DYKDDDDK), and HA (MYPYDVPDYA) tags were subcloned into the pCI mammalian expression vector as described previously (16). Olfr62 (mOR258-5) and Olfr599 (mOR23-1) open reading frames were amplified from mouse genomic DNA and subcloned into pCI expression vectors containing Rho or FLAG tags. RTP1S deletions, chimeras, mutations, and TM modifications were created using existing RTP1S and RTP4 plasmid constructs. The sequences of all plasmid constructions were verified by sequencing.
Cell Culture
HEK293T cells were maintained in minimal essential medium (HyClone) containing 10% fetal bovine serum (Invitrogen), 500 μg/ml penicillin/streptomycin (HyClone), and 6 μg/ml amphotericin B (Sigma) at 37 °C with 5% CO2.
Immunocytochemistry
Live-cell surface staining was performed as described previously (16). The primary antibodies used were mouse anti-rhodopsin (a generous gift of Dr. R. Molday and Millipore), mouse anti-HA (Roche Applied Science), and rabbit anti-HA (Sigma). The secondary antibodies used were Cy3-conjugated (Jackson ImmunoResearch Laboratories, Inc.) and Alexa Fluor 488-conjugated (Invitrogen) anti-rabbit and anti-mouse IgG.
For permeabilized staining, 24 h post-transfection, cells were fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.2% Triton X-100 at 4 °C for 10 min. The cells were blocked in 5% BSA diluted in phosphate-buffered saline and incubated in 5% BSA diluted in phosphate-buffered saline containing the primary antibody at room temperature for 45 min. The cells were then washed with phosphate-buffered saline, followed by incubation with secondary antibodies at room temperature for 30 min. Anti-calnexin antibody (Abcam) was used for ER staining. For Golgi staining, cells were incubated with Alexa Fluor 488-conjugated wheat germ agglutinin (Invitrogen) for 20 min following incubation with a secondary antibody. Slides were mounted with Mowiol and visualized by confocal microscopy (Leica TCS SP5).
To quantify the percentages of OR or RTP1S colocalization with markers for ER or the Golgi apparatus, cells were doubled-stained with the respective epitope tags for the OR or RTP (Cy3) and for ER or Golgi markers (Alexa Fluor 488). As assessed by a cotransfected blue fluorescent protein plasmid, we estimated the transfection efficiency of the system to be ∼40%; thus, for each experiment, we first counted 100 cells with Cy3 signals and subsequently recorded the number of cells in which Cy3 and Alexa Fluor 488 overlapped. To quantify the percentages of cells that were expressed on the cell surface, we cotransfected the cells with GFP, counted 100 cells with GFP, and recorded the numbers of cells that had punctate cell-surface signals.
In addition, we used the JACoP plug-in in ImageJ to calculate the Manders' coefficients (which range from 0 to 1 and in which M1 and M2 of 0.8 and 0.2 for a red-green pair imply that 80% of red pixels colocalize with green, but only 20% of green pixels colocalize with red) for three selected images from each experiment. M1 is the more meaningful coefficient in the context of this experiment, as the Cy3 signal depends on transfection efficiency.
FACS Analysis
FACS analysis was performed as described previously (16). Briefly, HEK293T cells were seeded in 35-mm dishes and then transfected with the same amount of plasmid DNA as used form immunocytochemistry. 2 ng of GFP expression vector was transfected per dish as a control for transfection efficiency. 24 h post-transfection, the cells were dissociated in CellstripperTM (Corning cellgro) and transferred to a tube for incubation with the anti-rhodopsin antibody as described for immunocytochemistry and then with phycoerythrin-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.). Fluorescence was analyzed using a FACSCalibur (BD Biosciences).
Luciferase Assay
The Dual-Glo® luciferase assay system (Promega) was used for luciferase assay essentially as described previously (16). HEK293T cells were plated on poly-d-lysine-coated 96-well plates (Greiner). Plasmid DNAs of ORs and accessory factors were transfected using Lipofectamine 2000 (Invitrogen). In addition, two luciferase constructs were used, including a firefly luciferase gene driven by a 4× cAMP-response element (CRE-Luc) and a Renilla luciferase gene driven by a constitutively active SV40 promoter (pRL-SV40; Promega) that was used as an internal control for cell viability and transfection efficiency. When ORs are activated, the downstream second messenger cAMP is produced, and binding of cAMP to the cAMP-response element region leads to luciferase gene transcription and luminescence. For each 96-well plate, 1 μg of CRE-Luc, 1 μg of pRL-SV40, 5 μg of OR, and 2 μg total of all accessory proteins (RTP1S variants) or the pCI empty vector were transfected. 24 h post-transfection, the medium was replaced with CD293 chemically defined medium (Invitrogen) and then incubated for 30 min at 37 °C. The medium was replaced with 25 μl of odorant solution diluted in CD293 and incubated for 4 h at 37 °C. We followed the manufacturer's protocols for measuring firefly luciferase (Luc) and Renilla luciferase (RL) activities. Luminescence was measured using a SpectraMax M5 plate reader (Molecular Devices). Normalized luciferase activity for RTP1S variants was calculated as (Luc/RLN − Luc/RLmin)/(Luc/RL(RTP1S) − Luc/RLmin), where Luc/RLN represents the mean value from the replicate wells of a certain sample, and Luc/RLmin represents the mean value from the minimal response in the experiment. Student's t test was used to compare the responses of RTP1S variants with that of RTP1S. Bonferroni corrections were applied to correct for problems associated with multiple comparisons.
Lipid Raft Fractionation
HEK293T cells grown to near confluence in four 100-mm dishes were transfected with N-terminally FLAG-tagged Olfr599 and C-terminally HA-tagged RTP1S or TM modifications. Lipid raft fractionation was performed as described in a published protocol (23). Anti-caveolin-1 (Cell Signaling) and anti-transferrin receptor (CD71; Abnova) antibodies were used for probing lipid rafts and non-rafts in Western blotting, respectively.
Immunoprecipitation
HEK293T cells in 60-mm dishes were transfected with N-terminally FLAG-tagged OR, C-terminally HA-tagged RTP1S variants, and/or the negative control, C-terminally HA-tagged ANP32B. 16 h after transfection, cells were lysed with lysis buffer (50 mm Tris (pH 7.4), 150 mm NaCl, 1% Nonidet P-40, 0.5 mm PMSF, and 1% protease inhibitor mixture). The lysates were incubated with anti-FLAG M2 affinity gel (Sigma) or anti-HA affinity matrix (Roche Applied Science) for 4 h at 4 °C and washed with lysis buffer. The bound proteins were eluted by incubation with 1× SDS sample buffer at room temperature for 2 h and at −80 °C overnight and then subjected to Western blotting. The intensity of the bands in each of the Western blots was quantified using Quantity One 1-D analysis software (Bio-Rad). The relative levels of interactions between FLAG-tagged OR and HA-tagged RTP1S deletion mutants and chimeras were calculated as the normalized ratios of immunoprecipitated proteins over protein lysis, blotted with both antibodies.
Western Blotting
Proteins from immunoprecipitation or lipid raft fractionation were resolved by SDS-PAGE using a mini-gel apparatus (Bio-Rad) and subsequently electrophoretically transferred onto nitrocellulose membranes. The membranes were blocked with blocking solution (Tris-buffered saline with 5% nonfat milk and 0.1% Tween 20) for 2 h at room temperature, incubated with the primary antibodies (anti-DYKDDDDK (FLAG; Cell Signaling) or anti-HA), dissolved in blocking solution overnight at 4 °C, and finally incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling) dissolved in blocking solution for 1.5 h at room temperature. The signals were detected using Immobilon Western chemiluminescent HRP substrate (Millipore) according to the manufacturer's instructions.
RESULTS
RTP1S N-terminal Domain and Middle Segments Are Crucial, whereas TM Domain Is Not Required for Functional Expression of ORs
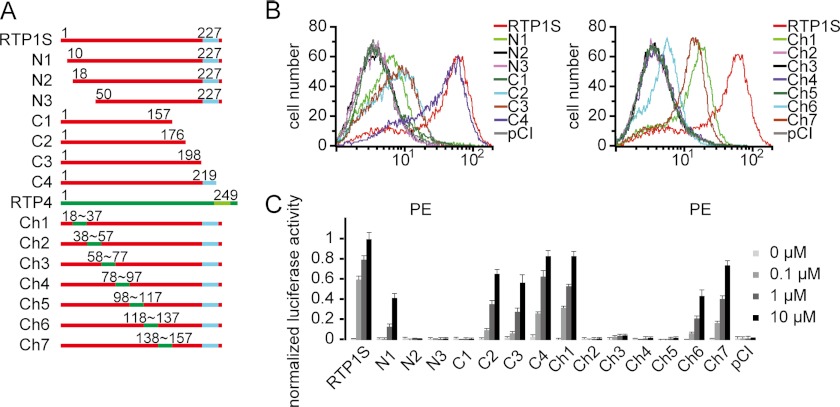
Scanning the amino acid sequence and the predicted secondary structure of RTP1S revealed very few known motifs or domains. To pinpoint the regions in RTP1S that are important for OR expression and function, we constructed a series of sequence modifications of RTP1S, including N- and C-terminal deletions, in which progressively longer fragments of up to 70 amino acids were truncated from both ends of the protein, and RTP1/RTP4 chimeras, in which 20-amino acid-long fragments in the middle of RTP1S were replaced with the corresponding fragments in RTP4 (Fig. 1A and supplemental Fig. S1), which is not expressed in the olfactory epithelium and does not promote OR functional expression (15). We intercalated the deletions and the chimeras so that all regions in the middle of the protein that were not covered by deletions were accounted for by the chimeras. The deletion mutants or chimeras, along with the mouse OR Olfr599, were transiently transfected into HEK293T cells. We then assayed for the cell-surface expression of Olfr599 by flow cytometry (Fig. 1B). We found that the OR could still be expressed on the cell surface when coexpressed with N1, C2, C3, C4, Ch1, and Ch7, but had little (Ch6) or no surface expression with the other variants, indicating that certain parts of the N terminus may be more important for RTP1S function. The activation of Olfr599 upon exposure to the ligand octanoic acid, as measured by cAMP-mediated luciferase reporter gene assays, was detected with the same constructs as those showing OR cell-surface expression (Fig. 1C). Notably, the C3 deletion is a shorter form of RTP1S missing only the TM domain. Olfr599 cotransfected with C3 retained at least 50% of the cell-surface expression and activation compared with RTP1S, suggesting that the TM domain is not essential for RTP1S function as an OR accessory protein. We also repeated the experiments using another mouse OR, Olfr62, and obtained similar results, corroborating that reduced OR response is always paralleled by loss of OR surface expression (supplemental Fig. S2).
FIGURE 1.
RTP1S N-terminal domain and middle segments are crucial, whereas TM domain is not required for functional expression of ORs. A, diagram of a series of RTP1S deletion mutants and RTP1S/RTP4 chimeras. The TM domain is shown in blue. Segments from RTP1S are shown in red, and segments from RTP4 in green. N denotes N-terminal deletions, and C denotes C-terminal deletions. Refer to supplemental Fig. S1 for the amino acid sequences of RTP1 and RTP4. B, flow cytometry analysis of the cell-surface expression of N-terminally Rho-tagged Olfr599 cotransfected with RTP1S deletion mutants and various RTP1S chimeras in HEK293T cells. Transfection with the pCI vector was used as a control. The intensity of phycoerythrin (PE) signal in the GFP-positive population was measured and plotted. C, normalized luciferase activities of Olfr599 cotransfected with RTP1S deletion mutants and chimeras stimulated with octanoic acid at various concentrations (0.1, 1, and 10 μm). The y axis represents normalized luciferase activity ± S.E. (n = 3).
Trp-60, Trp-62, and CXXC Motifs of RTP1S Are Essential for OR Functional Expression
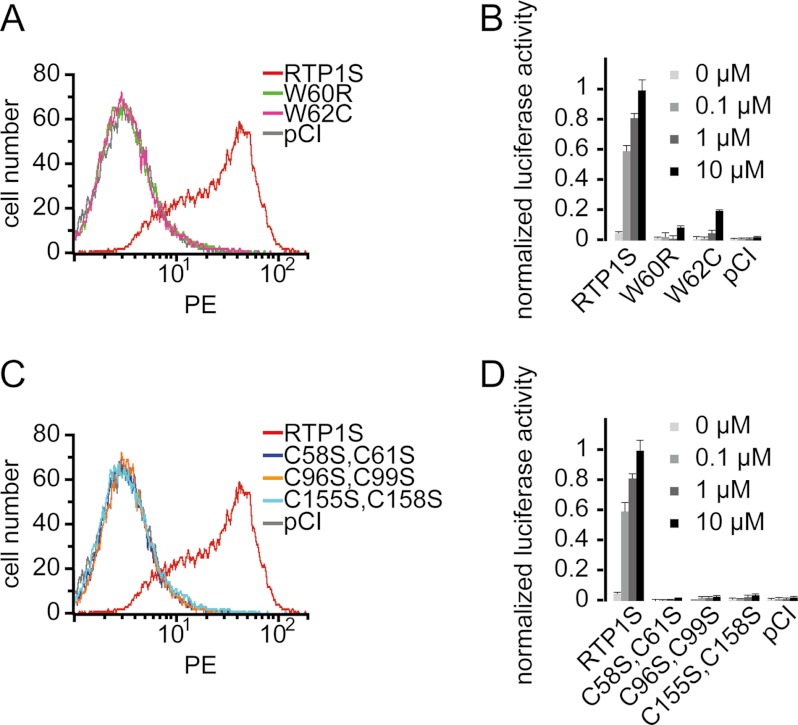
To further identify the residues that are key to RTP1S function, we carried out site-directed mutagenesis on 65 amino acids in the regions that abolished RTP1S function, namely N2, C1, and Ch2–Ch6 (blue in supplemental Fig. S1 and supplemental Table S1). This included all amino acid residues that are conserved in RTP1S and RTP2 but not in RTP3 and RTP4, and these were mutated to the corresponding residues in RTP4. We then measured the cell-surface expression and activation of Olfr599 when cotransfected with each of the RTP1S mutants (supplemental Fig. S3). The results showed that the W60R (M14) and W62C (M15) mutations led to the complete loss of OR surface expression and function (p < 0.0065) (Fig. 2, A and B, and supplemental Fig. S3), whereas mutations of some of the other residues (A52L (M12), K93G (M27), S107P (M36), S108K (M37), and M109F (M38)) caused partial disruption of RTP1S function (supplemental Fig. S3).
FIGURE 2.
Tryptophans 60 and 62, as well as hypothetical CXXC domains, are important for RTP1S function. A, flow cytometry analysis of the cell-surface expression of Rho-tagged Olfr599 cotransfected with various RTP1S single-residue mutants. PE, phycoerythrin. B, normalized luciferase activities of Olfr599 cotransfected with RTP1S single-residue mutants and stimulated with different concentrations of octanoic acid. C, flow cytometry analysis of the cell-surface expression of Rho-tagged Olfr599 cotransfected with various RTP1S double-residue Cys-to-Ser mutants at the cysteines of the hypothetical CXXC domain. D, normalized luciferase activities of Olfr599 cotransfected with RTP1S Cys-to-Ser mutants.
We next investigated the significance of a known CXXC motif that is seen three times throughout RTP1S and is conserved among all four members of the RTP family. We carried out site-directed double-residue mutagenesis of the three conserved pairs of cysteine residues (C58S and C61S, C96S and C99S, and C155S and C158S). As expected, we found that these mutations totally abolished both the cell-surface expression and function of the OR (Fig. 2, C and D).
RTP1S N Terminus Is Important for ER Exit of OR, and RTP1S Middle Segment Is Important for OR Trafficking from Golgi to Membrane
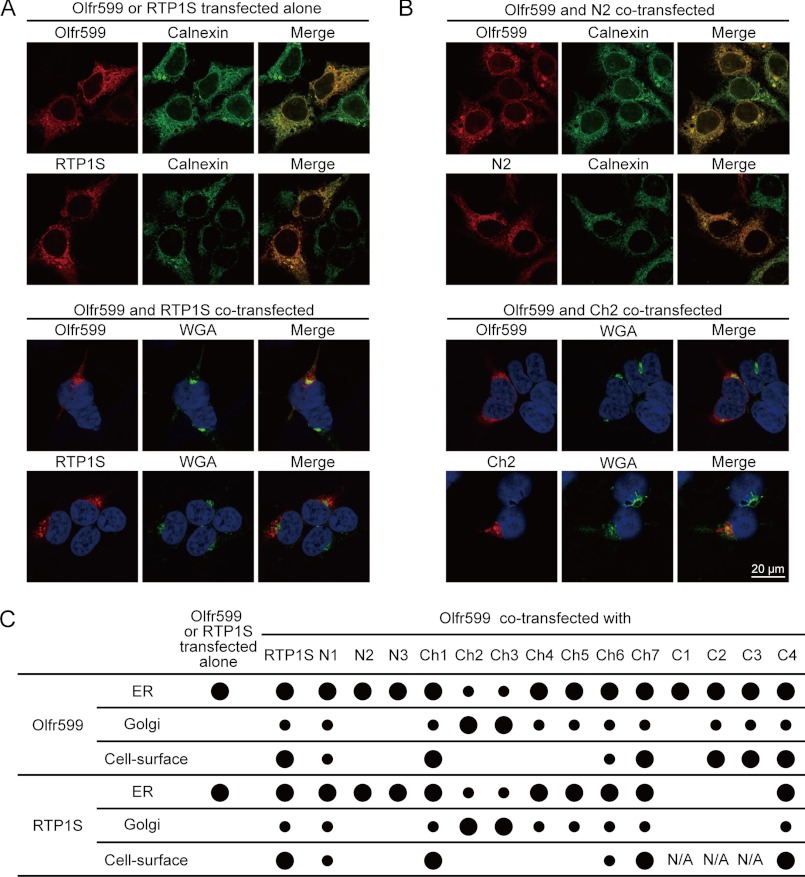
It has long been known that heterologously expressed ORs are retained in the ER (7), a problem that can be readily resolved when the OR and RTP1/RTP2 are cotransfected (15). In a previous study, we found that the majority of the signals for the OR and RTP1S colocalized (16), suggesting a possible association between the two proteins in intracellular compartments. Here, we used both live-cell staining and permeabilized staining to detect the subcellular localization of both Olfr599 and RTP1S in combinations with the markers for the ER and Golgi apparatus. We found that both Olfr599 and RTP1S colocalized with the ER marker calnexin when transfected on their own; both are found on the cell surface (15) and also colocalized with the Golgi marker when the two were cotransfected (Fig. 3A).
FIGURE 3.
Altering segments in RTP1S impacts cell-surface expression and subcellular localization of OR and RTP1S. Live-cell and permeabilized staining was performed to examine the cell-surface expression and subcellular localization of the OR and RTP1S deletion mutants and chimeras. A, subcellular localization of the OR and RTP1S when they were transfected alone or cotransfected. B, subcellular localization of Olfr599 when cotransfected with representative RTP1S variants, including N2 and Ch2. Calnexin is a marker for the ER, whereas wheat germ agglutinin marks the trans-Golgi apparatus. Blue signals in the lower panel are DAPI nuclear staining. C, quantification of OR and RTP1S subcellular localization when Olfr599 and RTP1S were transfected alone and when Olfr599 was cotransfected with various RTP1S deletion mutants and chimeras. Large dots represent >30% colocalization with markers for the ER or Golgi, and small dots represent 10–30% with these markers. The actual numerical values (mean ± S.D., n = 3) that were converted to dots are shown in supplemental Table S2. N/A, not applicable.
We next investigated the specific regions in RTP1S that are important for the trafficking of the OR using the deletion and chimera series. First, for all RTP1S deletion mutants and chimeras, RTP1S and Olfr599 immunofluorescence was almost always colocalized, reinforcing the reciprocal trafficking of the two molecules (Fig. 3, B and C, and supplemental Tables S2 and S3). Second, we found that, consistent with the cell-surface expression of the OR shown in Fig. 1B, chimeras Ch1 and Ch7, as well as most of the C-terminal deletion mutants, were presented at the cell-surface as punctate signals. In contrast, when Ch2–Ch5 were transfected with the OR, Olfr599 reached the Golgi but not the cell surface, whereas when cotransfected with deletion mutants N2 and C1, Olfr599 was found exclusively in the ER (Fig. 3, B and C). Interestingly, when cotransfected with Olfr599, the immunofluorescence of C2 and C3, which are C-terminal deletions lacking the TM domain, accumulated near the intracellular surface of the plasma membrane (supplemental Fig. S4), indicating that the C-terminal domain, including the TM domain, is not required for the Golgi exit of RTP1S or the OR.
Lipid Raft Fractions of RTP1S and Activation of ORs
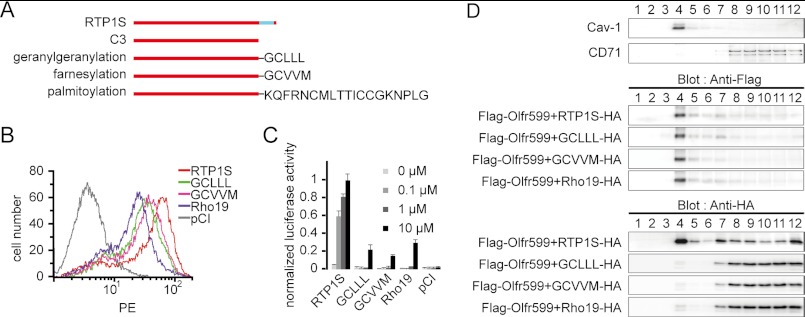
The results from the deletion series showed that the RTP1S TM domain is not required for the function of RTP1S. To explore the specific function of the TM domain, whether it is the mere anchoring of RTP1S to the membrane or other functions, we appended a series of lipid modifications to the C3 deletion, namely the signals for geranylgeranylation (GCLLL), farnesylation (GCVVM), and palmitoylation (KQFRNCMLTTICCGKNPLG, the last 19 amino acids from human rhodopsin Rho19), all of which would aid in the insertion of the protein into the plasma membrane (Fig. 4A). We found that lipid-modified RTP1S retained the ability to promote the cell-surface expression of OR (Fig. 4B). However, in contrast to other experiments in which OR cell-surface expression and odor-mediated responses were always in agreement with each other, Olfr599 cotransfected with lipid-modified RTP1S exhibited severely impaired activation by its cognate ligand (Fig. 4C).
FIGURE 4.
Recruitment of RTP1S to lipid raft microdomain is essential for odorant-mediated response. A, diagram of a series of RTP1S TM domain modifications. B, flow cytometry analysis of the cell-surface expression of Rho-tagged Olfr599 cotransfected with RTP1S TM modification mutants. PE, phycoerythrin. C, normalized luciferase activities of Olfr599 cotransfected with RTP1S TM domain modification mutants with different concentrations of octanoic acid. D, sucrose gradient separation of HEK293T cells transfected with FLAG-tagged Olfr599 and HA-tagged RTP1S or RTP1S TM domain modification mutants. 12 fractions were obtained and blotted for the lipid raft marker caveolin-1 (Cav-1) and the non-lipid raft marker CD71 (upper panel) and for anti-FLAG (middle panel) or anti-HA (lower panel) antibodies.
To address the apparent discrepancy in OR expression and function, we performed sucrose gradient centrifugation to probe the microdomain localization of RTP1S and Olfr599 on the plasma membrane. It is known that the addition of lipid modifications may not be sufficient for localization to the lipid rafts, and certain modifications, such as prenylation, are less likely to be found in the rafts due to the bulky branched nature of their lipid moiety (24, 25). If RTP1S plays an integral role in OR activation, mislocalization of RTP1S could affect the odorant-mediated response of the OR. Indeed, although wild-type RTP1S was clearly detected in both the lipid raft and non-raft microdomain fractions, all lipid-modified RTP1S variants were found exclusively in the non-raft fractions. Interestingly, ORs were consistently localized to the lipid raft fractions regardless of the RTP1S variants transfected (Fig. 4D).
OR Interacts with C-terminal Half of RTP1S
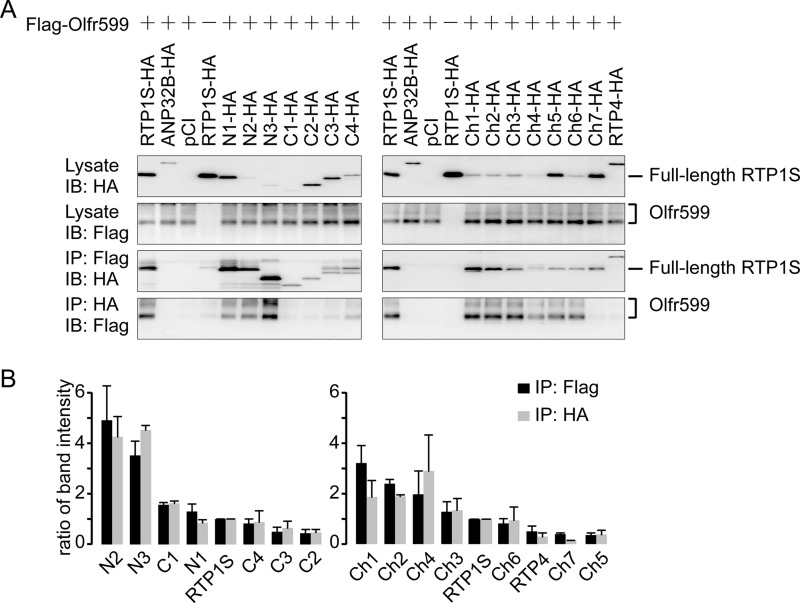
Finally, we investigated which regions of RTP1S mediate stable protein-protein interaction with ORs. We cotransfected RTP1S deletion mutants and chimeras with Olfr599 and used co-immunoprecipitation to assess protein-protein interaction. Despite the fact that the RTP1S N-terminal deletion mutants were, in general, expressed to a lesser degree than the C-terminal deletion mutants (Fig. 5, A (left panel) and B, and supplemental Fig. S5), Olfr599 always co-immunoprecipitated with the RTP1S N-terminal deletion mutants and vice versa, but not in the case of C-terminal deletion mutants (Fig. 5A, left panel). The same trend was seen for the RTP1S chimera series (Fig. 5, A (right panel) and B, and supplemental Fig. S5). Therefore, our results demonstrated that the OR physically interacted primarily with the C-terminal half of RTP1S.
FIGURE 5.
RTP1S C-terminal half is important for protein-protein interaction with ORs. A, left panel, interactions between Olfr599 and RTP1S deletion mutants. Right panel, interactions between Olfr599 and RTP1S chimeras. First and second panels, protein lysates of HEK293T cells transfected with FLAG-tagged Olfr599 and/or HA-tagged RTP1S deletion mutants and chimeras and blotted with anti-HA or anti-FLAG antibody. Third panels, co-immunoprecipitation of certain RTP1S deletion mutants and chimeras with anti-FLAG antibody. Fourth panels, co-immunoprecipitation of Olfr599 with anti-HA antibody when transfected with certain RTP1S deletion mutants and chimeras. Refer to supplemental Fig. S5 for the original blots. IB, immunoblot; IP, immunoprecipitation. ANP32B is a presumably non-interacting protein, and pCI is the empty vector. B, quantification of the co-immunoprecipitation assays with Olfr599 and RTP1S deletion mutants and chimeras in A. Immunoprecipitation efficiencies were calculated by dividing by the input amounts of both components and then normalizing to the interaction between Olfr599 and wild-type RTP1S. The results shown represent the mean ± S.D. from two independent experiments. Note that Ch4 presented unusually large ratios due to a small denominator (a small amount of proteins in protein lysates).
DISCUSSION
Past successes in the heterologous expression of ORs highlighted the importance of a group of specific accessory proteins that regulate the trafficking of ORs to the plasma membrane. Of these, RTP family members appear to most dramatically improve the cell-surface expression and function of many ORs. In this study, we identified key regions conferring OR functional expression. Specifically, the 17 amino acids in the RTP1S N-terminal domain are responsible for the ER exit of the OR, whereas a region of ∼80 amino acids in the middle of RTP1S is required for the OR to pass through the Golgi apparatus. We also showed that the identity of the TM domain is critical for the localization of RTP1S to the lipid rafts and consequently for the OR to signal at the cell surface.
What is the exact role of RTP1S in OR maturation, trafficking, and function? It is plausible to hypothesize that multiple steps are required for OR trafficking. In other words, the OR has to overcome several barriers along the secretory pathway to the membrane, and different domains of RTP1S are responsible for the OR to pass these steps. Our data are highly supportive of a reciprocal trafficking mechanism in which RTP1S and the OR rely on each other for trafficking to the membrane. As proposed in a previous study (15), RTPs may contribute to the folding and trafficking of the OR or function as co-receptors to the OR. Our data showed a possible sequential functionality of RTP1S in all of these steps. First, the RTP1S N-terminal domain stabilizes the OR by ensuring its correct folding in the ER, which is in turn required for successful ER exit. Next, RTP1S aids the OR in its vesicular transportation to the membrane. Our results showed some RTP1S/RTP4 chimeric proteins that are retained in the Golgi, and this is consistent with the notion that Golgi exit is another critical checkpoint in OR trafficking.
Once the OR is at the membrane, RTPs could function as co-receptors to the OR to carry out the proper signal transduction of ORs by localizing to the lipid raft microdomains where ORs are located. Our data showed that, when cotransfected with the lipid-modified versions of RTP1S, the association between the OR and RTP1S was lost at the cell surface, contributing to the loss of OR function. This is in contrast to a previous report in which the REEP2 accessory protein promoted the localization of sweet taste receptors to lipid rafts (20). It was proposed that lipid rafts act as a platform on which molecules in the G-protein-coupled receptor signal transduction pathway are brought to close proximity to selectively associate with each other, activating the downstream signal transduction cascade (26). We thus hypothesize that, in addition to its chaperone activities, the presence of RTP1S in the lipid rafts side by side with the OR is required to form a functional receptor complex with the OR. An analogous example would be the receptor activity-modifying proteins, which complex with the calcitonin receptor and calcitonin-like receptor to give receptor complexes with divergent ligand specificities (27). Due to its short extracellular C-terminal domain, it is unlikely that RTP1S contributes to OR ligand binding, but it may still modulate OR pharmacology through orthosteric or allosteric mechanisms that may eventually contribute to downstream signaling efficiency. Notably, wild-type RTP1S is also found in non-raft/cytoplasmic fractions, whereas the OR is found exclusively in lipid raft fractions. The exact role of non-raft RTP1S remains to be investigated.
Finally, the three pairs of cysteine residues conserved among RTP family members point to a possible common secondary structure. The loss-of-function mutations at these sites could be a result of structural perturbation, and this is consistent with the notion that the CXXC motif may contribute to protein conformation through the formation of disulfide bonds. For example, the CXXC motif in the protein-disulfide isomerase, a member of the thioredoxin family of chaperone proteins, can catalyze the formation of disulfide bonds in vitro (28). Interestingly, the two key tryptophan residues, Trp-60 and Trp-62, intercalate the first CXXC motif. The hydrophobicity of the tryptophans may be important for the proper orientation of the nearby cysteine residues required for disulfide bridge formation. Taken together, the CXXC motifs, as well as Trp-60 and Trp-62, may be important for the structural integrity of RTP1S and/or ORs by serving as the sites for stable intermolecular and intramolecular interactions. Future purification and crystallography studies of RTP family members will reveal specific structural features important for the function of and interaction with the OR.
Acknowledgments
We thank Drs. H. Amrein, D. Marchuk, D. Tracey, M. Caron, and E. Chin and our laboratory members for helpful discussions.
This work was supported, in whole or in part, by a National Institutes of Health Grant DC005782 from NIDCD (to H. M.). This work was also supported by National Natural Science Foundation of China Grants 30970981 and 31070972, 973 Program Grant 2012CB910401, Shanghai Pujiang Program Grant 09PJ1406900, the Program for Innovative Research Team of the Shanghai Municipal Education Commission, Chen Guang Project Grant 2009CG15 funded by the Shanghai Municipal Education Commission and the Shanghai Education Development Foundation, the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, and Leading Academic Discipline Project Grant J50201 from the Shanghai Municipal Education Commission (to H. Z.).

This article contains supplemental Figs. S1–S5 and Tables S1–S3.
- OR
- odorant receptor
- TM
- transmembrane
- ER
- endoplasmic reticulum
- RTP
- receptor-transporting protein
- REEP
- receptor expression-enhancing protein.
REFERENCES
- 1. Buck L., Axel R. (1991) A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187 [DOI] [PubMed] [Google Scholar]
- 2. Shepherd G. M. (1994) Discrimination of molecular signals by the olfactory receptor neuron. Neuron 13, 771–790 [DOI] [PubMed] [Google Scholar]
- 3. Mombaerts P. (1999) Odorant receptor genes in humans. Curr. Opin. Genet. Dev. 9, 315–320 [DOI] [PubMed] [Google Scholar]
- 4. Firestein S. (2001) How the olfactory system makes sense of scents. Nature 413, 211–218 [DOI] [PubMed] [Google Scholar]
- 5. Zhang X., Firestein S. (2002) The olfactory receptor gene superfamily of the mouse. Nat. Neurosci. 5, 124–133 [DOI] [PubMed] [Google Scholar]
- 6. Matsunami H., Mainland J. D., Dey S. (2009) Trafficking of mammalian chemosensory receptors by receptor-transporting proteins. Ann. N.Y. Acad. Sci. 1170, 153–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McClintock T. S., Landers T. M., Gimelbrant A. A., Fuller L. Z., Jackson B. A., Jayawickreme C. K., Lerner M. R. (1997) Functional expression of olfactory-adrenergic receptor chimeras and intracellular retention of heterologously expressed olfactory receptors. Brain Res. Mol. Brain Res. 48, 270–278 [DOI] [PubMed] [Google Scholar]
- 8. Lu M., Echeverri F., Moyer B. D. (2003) Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein-coupled receptors. Traffic 4, 416–433 [DOI] [PubMed] [Google Scholar]
- 9. Krautwurst D., Yau K. W., Reed R. R. (1998) Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell 95, 917–926 [DOI] [PubMed] [Google Scholar]
- 10. Hague C., Uberti M. A., Chen Z., Bush C. F., Jones S. V., Ressler K. J., Hall R. A., Minneman K. P. (2004) Olfactory receptor surface expression is driven by association with the β2-adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A. 101, 13672–13676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y. R., Matsunami H. (2011) Activation state of the M3 muscarinic acetylcholine receptor modulates mammalian odorant receptor signaling. Sci. Signal. 4, ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gimelbrant A. A., Haley S. L., McClintock T. S. (2001) Olfactory receptor trafficking involves conserved regulatory steps. J. Biol. Chem. 276, 7285–7290 [DOI] [PubMed] [Google Scholar]
- 13. Von Dannecker L. E., Mercadante A. F., Malnic B. (2005) Ric-8B, an olfactory putative GTP exchange factor, amplifies signal transduction through the olfactory-specific G-protein Gαolf. J. Neurosci. 25, 3793–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Von Dannecker L. E., Mercadante A. F., Malnic B. (2006) Ric-8B promotes functional expression of odorant receptors. Proc. Natl. Acad. Sci. U.S.A. 103, 9310–9314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saito H., Kubota M., Roberts R. W., Chi Q., Matsunami H. (2004) RTP family members induce functional expression of mammalian odorant receptors. Cell 119, 679–691 [DOI] [PubMed] [Google Scholar]
- 16. Zhuang H., Matsunami H. (2007) Synergism of accessory factors in functional expression of mammalian odorant receptors. J. Biol. Chem. 282, 15284–15293 [DOI] [PubMed] [Google Scholar]
- 17. Saito H., Chi Q., Zhuang H., Matsunami H., Mainland J. D. (2009) Odor coding by a mammalian receptor repertoire. Sci. Signal. 2, ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller A., Zhuang H., Chi Q., Vosshall L. B., Matsunami H. (2007) Genetic variation in a human odorant receptor alters odor perception. Nature 449, 468–472 [DOI] [PubMed] [Google Scholar]
- 19. Behrens M., Bartelt J., Reichling C., Winnig M., Kuhn C., Meyerhof W. (2006) Members of RTP and REEP gene families influence functional bitter taste receptor expression. J. Biol. Chem. 281, 20650–20659 [DOI] [PubMed] [Google Scholar]
- 20. Ilegems E., Iwatsuki K., Kokrashvili Z., Benard O., Ninomiya Y., Margolskee R. F. (2010) REEP2 enhances sweet receptor function by recruitment to lipid rafts. J. Neurosci. 30, 13774–13783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Décaillot F. M., Rozenfeld R., Gupta A., Devi L. A. (2008) Cell-surface targeting of μ-δ opioid receptor heterodimers by RTP4. Proc. Natl. Acad. Sci. U.S.A. 105, 16045–16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mast T. G., Brann J. H., Fadool D. A. (2010) The TRPC2 channel forms protein-protein interactions with Homer and RTP in the rat vomeronasal organ. BMC Neurosci 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zuo W., Chen Y. G. (2009) Specific activation of mitogen-activated protein kinase by transforming growth factor-β receptors in lipid rafts is required for epithelial cell plasticity. Mol. Biol. Cell 20, 1020–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melkonian K. A., Ostermeyer A. G., Chen J. Z., Roth M. G., Brown D. A. (1999) Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274, 3910–3917 [DOI] [PubMed] [Google Scholar]
- 25. Wang T. Y., Leventis R., Silvius J. R. (2001) Partitioning of lipidated peptide sequences into liquid-ordered lipid domains in model and biological membranes. Biochemistry 40, 13031–13040 [DOI] [PubMed] [Google Scholar]
- 26. Ostrom R. S., Insel P. A. (2004) The evolving role of lipid rafts and caveolae in G-protein-coupled receptor signaling: implications for molecular pharmacology. Br. J. Pharmacol. 143, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McLatchie L. M., Fraser N. J., Main M. J., Wise A., Brown J., Thompson N., Solari R., Lee M. G., Foord S. M. (1998) RAMPs regulate the transport and ligand specificity of the calcitonin receptor-like receptor. Nature 393, 333–339 [DOI] [PubMed] [Google Scholar]
- 28. Chivers P. T., Laboissière M. C., Raines R. T. (1996) The CXXC motif: imperatives for the formation of native disulfide bonds in the cell. EMBO J. 15, 2659–2667 [PMC free article] [PubMed] [Google Scholar]