Background: Muscarinic acetylcholine receptor (mAchR) activation enhances expression of Arc, a key gene for synaptic plasticity and memory.
Results: Carbachol-evoked Arc expression is abruptly curtailed by translation-dependent RNA decay and proteasomal degradation.
Conclusion: Short, rapid eye movement sleep-like, bursts of cholinergic activity induce maximal Arc expression.
Significance: Cholinergic epoch pattern may be a critical determinant of Arc expression and function in synaptic plasticity and memory.
Keywords: Cholinergic Receptor, Gene Expression, Neurobiology, Protein Degradation, Protein Synthesis, Signal Transduction, Synaptic Plasticity, Sleep
Abstract
Cholinergic signaling induces Arc/Arg3.1, an immediate early gene crucial for synaptic plasticity. However, the molecular mechanisms that dictate Arc mRNA and protein dynamics during and after cholinergic epochs are little understood. Using human SH-SY5Y neuroblastoma cells, we show that muscarinic cholinergic receptor (mAchR) stimulation triggers Arc synthesis, whereas translation-dependent RNA decay and proteasomal degradation strictly limit the amount and duration of Arc expression. Chronic application of the mAchR agonist, carbachol (Cch), induces Arc transcription via ERK signaling and release of calcium from IP3-sensitive stores. Arc translation requires ERK activation, but not changes in intracellular calcium. Proteasomal degradation of Arc (half-life ∼37 min) was enhanced by thapsigargin, an inhibitor of the endoplasmic calcium-ATPase pump. Similar mechanisms of Arc protein regulation were observed in cultured rat hippocampal slices. Functionally, we studied the impact of cholinergic epoch duration and temporal pattern on Arc protein expression. Acute Cch treatment (as short as 2 min) induces transient, moderate Arc expression, whereas continuous treatment of more than 30 min induces maximal expression, followed by rapid decline. Cholinergic activity associated with rapid eye movement sleep may function to facilitate long term synaptic plasticity and memory. Employing a paradigm designed to mimic intermittent rapid eye movement sleep epochs, we show that application of Cch in a series of short bursts generates persistent and maximal Arc protein expression. The results demonstrate dynamic, multifaceted control of Arc synthesis during mAchR signaling, and implicate cholinergic epoch duration and repetition as critical determinants of Arc expression and function in synaptic plasticity and behavior.
Introduction
Cholinergic afferents from the medial septum to the hippocampus are critical for the encoding of episodic memories and induction of several forms of activity-dependent synaptic plasticity (1). mAchR antagonists impair spatial learning and induction of long term potentiation (LTP)2 (2, 3), whereas cholinergic agonists facilitate LTP induction (4–9). Application of the mAchR agonist carbachol (Cch) to in vitro hippocampal slices can induce LTP or long term depression (LTD) depending on the timing of glutamatergic pathway activation relative to the cycle of Cch-induced theta rhythm (10–13).
Consolidation of synaptic plasticity and memory depends on de novo gene expression and protein synthesis. Although cholinergic facilitation of LTP induction is now well documented, there is also evidence for cholinergic modulation of late, protein synthesis-dependent plasticity. Thus, Cch application can convert early LTP into late LTP (14) and stimulate protein synthesis in dendrites of hippocampal CA1 pyramidal neurons (15).
Acetylcholine levels increase in the hippocampus during behavioral states predominated by the EEG theta rhythm, namely active waking and rapid eye movement (REM) sleep (16, 17). Enhanced cholinergic signaling maintained during these states may function in synaptic consolidation and memory formation (18, 19). Procedures that selectively disrupt REM sleep impair LTP consolidation (20–23). Following some learning tasks, the duration of REM sleep epochs in the post-learning period is enhanced and necessary for memory formation (17, 24). Furthermore, post-learning REM sleep is associated with transcription of several genes linked to long term synaptic plasticity, one of which is activity-regulated cytoskeleton-associated protein Arc/Arg3.1 (hereafter Arc) (25–27).
Arc has emerged as key regulator of protein synthesis-dependent synaptic plasticity, memory formation, and postnatal cortical development (28, 29). Gene knock-out or acute inhibition of Arc translation inhibits long term memory formation (30, 31) and impairs several protein synthesis-dependent forms of synaptic plasticity, including LTP, LTD, and homeostatic scaling (31–39). In Angelman syndrome, deficient proteasomal degradation of Arc is associated with pathological accumulation of Arc at synapses and impaired synaptic transmission (40). Furthermore, Arc mRNA has been identified as a natural target for nonsense-mediated decay (NMD), a mechanism thought to ensure the rapid destruction of transcripts upon translation (41–43). LTP consolidation in the dentate gyrus of live rats requires a period of sustained Arc translation, during which Arc mRNA and protein are both rapidly degraded (32, 44). Taken together, evidence from disparate preparations and model systems suggests tight control of Arc mediated at multiple levels in the gene expression pathway. However, little is known about the coordination of Arc synthesis and degradation as studied in any single preparation.
Activation of mAchR triggers Arc expression in vitro and in vivo (45–47) and behavioral Arc expression in the rat hippocampus is at least partly induced by the septal cholinergic system (47–49). The present study was aimed at elucidating the molecular mechanisms that regulate Arc protein expression dynamics during and after muscarinic cholinergic activation. Using human SH-SY5Y neuroblastoma cells as our main model system, we find that Cch treatment induces transient Arc mRNA expression mediated by mAchR-coupled ERK activation and intracellular calcium release. Arc protein translation also requires ERK signaling, but not intracellular calcium. As the duration of Cch stimulation was extended beyond a critical time window of 30 min, Arc mRNA expression is prolonged, resulting in marked enhancement of protein expression. Concurrently with de novo synthesis, Arc mRNA is subject to rapid translation-dependent decay, whereas Arc protein is ubiquitinated and targeted for proteasomal degradation. Beyond describing the mechanisms underlying Arc dynamics, we find that maximal Arc expression can be induced by short, repeated epochs of Cch treatment, using a paradigm that mimics naturally occurring REM sleep epochs. Taken together, our study demonstrates coordinate, multifaceted regulation of Arc protein expression in response to cholinergic signaling and points to cholinergic epoch duration and reiteration as critical determinants of Arc modulation during behavior.
EXPERIMENTAL PROCEDURES
Cell Culture
Human SH-SY5Y cells were grown in Dulbecco's modified Eagle's medium (DMEM, Sigma) supplemented with 10% fetal bovine serum, penicillin/streptomycin, and l-glutamine. Cells were seeded in 97-mm Petri dishes or 6-well plates. As previously described (45), cells were serum-starved overnight before experiments were carried out. DMEM supplemented with only 0.5% fetal bovine serum, penicillin/streptomycin, and l-glutamine was used for serum starvation and for experiments involving washout or medium change.
Organotypic Hippocampal Slice Preparation
Organotypic hippocampal tissue slices (OTSC) were prepared after the method of Marrs et al. (50). Briefly, hippocampi were dissected from Wistar rats aged postnatal days 5 to 9 (P5–P9) into ice-cold Gey's balanced salt solution (Sigma) containing 0.5% d-glucose. Hippocampi were cut into 400-μm thick transverse slices using a McIlwain tissue chopper. Hippocampal slices were placed on Millicell membrane culture inserts (Millipore) and maintained in culture media (50% minimal essential medium (Invitrogen), 25% Earle's buffered salt solution (Invitrogen), 25% heat-inactivated horse serum (Sigma), 1% glucose (Sigma), 2.5% B27 (Invitrogen), and 10 units/ml of penicillin/streptomycin) in a 95% O2, 5% CO2 humidified incubator at 36 °C for 7 days. The culture medium was replaced every 2–3 days. Experiments were carried out 3 days after the latest medium change.
Drugs and Concentrations
The following drugs were used in this study: carbachol (Sigma, 50 μm), U0126 (Promega, 10 μm), thapsigargin (Calbiochem, 1 μm), rapamycin (LC Laboratories, 200 nm), wortmannin (Tocris, 10 μm), atropine (Nicomed, 1 μg/ml), actinomycin D (Sigma, 5 μg/ml), MG-132 (Sigma, 10 μm), anisomycin (Sigma, 50 μg/ml), and BAPTA-AM (Sigma, 10 μm).
Antibodies for Western Blotting
Primary antibodies were mouse anti-Arc (Santa Cruz, C7, sc-17839), rabbit anti-Arc (Santa Cruz, H300, sc-15325), and mouse anti-GAPDH (Santa Cruz, 6C5, sc-32223). HRP-conjugated anti-mouse and anti-rabbit secondary antibodies were from Calbiochem.
SDS-PAGE and Western Blotting
OTSC and cells were lysed in PBS + 0.1% Triton X-100 containing 1 mm PMSF and supplemented with Roche Complete Protease Inhibitor Mixture. 40 μg of protein were separated on SDS-PAGE gels (10%) and transferred onto a nitrocellulose membrane (Hybond-C, Amersham Biosciences). Membranes were blocked for 1 h at room temperature in TBST (Tris-buffered saline, 0.1% Tween 20) and 3% nonfat dry milk. Primary antibodies were diluted in blocking buffer containing TBST and 5% BSA and applied on membranes overnight at 4 °C with constant shaking. Following three washes with TBST, blots were incubated for 1 h at room temperature in horseradish peroxidase-conjugated secondary antibody diluted in TBST. Blots were then visualized using enhanced chemiluminescence (Pierce, ECL Western blotting Substrate). Optical density values for each protein were estimated using the GelDoc XRS system (Bio-Rad).
Immunoprecipitation
Protein G-Sepharose 4 Fast Flow beads (GE Healthcare) were washed in PBS and then incubated with 2.5 μg of Arc C7 antibody for 1 h at room temperature. Beads were again washed in PBS and incubated with 500 μg of total protein overnight at 4 °C. Bound fractions were washed in PBS and analyzed by Western blot. Polyubiquitinated proteins were detected with polyubiquitinated conjugates, mAb (FK1, 1:1000, Enzo Life Sciences) and goat anti-mouse IgM, polyclonal Ab (1:2500, Enzo Life Sciences).
Immunohistochemistry
After completion of the experiment, slices were fixed in PBS + 4% paraformaldehyde for 20 min at 4 °C. Slices were washed in PBT (PBS + 0.1% Tween 20) then permeabilized in PBT + 0.5% Triton X-100 for 30 min at room temperature. Slices were incubated in blocking buffer consisting of PBT + 2% BSA + 2% horse serum for 30–60 min, then incubated with anti-Arc (H300) overnight at 4 °C. After several washes, slices were incubated with secondary HRP-conjugated antibodies diluted in blocking buffer for 1 h at room temperature. Staining was completed using 3,3-diaminobenzidine (Sigma).
mRNA Isolation, cDNA Synthesis, and Semiquantitative Real-time PCR
mRNA was isolated using Dynabeads mRNA direct kit (Invitrogen) according to the manufacturer's instructions. Concentration and quality of the mRNA was determined with a Nano Drop® Spectrophotometer ND-1000. cDNA was synthesized using SuperScript III (Invitrogen) following the manufacturer's instructions. 60 ng of mRNA was used in the reaction and the synthesized cDNA was diluted 10-fold prior to real-time PCR. Semiquantitative real-time PCR was performed in a Light Cycler 480 with SYBR Green (Roche Applied Science). Hypoxanthine-guanine phosphoribosyltransferase (forward, tgacactggcaaaacaatgca, reverse, ggtccttttcaccagcaagct) and Cyclopholine (forward, caagacggagtggttggatg, reverse, tggtggtcttcttgctggtc) were used for normalization, and amplification was done at 60 °C. The relative amount of Arc mRNA (forward, tgagtcctcaaatccggctgag, reverse, tgtgggaaccttgagacctgttg) was calculated by the second derivative method. The efficiency of each primer pair was determined by standard curve.
Data Presentation and Statistical Analysis
All data are presented as mean ± S.E. Statistical analysis was based on one-way analysis of variance or t test where the significance level was set to p < 0.05.
RESULTS
Carbachol Induces mAchR-specific, de Novo Arc Protein Synthesis in SH-SY5Y Cells
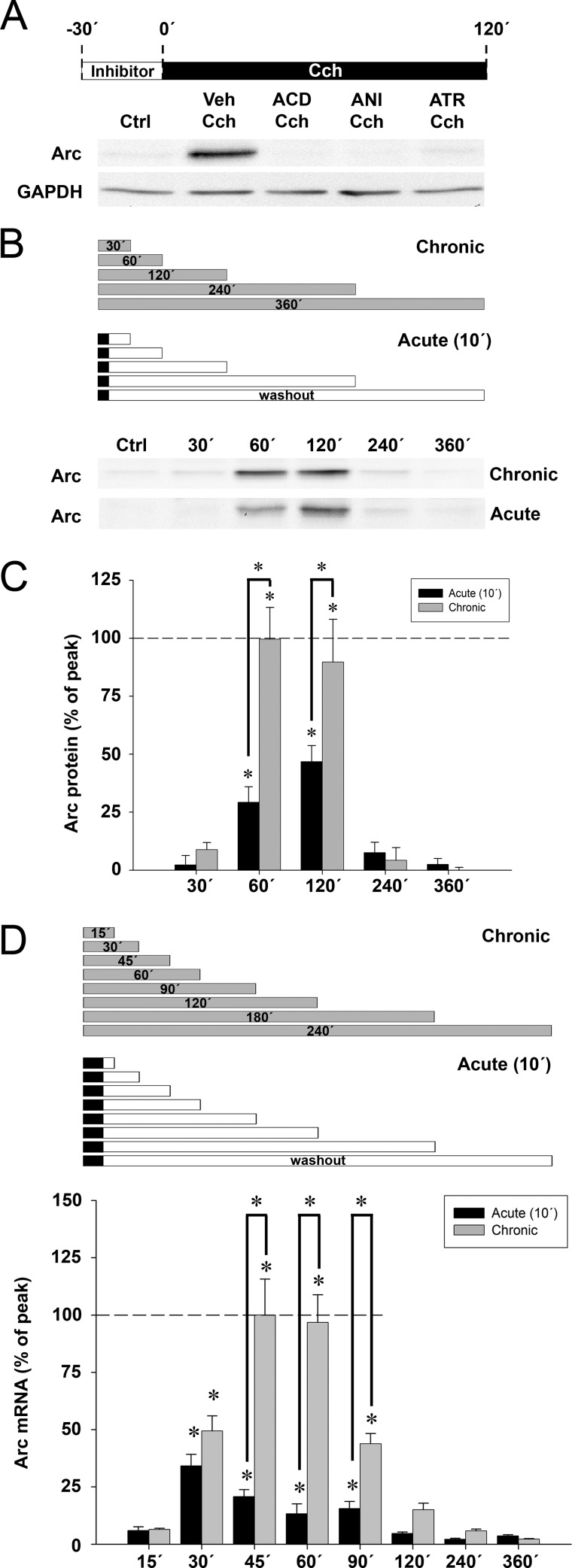
Human SH-SY5Y neuroblastoma cells are responsive to cholinergic stimulation by Cch and express Arc mRNA upon activation of M1/M3 mAchR subtypes (45, 51). The technical simplicity of this cellular model allows for detailed pharmacological study of the signaling pathways regulating Arc expression. First, we found that continuous treatment with Cch (50 μm) for 120 min triggered Arc protein expression (Fig. 1A). Pretreatment with the transcriptional inhibitor, actinomycin D (ACD), the protein synthesis inhibitor, anisomycin, or the specific mAchR antagonist, atropine, abolished Cch-induced Arc protein synthesis (Fig. 1A). Thus, Cch triggers mAchR-specific, de novo Arc protein synthesis in SH-SY5Y cells.
FIGURE 1.
Carbachol induces mAchR-mediated rapid, transient Arc mRNA and protein expression in human SH-SY5Y neuroblastoma cells. A, representative Western blots show expression of Arc protein in SH-SY5Y cells pretreated for 30 min with ACD, anisomycin (ANI), atropine (ATR), or dimethyl sulfoxide vehicle (Veh), then stimulated with carbachol (Cch, 50 μm) for 2 h (Ctrl: untreated cells). GAPDH served as a loading control. B, representative Western blots show Arc expression at 30, 60, 120, 240, and 360 min after the start of chronic Cch treatment (upper panel) or acute, 10-min long Cch treatment followed by washout (lower panel). C, quantitative analysis of Western blots shows expression of Arc protein resulting from acute (black boxes) or prolonged (gray boxes) Cch treatment. Arc levels are normalized to GAPDH and expressed in percent of the maximum Arc expression relative to untreated control cells. Asterisks indicate significant increase relative to Ctrl or between time groups (p < 0.05; n = 3–4). D, quantitative analysis of Arc mRNA expression by real-time semiquantitative PCR in SH-SY5Y cells acutely (black boxes) or continuously (gray boxes) treated with Cch at time points ranging from 15 to 360 min after Cch application. Arc levels are normalized to both hypoxanthine-guanine phosphoribosyltransferase and cyclopholin and expressed in percent of the maximum Arc expression relative to untreated cells. Asterisks indicate a significant increase relative to Ctrl (p < 0.05; n = 5–6).
Differential Effects of Acute and Chronic Cch Treatment on Arc Protein Expression
We sought to characterize the time course of Arc protein expression during continuous, chronic Cch treatment. Arc levels were investigated at five time points ranging from 30 min to 6 h (Fig. 1, B and C, gray boxes). Low, constitutive Arc protein expression was detected in untreated cells (Ctrl). Cch-induced Arc protein expression increased abruptly after 30 min of Cch application, reaching a peak increase of ∼5.6-fold at 1 to 2 h. Surprisingly, however, Arc protein levels declined rapidly to control levels by 4 h.
During sleep in humans, cats, and rodents, release of acetylcholine occurs predominantly during REM sleep epochs that is usually restricted to a few minutes. Thus, we investigated the time course of Arc protein synthesis following acute (10 min) exposure to Cch followed by washout (Fig. 1, B and C, black boxes). Surprisingly, acute Cch treatment evoked Arc expression with the same time course as chronic treatment. However, the amount of Arc protein expressed was ∼2-fold greater during chronic Cch treatment.
Chronic Cch Treatment Induces Peak Arc Protein Expression via Prolonged Transcription
To determine whether the boost in Arc protein expression during chronic Cch treatment reflects enhanced transcription, we first measured the time course of Arc mRNA expression after acute (10 min) Cch treatment and compared this with the effects of various durations of chronic, continuous Cch treatment (Fig. 1D). Acute mAchR stimulation (Fig. 1D, black boxes) led to a rapid, transient elevation in Arc mRNA levels, peaking at 30 min with a ∼22-fold change, and returning to the level of control, untreated cells 2 h after washout. Chronic Cch treatment evoked similar increases in Arc mRNA levels at 30 min. After this time, however, Arc mRNA levels climbed rapidly to reach a peak ∼66-fold increase at 45–60 min (Fig. 1D, gray boxes). After 2 h in the presence of Cch, Arc mRNA declined to control levels. Thus, Cch stimulation lasting more than 30 min boosts Arc mRNA and protein levels, although the persistence of the expression remains strongly curtailed.
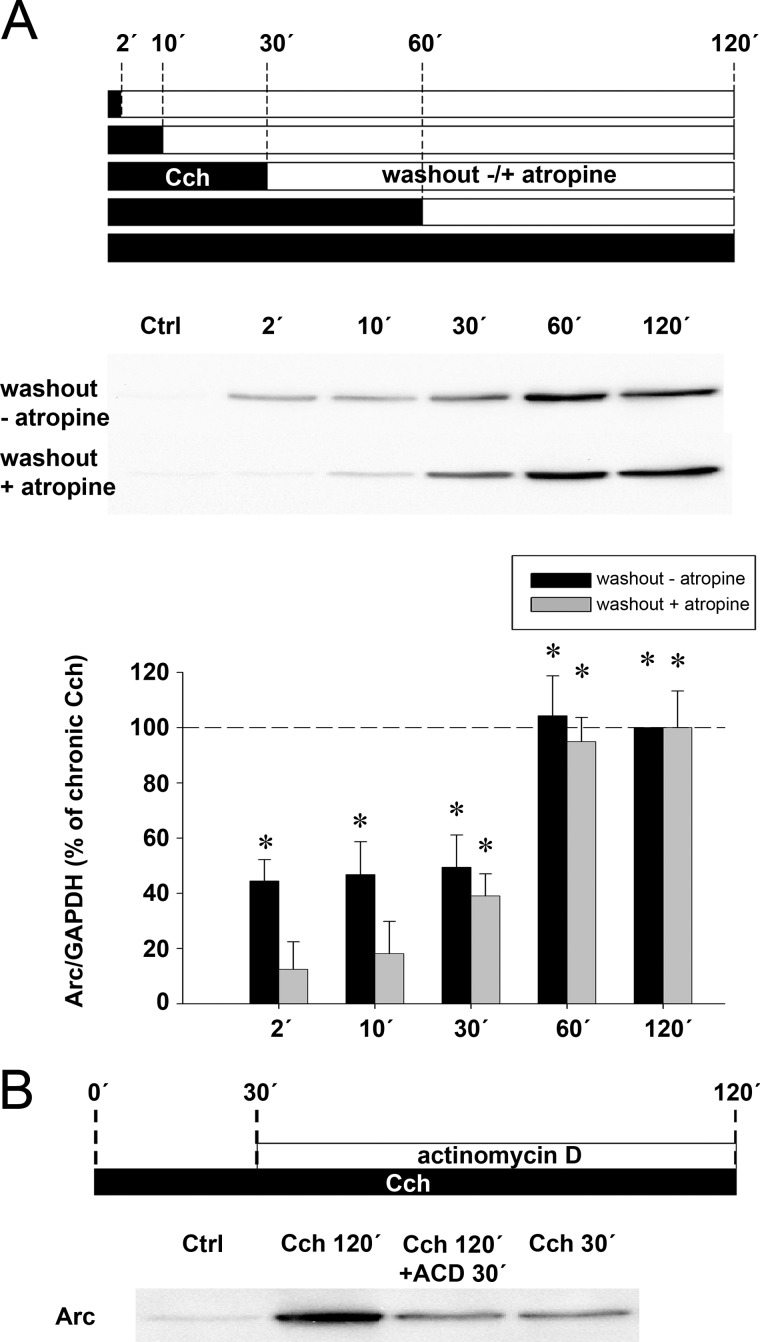
Next we investigated the effect of 5 different durations of acute Cch stimulation on Arc protein expression. Cch was applied for 2, 10, 30, 60, or 120 min before washout and cells were collected 120 min after the start of Cch treatment (Fig. 2A, black boxes). Peak increases of Arc expression of 5.2-fold were observed in the 60- and 120-min treatment groups. Exposure to Cch for shorter time periods of 2, 10, or 30 min all produced a significant increase in Arc protein expression of ∼45% of that induced by 60 min of Cch treatment.
FIGURE 2.
Chronic Cch treatment boosts Arc protein expression via a transcription-dependent mechanism. A, representative Western blots and quantitative analysis show Arc protein expression in cells treated with Cch for 2, 10, 30, 60, or 120 min (black boxes) followed by washout (white boxes) with or without atropine until lysate preparation. All lysates were prepared 120 min after beginning the Cch treatment. Arc levels are normalized to GAPDH and expressed in percent of Arc expression induced by chronic Cch treatment. Asterisks indicate significant increase relative to Ctrl (p < 0.05; n = 5). B, representative Western blot shows expression of Arc protein in SH-SY5Y cells stimulated with Cch for 30 or 120 min. Cells stimulated for 120 min additionally received ACD or vehicle 30 min after the onset of stimulation (Ctrl, unstimulated cells).
We hypothesized that the two distinct plateaus of Arc protein expression may be the result of temporally distinct early and late transcriptional events. Supporting this idea, addition of ACD to the medium 30 min after the onset of chronic Cch treatment reduced Arc protein expression to levels obtained by an acute, 30-min exposure to Cch (Fig. 2B, see also Fig. 4D). Hence, there appears to be an inflection point in the Arc transcriptional response, such that Cch treatment beyond 30 min markedly boosts transcription and approximately doubles the amount of Arc mRNA and protein expressed.
FIGURE 4.
ERK signaling and intracellular calcium release differentially control Cch-induced Arc protein expression at transcriptional and translational levels. A, representative Western blot and quantitative analysis show Arc protein expression in SH-SY5Y cells pretreated for 30 min with U0126, rapamycin (rapa), wortmannin (wort), or vehicle (veh), then stimulated with Cch for 120 min (n = 4). B, representative Western blot and quantitative analysis show Arc protein expression in cells pretreated for 30 min with BAPTA-AM (bapta), thapsigargin (thapsi), or vehicle (veh), then stimulated with Cch for 120 min (n = 4). Arc levels are normalized to GAPDH and expressed in percent of vehicle pretreatment. C, real-time semiquantitative PCR shows Arc mRNA expression in cells pretreated for 30 min with U0126, BAPTA-AM, thapsigargin, anisomycin (ANI), or vehicle, then stimulated with Cch for 30 min (n = 5–6). Arc levels are normalized to both hypoxanthine-guanine phosphoribosyltransferase and cyclopholin and expressed in percent of vehicle pretreatment. Asterisks indicate a significant change relative to vehicle pretreatment (p < 0.05). D, representative Western blot and quantitative analysis show Arc protein expression in Cch-stimulated cells treated with U0126, BAPTA-AM, thapsigargin, ACD, atropine (ATR), or vehicle 30 min after the start of Cch treatment (n = 4). Arc levels are normalized to GAPDH and expressed in percent of vehicle pretreatment. Asterisks indicate a significant change relative to vehicle pretreatment or between treatment groups (p < 0.05).
Acute Cch Triggers Prolonged, Atropine-sensitive mAchR Signaling
We were surprised by the fact that 2, 10, and 30 min of Cch exposure produced an equivalent enhancement of Arc protein expression. Previous work performed in isolated heart membranes showed that binding of Cch to mAchR induces formation of mAchR-G protein complexes that are stable beyond removal of the agonist but dissociate upon atropine treatment (52). Such stable mAchR signaling might explain our observations. First we confirmed earlier reports (53–55), showing that treatment with Cch for 2 min triggers ERK phosphorylation lasting at least 30 min (supplemental Fig. S1A). We then examined the effect of applying atropine during the washout period in cells exposed to Cch for 2 or 10 min. As shown in supplemental Fig. S1B, washout with atropine rapidly inhibited ERK phosphorylation. Thus, acute Cch induces sustained, atropine-sensitive ERK signaling.
We then sought to determine the impact of atropine-sensitive signaling on Arc expression. As previously performed (Fig. 2A, black boxes), Cch was applied for durations of 2, 10, 30, 60, or 120 min, but this time treatment was followed by atropine washout. Cells were collected 120 min after the start of Cch treatment (Fig. 2A, gray boxes). Arc expression was not affected by atropine in the 30- and 60-min Cch treatment groups (compare with black boxes), yet it blocked the increase in Arc expression induced by 2- and 10-min long Cch exposure. These results show that acute 2- or 10-min long Cch treatments drive Arc protein synthesis via sustained mAchR signaling that lasts between 10 and 30 min beyond agonist removal.
Cch Application Mimicking REM Sleep Bursts Induces Maximal Arc Protein Expression
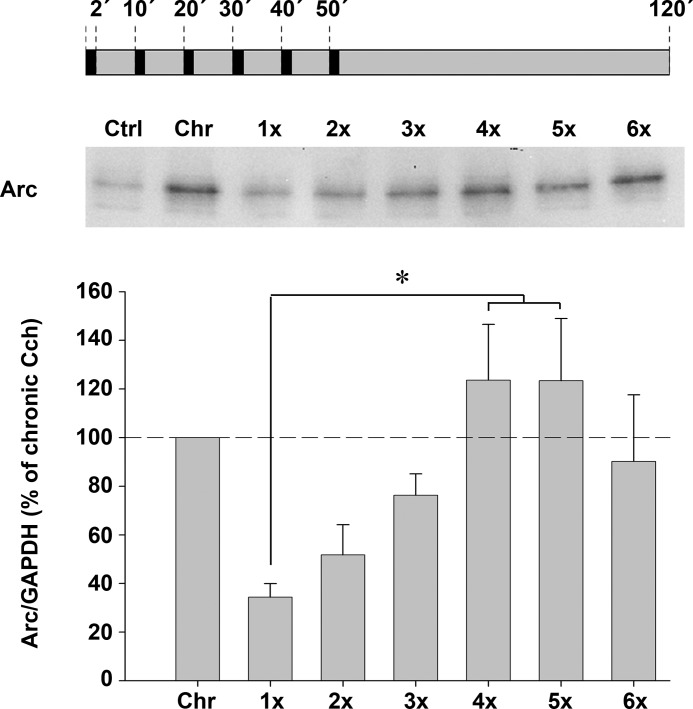
We were intrigued by a possible analogy between our finding that Arc protein expression could be initiated by cholinergic epochs as short as 2 min and the occurrence of short REM sleep episodes in rodents. Previous studies of sleep architecture in rats showed that most REM sleep epochs last for ∼0.5 to 2 min and reoccur with a frequency of ∼10 min (56, 57). Modeling the recurrent cholinergic epochs of natural REM sleep, we designed a paradigm where SH-SY5Y cells were exposed to up to 6 consecutive, 2-min long Cch applications separated by 8-min long washout periods. Arc protein levels were assessed in lysates prepared 2 h after the onset of the initial stimulation (Fig. 3). We found that Arc expression progressively increased with each Cch epoch, reaching a plateau after the fourth episode (Fig. 3). Interestingly, maximal Arc protein levels were obtained by 4 to 6 acute, consecutive Cch applications. The increase in Arc expression matched that induced by chronic, 2-h-long mAchR stimulation.
FIGURE 3.
Repeated, acute Cch treatment maximizes Arc protein expression. Representative Western blot and quantitative analysis show Arc protein expression in cells treated repeatedly (up to 6 times) with Cch followed by washout. Each Cch application lasted for 2-min, and 10-min intervals separated the onset of consecutive applications. All lysates were prepared 120 min after beginning the initial Cch application. Arc levels are normalized to GAPDH and expressed in percent of Arc expression induced by chronic Cch treatment. Asterisks indicate a significant increase relative to single, acute Cch stimulation (p < 0.05; n = 4).
Cch-induced Arc mRNA and Protein Expression Depends on ERK, but Not PI3K or mTOR, Signaling
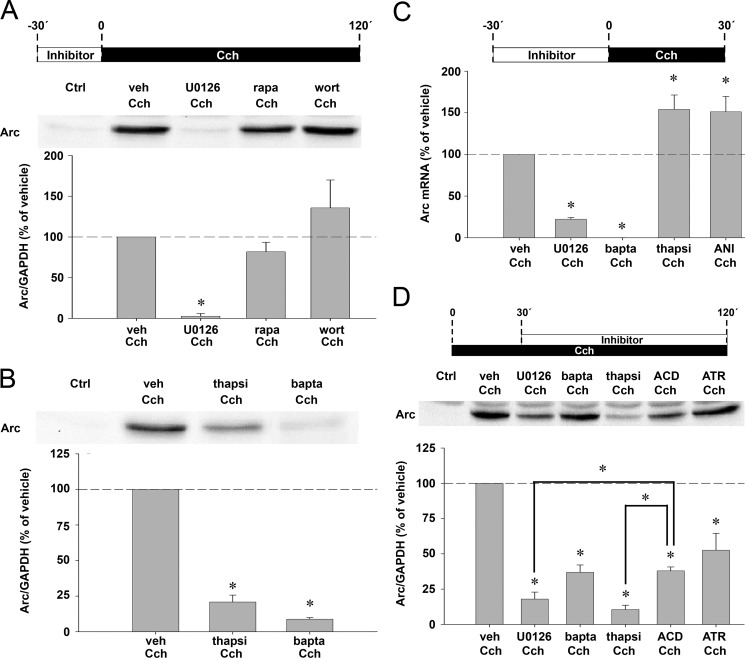
mAchR stimulation by Cch was previously shown to activate ERK, PI3K, and mTOR kinase. These major signaling hubs have multiple roles in regulation of gene expression at the transcriptional and translational levels. We find that pretreatment with the MEK inhibitor U0126 blocks ERK phosphorylation and inhibits Cch-induced Arc mRNA and protein expression by 80 (Fig. 4C) and 98% (Fig. 4A), respectively. Treatment with the mTOR inhibitor rapamycin had no detectable effect on Cch-induced Arc synthesis (Fig. 4A), although it abolished enhanced phosphorylation of ribosomal protein S6, a major downstream target of mTOR (supplemental Fig. S2A). Similarly, Arc synthesis was unaffected by the PI3K inhibitor wortmannin (Fig. 4A), whereas phosphorylation of Akt, a direct target of PI3K, was strongly reduced (supplemental Fig. S2B). In contrast, the protein synthesis inhibitor anisomycin completely prevented Arc protein expression (Fig. 1A). Altogether, these results indicate that Arc protein expression depends on ERK, but not mTOR or PI3K, signaling.
Intracellular Calcium Is Necessary for Cch-induced Arc Transcription
Cch induces rapid, transient calcium release from IP3-sensitive intracellular stores (58, 59). Moreover, we noted that each of four consecutive, acute Cch epochs also lead to rapid release of intracellular calcium with comparable intensity (supplemental Fig. S3C). See supplementary Material for corresponding materials and methods.
We then investigated the possible contribution of intracellular calcium to Cch-induced Arc expression. Treatment with the membrane-permeable calcium chelator, BAPTA-AM, led to a ∼91% inhibition of Arc protein expression (Fig. 4B) and essentially eliminated Cch-evoked Arc transcription (Fig. 4C). In line with previous studies (54, 55), we noted that chelation of intracellular calcium by BAPTA-AM did not prevent Cch-induced ERK phosphorylation (supplemental Fig. S4). Thus, mAchR induces Arc transcription via two independent and convergent pathways requiring ERK signaling and calcium elevation, respectively.
Thapsigargin, a specific inhibitor of the endoplasmic reticulum calcium ATPase pump, depletes almost completely IP3-releasable intracellular calcium pools within 15 min of treatment. As a consequence, calcium accumulates at high concentrations in the cytosol (58, 60, 61). Consistent with calcium sensitivity of the Arc promoter (62), thapsigargin pretreatment increased Cch-induced Arc mRNA expression by ∼50% (Fig. 4C). In surprising contrast, thapsigargin also reduced Cch-induced Arc protein levels by 78% (Fig. 4B).
U0126 and Thapsigargin, but Not BAPTA-AM, Inhibit Arc Expression at the Post-transcriptional Level
We asked whether post-transcriptional mechanisms contribute to regulation of Arc expression by ERK and calcium. Taking advantage of the time course of Arc mRNA and protein expression (Fig. 1, D and C, respectively), U0126, thapsigargin, and BAPTA-AM were applied at an early time point (30 min) when Arc mRNA levels are elevated but Arc protein is still low. Inhibitors were thus applied 30 min after the start of Cch treatment and lysates were prepared after 2 h of continuous Cch exposure (Fig. 4D). Time-matched ACD treatment reduced Arc levels by 62% relative to vehicle, thus defining the transcriptional component in this experimental paradigm. We find that U0126 and thapsigargin significantly decrease Arc protein expression by 84 and 91%, respectively. The inhibition of Arc expression by U0126 and thapsigargin was significantly greater than that observed after ACD treatment. In contrast, BAPTA-AM had the same effect as ACD, reducing Arc expression to 63% relative to vehicle. Thus, ERK signaling and a thapsigargin-triggered mechanism significantly regulate Arc expression at the post-transcriptional level.
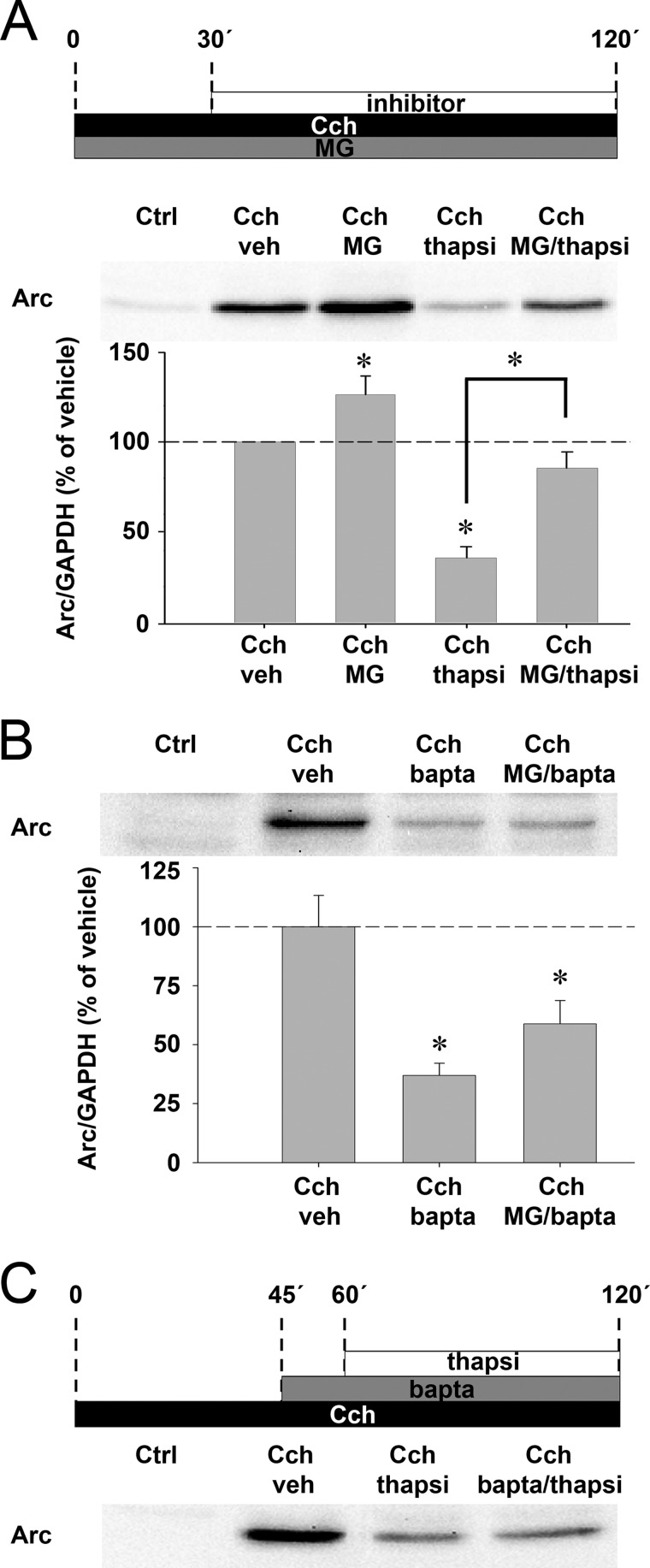
Thapsigargin Triggers Proteasomal Degradation of Cch-induced Arc Protein
Next, we sought to determine whether thapsigargin curtails Arc protein expression by translational repression or protein degradation. SH-SY5Y cells were stimulated with Cch in the presence of the proteasomal inhibitor, MG-132, then treated with thapsigargin 30 min later (Fig. 5A). MG-132 blocked the inhibitory effect of thapsigargin on Arc protein expression, suggesting that thapsigargin stimulates proteasomal degradation of Arc. Because thapsigargin releases calcium from internal stores, we hypothesized that Arc is targeted for degradation through a calcium-dependent mechanism. Using the same protocol, we replaced thapsigargin with BAPTA-AM. BAPTA-AM led to a strong decrease in Cch-evoked Arc expression (as also seen in Fig. 4B), but this decrease was not rescued by MG-132 (Fig. 5B). Similarly, treatment with BAPTA-AM 15 min prior to thapsigargin treatment failed to rescue Arc expression (Fig. 5C). Thus, induction of Arc proteasomal degradation by thapsigargin must be induced by a mechanism other than acute calcium elevation.
FIGURE 5.
Thapsigargin triggers rapid proteasomal degradation of Cch-induced Arc protein. A, representative blot and quantitative analysis show Arc protein expression in cells stimulated with Cch and treated simultaneously with MG-132 (MG) or vehicle (veh). Cells additionally received thapsigargin (thapsi) or vehicle 30 min after the start of Cch stimulation. B, representative blot and quantitative analysis show Arc protein expression in cells stimulated with Cch and treated simultaneously with MG-132 or vehicle. Cells additionally received BAPTA-AM (bapta) or vehicle 30 min after the start of Cch stimulation. Arc levels are normalized to GAPDH and expressed in percent of vehicle treatment. Asterisks indicate a significant change relative to vehicle or between groups (p < 0.05; n = 4). C, representative blot shows Arc protein expression in cells stimulated with Cch, then treated with BAPTA-AM and thapsigargin, respectively. Additional controls are provided in Fig. S5.
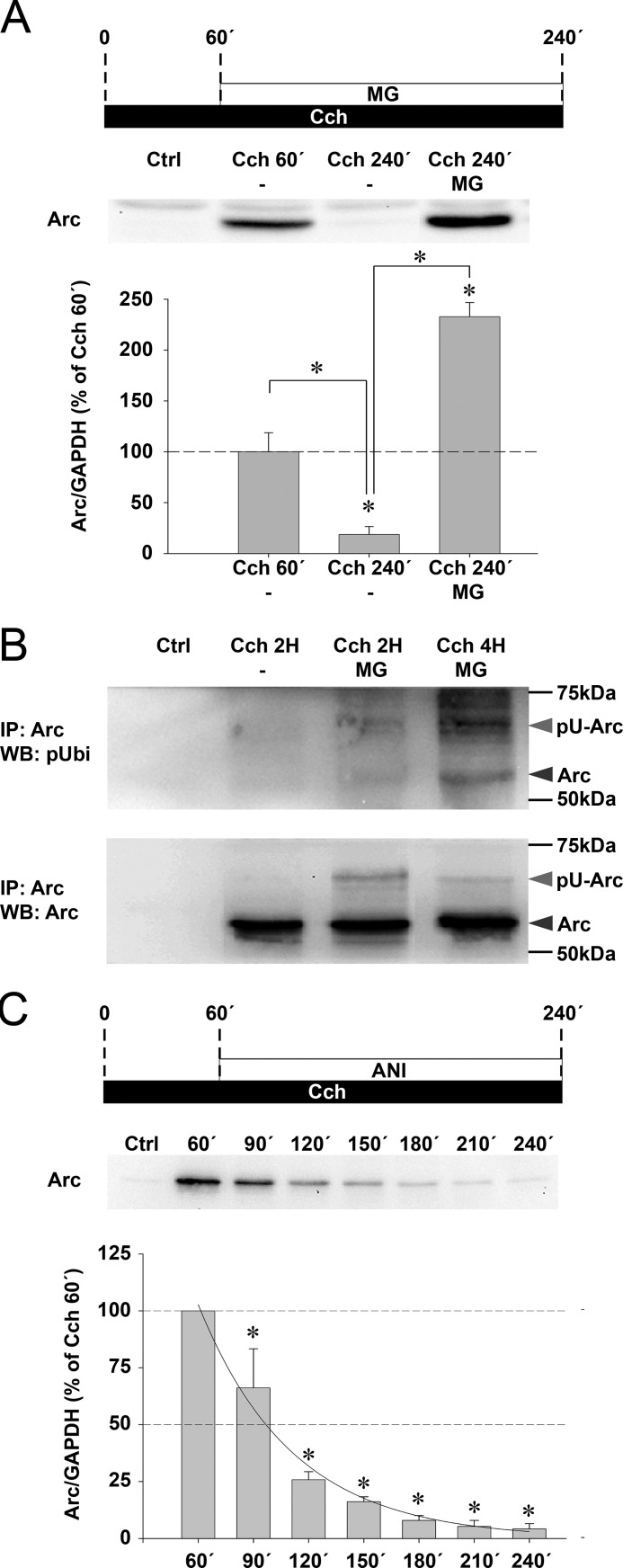
Proteasomal Degradation Regulates Turnover of Cch-induced Arc Protein
As shown in Fig. 1C, Cch-induced Arc protein expression peaks between 1 and 2 h, but returns to control levels by 4 h in the continuous presence of Cch. We investigated whether this rapid loss of Arc protein is mediated by proteasomal degradation. SH-SY5Y cells were stimulated with Cch and treated 1 h later with MG-132 (Fig. 6A). MG-132 treatment led to a significant 12.4-fold increase in Arc levels relative to the time-matched Cch-treated control (240 min), and a 2.3-fold rise relative to the 60-min Cch treatment group. Arc immunoprecipitates were immunoblotted with a polyubiquitin antibody for detection of ubiquitin-conjugated Arc. Proteasome inhibition by MG-132 resulted in accumulation of ubiquitinated Arc in cells stimulated with Cch for 2 and 4 h (Fig. 6B). The results implicate the ubiquitin-proteasome system in Arc degradation during Cch stimulation.
FIGURE 6.
Cch-induced Arc protein expression is strictly limited by ubiquitin proteasome-mediated degradation. A, representative Western blot and quantitative analysis show Arc protein expression 60 or 240 min after the start of continuous Cch treatment. Cells stimulated for 240 min additionally received MG-132 (MG) or vehicle 60 min after Cch application. Arc protein levels are normalized to GAPDH and expressed in percent of Arc expression at 60 min post-Cch application. Asterisks indicate a significant change between groups (n = 4, p < 0.05). B, cells were stimulated with Cch for 2 and 4 h in the presence of MG-132 or for 2 h in the presence of vehicle. Lysates were processed for Arc immunoprecipitation. Western blots (WB) using a polyubiquitin antibody (pUbi, upper panel) or an alternative Arc antibody (lower panel) reveal the accumulation of both Arc (dark gray arrowhead) and polyubiquitinated Arc protein (pU-Arc, light gray arrowhead) in MG-132-treated cells. Ctrl lane corresponds to a negative control where SH-SY5Y cell lysate was omitted during the immunoprecipitation (IP). C, representative Western blot and quantitative analysis show a time course of Arc protein expression induced by chronic Cch treatment and where anisomycin is applied onto the cells after 60 min of stimulation. Arc protein levels are normalized to GAPDH and expressed in percent of Arc expression at 60 min of post-Cch stimulation. Asterisks indicate a significant change relative to maximum Arc expression (n = 4, p < 0.05). Solid line represents the corresponding regression curve calculated from the last 3 h of the experiment. Additional controls are provided in Fig. S5.
We next sought to evaluate the contribution of proteasomal degradation versus protein synthesis to the lifespan of Cch-induced Arc protein. We applied anisomycin at the peak of Arc protein synthesis (i.e. after 1 h) and retrieved samples every 30 min for the next 3 h. After 30 and 60 min of translation blockade, Arc protein levels rapidly decreased to ∼66 and ∼25%, respectively (Fig. 6C, compare with Fig. 1C, gray boxes). Based on a regression analysis of Arc protein levels, the half-life of Arc protein is estimated to be ∼37 min under these experimental conditions.
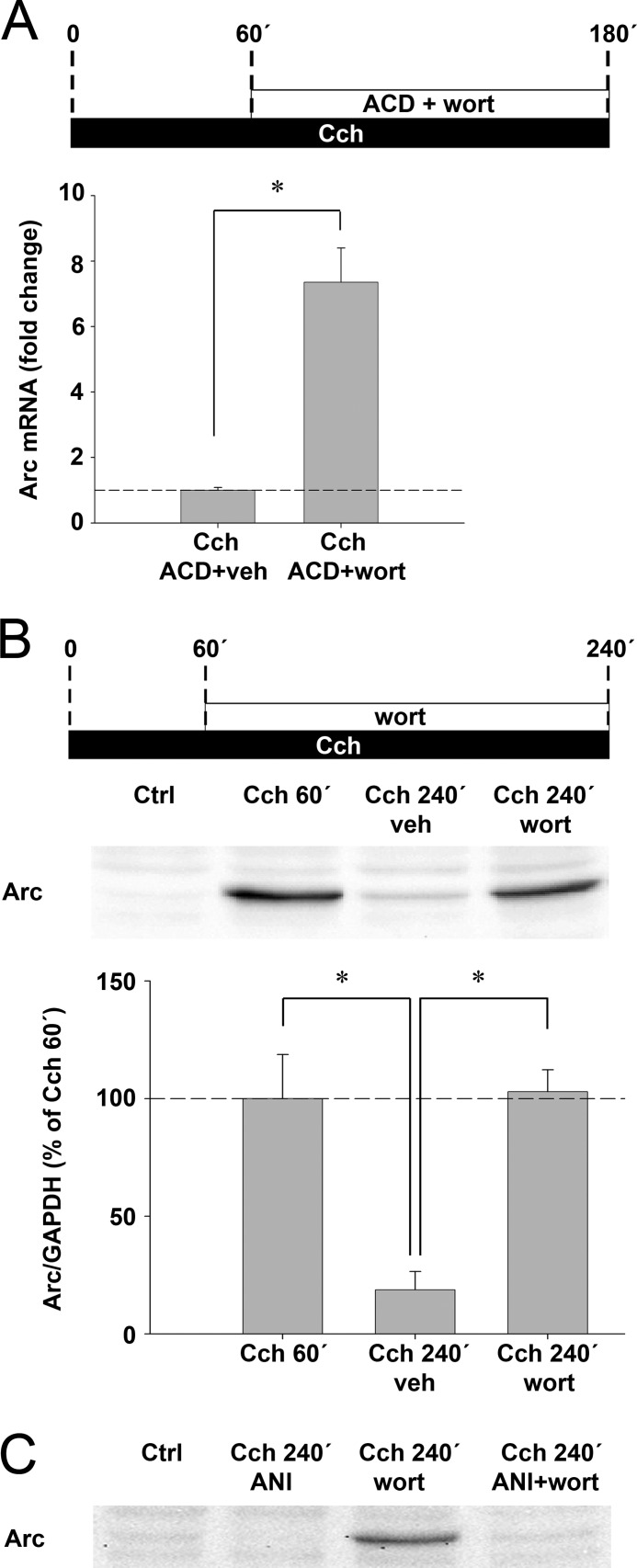
Regulation of Arc Protein Expression by Translation-dependent RNA Decay
Evidence suggests that Arc mRNA is destabilized through nonsense-mediated decay (NMD) (42, 43, 63). Consistent with this form of translation-dependent decay, increased Cch-induced Arc mRNA levels were observed under anisomycin treatment (Fig. 4C). hSMG-1, a key constituent of the NMD machinery, is directly repressed by wortmannin (64). SH-SY5Y cells were treated with wortmannin and ACD or ACD alone 1 h after onset of Cch exposure and processed for real-time PCR at 3 h (Fig. 7A). ACD was added in these experiments to rule out the possibility that wortmannin enhances Arc transcription. As shown in Fig. 7A, wortmannin increased Arc mRNA levels by 7.3-fold relative to vehicle control, indicating a strong stabilizing effect on Arc transcripts. In a similar manner, accumulation of Arc protein was observed 4 h after Cch application in the presence of wortmannin (Fig. 7B). To confirm that wortmannin-induced enhancement of Arc protein expression was due to extended translation as opposed to inhibition of protein degradation, Cch-stimulated cells were treated with wortmannin in combination with the translation inhibitor anisomycin. As shown in Fig. 7C, wortmannin failed to enhance Arc protein expression during translational block. We conclude that cholinergically induced Arc is subjected to wortmannin-sensitive translation-dependent mRNA decay.
FIGURE 7.
Arc mRNA is destabilized by translation-dependent RNA decay. A, quantitative analysis of Arc mRNA expression by real-time semiquantitative PCR shows Arc levels in cells stimulated with Cch for 3 h. Actinomycin D was applied in combination with wortmannin (wort) or vehicle (veh) 60 min after the start of continuous Cch treatment. Arc mRNA levels are normalized to both hypoxanthine-guanine phosphoribosyltransferase and cyclopholin and expressed as fold-change relative to vehicle treatment (p < 0.05; n = 6). B, representative Western blot and quantitative analysis show Arc protein expression 60 or 240 min after the start of continuous Cch treatment. Cells received wortmannin or vehicle 60 min after the start of Cch treatment. Arc protein levels are normalized to GAPDH and expressed in percent of Arc expression at 60 min post-Cch stimulation. Asterisks indicate a significant change between groups (n = 4, p < 0.05). C, representative Western blot shows Arc protein expression in cells stimulated with Cch for 240 min. Cells additionally received wortmannin and/or anisomycin (ANI) 1 h after onset of stimulation. Additional controls are provided in Fig. S5.
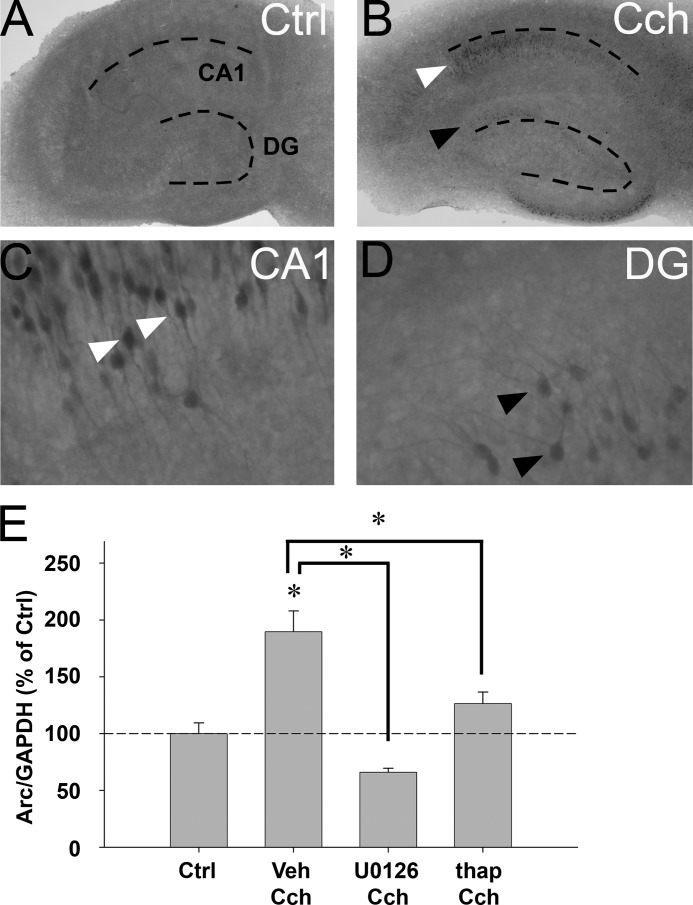
Cch Induces Arc Protein Expression in Organotypic Hippocampal Slice Cultures
Septohippocampal afferents, which combine GABAergic and cholinergic projections, are important for behavioral induction of Arc expression in the hippocampus (48). Lesions of cholinergic neurons in medial septum decrease hippocampal Arc expression (47). We investigated Arc protein expression in OTSCs during Cch treatment. Although Arc immunoreactivity was virtually absent in untreated slices (Fig. 8A), Arc-positive CA1 pyramidal cells were clearly visible after 2 h of continuous Cch (50 μm) treatment (Fig. 8, B and C). Arc-positive granule cells were also sparsely detected in the dentate gyrus (Fig. 8, B and D).
FIGURE 8.
Cch induces Arc protein expression in rat organotypic hippocampal slice culture. A and B, immunohistochemistry reveals Arc protein expression in untreated slices (A, ctrl) and slices stimulated with Cch (50 μm) for 2 h (B, Cch). Arc-positive cells are visible in CA1 (white arrowhead) and dentate gyrus (DG, black arrowhead). Dotted lines indicate the DG- and CA1 cell layers. C and D, high magnification reveals Arc-positive CA1 pyramidal cells (C, white arrowheads) and dentate granule cells (D, black arrowheads). Note that Arc protein is found in dendrites, the somatic cytoplasm, and nucleus of granule cells and CA1 pyramidal cells. E, quantitative analysis of Western blots shows Arc protein expression in slices pretreated with U0126, thapsigargin (thapsi), or vehicle (veh) and then stimulated with Cch for 2 h. Arc protein levels are normalized to GAPDH and expressed in percent of Arc expression in untreated slices (Ctrl). Asterisks indicate a significant change relative to Ctrl and between groups (n = 4–5, p < 0.05).
Cch-induced Arc Expression in Hippocampal Slices Is Sensitive to U0126 and Thapsigargin
We finally sought to verify whether Cch-induced Arc protein synthesis in OTSCs and SH-SY5Y cells shared the requirement for ERK signaling and sensitivity to thapsigargin. OTSC were pretreated with vehicle, U0126, or thapsigargin for 30 min and Cch was then applied for 2 h (Fig. 8E). Vehicle-treated slices exhibited a 1.9-fold increase in Arc protein levels relative to untreated slices. Cch-induced Arc protein expression was abolished by U0126 and significantly reduced by ∼71% by thapsigargin (Fig. 8E).
DISCUSSION
Over 15 years of research have revealed Arc as a molecular keystone for diverse forms of activity-induced synaptic plasticity (LTP, LTD, and homeostatic scaling), long term memory, and postnatal cortical development (28, 29).
Evidence from disparate preparations and paradigms has identified mechanisms underlying Arc expression at multiple levels (transcription, translation, mRNA and protein stability; for a review, see Ref. 28). Our study describes for the first time how a single paradigm, namely muscarinic receptor activation, puts each of these machineries into action to finely tune the levels and lifespan of Arc protein. Fig. 9 summarizes the regulatory mechanisms and their estimated dynamics. First, mAchR stimulation triggers ERK signaling and release of calcium from intracellular IP3-sensitive stores, resulting in rapid Arc transcription. The subsequent levels of Arc mRNA reflect a balance between transcriptional drive and translation-dependent mRNA decay. mRNA expression kinetics following acute and chronic Cch application are similar, but chronic stimulation beyond 30 min boosts transcriptional activity and doubles the amount of Arc protein expressed. Translation of Arc transcripts is rapidly put into action via ERK signaling, independently of intracellular calcium and activation of the PI3K-Akt-mTOR pathway. Finally, the lifespan of the Arc protein is curtailed by proteasomal degradation, concurrently with mRNA decay.
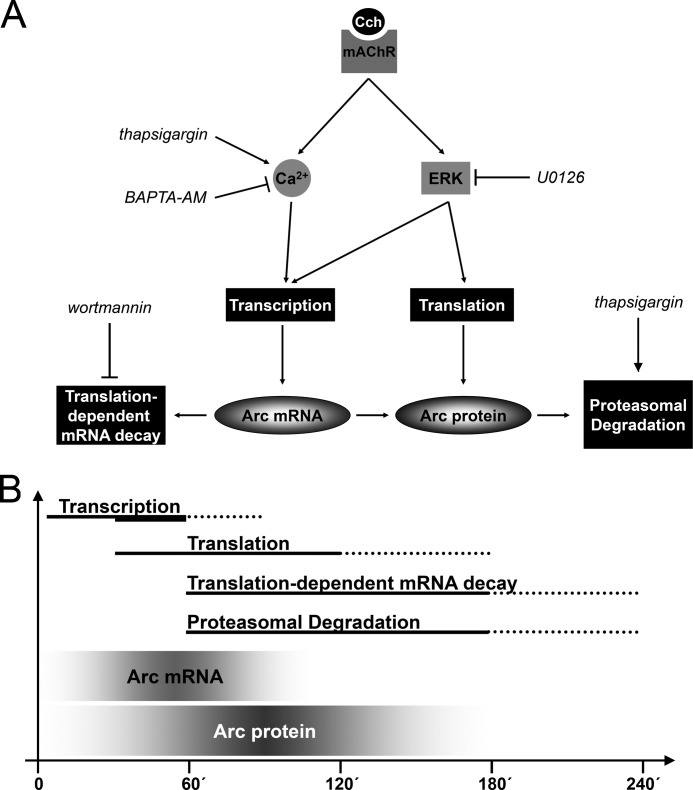
FIGURE 9.
Model of mAchR-mediated regulation of Arc expression. A, this model shows the major cellular pathways and regulatory mechanisms governing synthesis and degradation of Arc mRNA and protein. Stimulation of mAchR by Cch activates Arc transcription via two independent pathways involving ERK phosphorylation and release of calcium from IP3-sensitive intracellular stores. Cch-induced ERK activation also modulates translation of Arc mRNA. Destabilization of Arc mRNA is mediated by wortmannin-sensitive, translation-dependent decay. Finally, Arc protein is ubiquitinated and targeted for proteasomal degradation. Thapsigargin induces Arc degradation, suggesting regulation of Arc degradation during ER stress. B, sketch of Arc mRNA and protein dynamics and regulatory mechanisms. Acute Cch treatment (<30 min) and chronic Cch treatment both trigger rapid and transient Arc mRNA expression. However, chronic treatment triggers a delayed boost in transcription (thick solid line) resulting in enhanced protein expression. Translation of Arc transcripts is also rapidly initiated as Cch-induced Arc protein is detectable as soon as 30 min of treatment. Arc protein expression peaks after 1–2 h and returns to basal levels after 4 h. The reversal of Arc levels is the result of combined destabilization of Arc mRNA and protein by translation-dependent decay and proteasomal degradation, respectively. Solid lines show the time course based on the data obtained. Dotted lines are estimates of the complete time course.
mAchR stimulation induces prolonged ERK activation in neurons (53, 65), resulting in Arc transcription and translational activation (Figs. 1 and 4 and supplemental Fig. S1). Following brief, acute exposure to Cch, the formation of stable atropine-sensitive mAchR·G-protein complexes allows sustained ERK phosphorylation and enhanced Arc expression. mAchR-induced calcium mobilization also plays an important role in Arc expression because both chelation of intracellular calcium and depletion of IP3-sensitive stores dramatically alters Arc transcription (Fig. 4). Previous studies have shown that M3 receptors, one of five subtypes of mAchR, activate ERK in a calcium-independent manner (54, 55). M3 receptors activate PKC-dependent phosphorylation of ERK (54, 55) and Arc transcription (45). We similarly found that Cch-induced ERK phosphorylation was insensitive to calcium chelation by BAPTA-AM (supplemental Fig. S4). The mechanism of ERK and calcium pathway convergence on cholinergically evoked Arc transcription is beyond the framework of the present study, but is likely to involve known calcium and ERK-responsive elements in the Arc promoter (62, 66, 67).
The amount of protein expressed from nascent Arc transcripts during cholinergic signaling is strictly limited by concurrent destabilization of Arc mRNA and protein. As discovered by Giorgi et al. (42, 43), the presence of two introns in the Arc 3′ UTR make Arc a natural substrate for NMD. In NMD, transcripts are destabilized after one, or only a few rounds, of translation (42, 43, 68). The NMD pathway involves three proteins in the UPF family (UPF1, -2, and -3; a.k.a. SMG-2–4) (69). One of these proteins, UPF1, is activated by SMG-1, a member of the phosphatidylinositol kinase-related family, and this kinase is inhibited by wortmannin (64). The present work shows that wortmannin increases Arc protein levels by stabilizing Arc mRNA (Fig. 7). In agreement with Ref. 70, inhibition of protein synthesis by anisomycin led to accumulation of Arc transcripts. The fact that inhibition of translation blocked the stabilizing effect of wortmannin on Arc mRNA attests to translation-dependent decay (Fig. 7). We therefore conclude that Cch-induced Arc mRNA is rapidly degraded by wortmannin-sensitive translation-dependent decay. However, it remains to be determined whether NMD is the mechanism of decay.
The impact of proteasomal degradation on Arc expression levels is profound. In the continuous presence of Cch, Arc protein drops to nonstimulated control levels within 2 h of peak expression. In the presence of MG-132, Arc levels at the 2-h time point are not only enhanced relative to control, but exceed peak expression by 2-fold, as levels of polyubiquitinated Arc rise dramatically (Figs. 1 and 6). Conversely, blockade of translation at the peak of Cch-induced Arc protein expression leads to a rapid decrease in Arc protein levels, thus revealing a half-life of ∼37 min (Fig. 6). By counterbalancing synthesis, rapid turnover is likely important for concentration-dependent and time-dependent actions of Arc, while preventing pathological effects of Arc accumulation.
Recent work suggests that dysregulation of Arc protein degradation has serious deleterious effects on brain function (40). Angelman syndrome is caused by lack of the E3 ubiquitin ligase, Ube3a. Ube3a polyubiquitinates Arc, targeting the protein for proteasomal removal. In Angelman syndrome, the pathological accumulation of Arc causes excessive endocytosis of AMPA-type glutamate receptors from the postsynaptic membrane, so impairing synaptic function.
We show that Cch induces Arc expression in CA1 pyramidal cells and dentate granule cells of cultured hippocampal slices. As in neuroblastoma cells, Cch-induced Arc expression in hippocampal slices is ERK-dependent and reduced by thapsigargin. The simplicity of the neuroblastoma preparation is advantageous for uncovering receptor-mediated regulation of Arc as a stepping stone toward unraveling the mechanisms in the intact cholinergic system of behaving animals. Further work is also needed to determine whether Arc mRNA decay and proteasomal degradation activity are modulated by cholinergic signaling, or whether these mechanisms are purely constitutive. Arc mRNA decay and proteasomal degradation in primary hippocampal neuronal cultures were not altered by neuronal activity, suggesting that these mechanisms may be primarily constitutive (43, 71).
LTP consolidation in the dentate gyrus of live rats requires a critical period of Arc synthesis (28). Arc mRNA and protein during this 2–4 time window are both rapidly turned over, such that consolidation depends on the continued supply of new Arc mRNA (32). ERK has been identified as a major regulator of Arc synthesis in a variety of preparations and experimental paradigms (44, 62, 71–74). During LTP consolidation, ERK dually controls Arc transcription and translation (44). As in the present study in neuroblastoma cells, Arc translation during LTP was insensitive to inhibition of the mTOR signaling pathway.
Sleep is necessary for memory consolidation and probably involves slow-wave sleep-specific and REM sleep-specific effects, and cyclical interactions between them (17, 26, 75). Cholinergic activity associated with REM sleep theta activity and phasic pontine-waves provides a cellular context conducive for LTP maintenance and expression of several immediate-early genes, including Arc (17, 25, 26, 76, 77). Cholinergic transmission, when appropriately paired with glutamatergic activity, supports late phase LTP and dendritic protein synthesis (14, 15). Whether reactivation of learning related synaptic activity patterns (memory traces) occurs during SWS or REM sleep (17, 78), the time course of synaptic consolidation on the order of hours seems to necessitate active processing across multiple sleep stages.
REM sleep epochs in rodents generally last no more than 2 min and occur in regular intervals of about 10 min (56, 57). After procedural learning tasks and active avoidance learning, the duration and frequency of REM sleep epochs is increased and disruption of this REM sleep impairs memory formation (17, 24). Thus, a particularly intriguing observation from the present work is that brief (2 min) cholinergic epochs are sufficient to induce Arc expression (Fig. 2), and patterned repetition of these short epochs generates maximal Arc protein expression (Fig. 3). To the extent these findings from SH-SY5Y cell cultures can be applied to the in vivo situation, we predict that isolated, short REM epochs occurring early during sleep are sufficient for inducing Arc expression, whereas successive periods of REM sleep maximize Arc protein expression. In both events cholinergic facilitation of Arc-dependent synaptic plasticity is predicted. However, it is also possible that differences in Arc abundance will alter plasticity thresholds or shift the balance between synaptic potentiation and depression.
Finally, the present work revealed that Arc turnover is enhanced at the post-transcriptional level by thapsigargin (Figs. 4 and 5), a drug widely used to induce ER stress by chronic depletion of calcium stores (79, 80). ER stress results in accumulation of misfolded proteins that can be compromising for ER function and cell survival. Through a process known as endoplasmic reticulum-associated degradation, these proteins are retrotranslocated to the cytoplasm for rapid elimination by the ubiquitin-proteasome system (81). Thapsigargin leads to specific degradation of several proteins such as ATF6 and Smurf1 (82, 83). Our results show that thapsigargin promotes proteasomal degradation of Arc independently of thapsigargin-induced calcium release (Fig. 5). These findings support a role for the ER in regulation of Arc degradation, at least under conditions of calcium store depletion. The ER extends into the dendritic arborescence of hippocampal neurons and is selectively targeted to large spines containing strong synapses (84, 85). Although speculative at the moment, it is conceivable that ER compartments within dendrites and spines cooperate with the ubiquitin-proteasome system in the local control of Arc protein degradation.
This work was supported by Research Council of Norway Grants 191912, 186115, and 204861, the Western Norway Regional Health Authority, and the University of Bergen.

This article contains supplemental Methods and Figs. S1–S5.
- LTP
- long term potentiation
- LTD
- long term depression
- ACD
- actinomycin D
- OTSC
- organotypic hippocampal slice cultures
- Cch
- carbachol
- REM
- rapid eye movement
- NMD
- nonsense-mediated decay
- mAchR
- muscarinic acetylcholine receptor
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl ester).
REFERENCES
- 1. Hasselmo M. E. (2006) The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 16, 710–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blokland A., Honig W., Raaijmakers W. G. (1992) Effects of intra-hippocampal scopolamine injections in a repeated spatial acquisition task in the rat. Psychopharmacology 109, 373–376 [DOI] [PubMed] [Google Scholar]
- 3. Rogers J. L., Kesner R. P. (2003) Cholinergic modulation of the hippocampus during encoding and retrieval. Neurobiol. Learn Mem. 80, 332–342 [DOI] [PubMed] [Google Scholar]
- 4. Adams S. V., Winterer J., Müller W. (2004) Muscarinic signaling is required for spike-pairing induction of long term potentiation at rat Schaffer collateral-CA1 synapses. Hippocampus 14, 413–416 [DOI] [PubMed] [Google Scholar]
- 5. Shinoe T., Matsui M., Taketo M. M., Manabe T. (2005) Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J. Neurosci. 25, 11194–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernández de Sevilla D., Núñez A., Borde M., Malinow R., Buño W. (2008) Cholinergic-mediated IP3-receptor activation induces long lasting synaptic enhancement in CA1 pyramidal neurons. J. Neurosci. 28, 1469–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung L. S., Shen B., Rajakumar N., Ma J. (2003) Cholinergic activity enhances hippocampal long term potentiation in CA1 during walking in rats. J. Neurosci. 23, 9297–9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchanan K. A., Petrovic M. M., Chamberlain S. E., Marrion N. V., Mellor J. R. (2010) Facilitation of long term potentiation by muscarinic M1 receptors is mediated by inhibition of SK channels. Neuron 68, 948–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giessel A. J., Sabatini B. L. (2010) M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron 68, 936–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Auerbach J. M., Segal M. (1994) A novel cholinergic induction of long term potentiation in rat hippocampus. J. Neurophysiol. 72, 2034–2040 [DOI] [PubMed] [Google Scholar]
- 11. Natsume K., Kometani K. (1997) θ Activity-dependent and -independent muscarinic facilitation of long term potentiation in guinea pig hippocampal slices. Neurosci. Res. 27, 335–341 [DOI] [PubMed] [Google Scholar]
- 12. Huerta P. T., Lisman J. E. (1995) Bidirectional synaptic plasticity induced by a single burst during cholinergic θ oscillation in CA1 in vitro. Neuron 15, 1053–1063 [DOI] [PubMed] [Google Scholar]
- 13. Auerbach J. M., Segal M. (1996) Muscarinic receptors mediating depression and long term potentiation in rat hippocampus. J. Physiol. 492, 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Navakkode S., Korte M. (2012) Cooperation between cholinergic and glutamatergic receptors are essential to induce BDNF-dependent long lasting memory storage. Hippocampus 22, 335–346 [DOI] [PubMed] [Google Scholar]
- 15. Feig S., Lipton P. (1993) Pairing the cholinergic agonist carbachol with patterned Schaffer collateral stimulation initiates protein synthesis in hippocampal CA1 pyramidal cell dendrites via a muscarinic, NMDA-dependent mechanism. J. Neurosci. 13, 1010–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marrosu F., Portas C., Mascia M. S., Casu M. A., Fà M., Giagheddu M., Imperato A., Gessa G. L. (1995) Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 671, 329–332 [DOI] [PubMed] [Google Scholar]
- 17. Diekelmann S., Born J. (2010) The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 [DOI] [PubMed] [Google Scholar]
- 18. Poe G. R., Walsh C. M., Bjorness T. E. (2010) Cognitive neuroscience of sleep. Prog. Brain Res. 185, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graves L., Pack A., Abel T. (2001) Sleep and memory. A molecular perspective. Trends Neurosci. 24, 237–243 [DOI] [PubMed] [Google Scholar]
- 20. Lopez J., Roffwarg H. P., Dreher A., Bissette G., Karolewicz B., Shaffery J. P. (2008) Rapid eye movement sleep deprivation decreases long term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience 153, 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Romcy-Pereira R., Pavlides C. (2004) Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur. J. Neurosci. 20, 3453–3462 [DOI] [PubMed] [Google Scholar]
- 22. Ravassard P., Pachoud B., Comte J. C., Mejia-Perez C., Scoté-Blachon C., Gay N., Claustrat B., Touret M., Luppi P. H., Salin P. A. (2009) Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep 32, 227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishikawa A., Kanayama Y., Matsumura H., Tsuchimochi H., Ishida Y., Nakamura S. (2006) Selective rapid eye movement sleep deprivation impairs the maintenance of long term potentiation in the rat hippocampus. Eur. J. Neurosci. 24, 243–248 [DOI] [PubMed] [Google Scholar]
- 24. Smith C. (1995) Sleep states and memory processes. Behav. Brain Res. 69, 137–145 [DOI] [PubMed] [Google Scholar]
- 25. Ulloor J., Datta S. (2005) Spatio-temporal activation of cyclic AMP response element-binding protein, activity-regulated cytoskeletal-associated protein and brain-derived nerve growth factor. A mechanism for pontine-wave generator activation-dependent two-way active-avoidance memory processing in the rat. J. Neurochem. 95, 418–428 [DOI] [PubMed] [Google Scholar]
- 26. Ribeiro S. (2012) Sleep and plasticity. Pflugers Arch 463, 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Datta S., Li G., Auerbach S. (2008) Activation of phasic pontine-wave generator in the rat. A mechanism for expression of plasticity-related genes and proteins in the dorsal hippocampus and amygdala. Eur. J. Neurosci. 27, 1876–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bramham C. R., Alme M. N., Bittins M., Kuipers S. D., Nair R. R., Pai B., Panja D., Schubert M., Soule J., Tiron A., Wibrand K. (2010) The Arc of synaptic memory. Exp. Brain Res. 200, 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shepherd J. D., Bear M. F. (2011) New views of Arc, a master regulator of synaptic plasticity. Nat. Neurosci. 14, 279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guzowski J. F., Lyford G. L., Stevenson G. D., Houston F. P., McGaugh J. L., Worley P. F., Barnes C. A. (2000) Inhibition of activity-dependent Arc protein expression in the rat hippocampus impairs the maintenance of long term potentiation and the consolidation of long term memory. J. Neurosci. 20, 3993–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plath N., Ohana O., Dammermann B., Errington M. L., Schmitz D., Gross C., Mao X., Engelsberg A., Mahlke C., Welzl H., Kobalz U., Stawrakakis A., Fernandez E., Waltereit R., Bick-Sander A., Therstappen E., Cooke S. F., Blanquet V., Wurst W., Salmen B., Bösl M. R., Lipp H. P., Grant S. G., Bliss T. V., Wolfer D. P., Kuhl D. (2006) Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52, 437–444 [DOI] [PubMed] [Google Scholar]
- 32. Messaoudi E., Kanhema T., Soulé J., Tiron A., Dagyte G., da Silva B., Bramham C. R. (2007) Sustained Arc/Arg3.1 synthesis controls long term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J. Neurosci. 27, 10445–10455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peebles C. L., Yoo J., Thwin M. T., Palop J. J., Noebels J. L., Finkbeiner S. (2010) Arc regulates spine morphology and maintains network stability in vivo. Proc. Natl. Acad. Sci. U.S.A. 107, 18173–18178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Béïque J. C., Na Y., Kuhl D., Worley P. F., Huganir R. L. (2011) Arc-dependent synapse-specific homeostatic plasticity. Proc. Natl. Acad. Sci. U.S.A. 108, 816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCurry C. L., Shepherd J. D., Tropea D., Wang K. H., Bear M. F., Sur M. (2010) Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation. Nat. Neurosci. 13, 450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rial Verde E. M., Lee-Osbourne J., Worley P. F., Malinow R., Cline H. T. (2006) Increased expression of the immediate-early gene Arc/Arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron 52, 461–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shepherd J. D., Rumbaugh G., Wu J., Chowdhury S., Plath N., Kuhl D., Huganir R. L., Worley P. F. (2006) Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52, 475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park S., Park J. M., Kim S., Kim J. A., Shepherd J. D., Smith-Hicks C. L., Chowdhury S., Kaufmann W., Kuhl D., Ryazanov A. G., Huganir R. L., Linden D. J., Worley P. F. (2008) Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59, 70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Waung M. W., Pfeiffer B. E., Nosyreva E. D., Ronesi J. A., Huber K. M. (2008) Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59, 84–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greer P. L., Hanayama R., Bloodgood B. L., Mardinly A. R., Lipton D. M., Flavell S. W., Kim T. K., Griffith E. C., Waldon Z., Maehr R., Ploegh H. L., Chowdhury S., Worley P. F., Steen J., Greenberg M. E. (2010) The Angelman syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 140, 704–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bramham C. R., Worley P. F., Moore M. J., Guzowski J. F. (2008) The immediate early gene Arc/Arg3.1. Regulation, mechanisms, and function. J. Neurosci. 28, 11760–11767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giorgi C., Moore M. J. (2007) The nuclear nurture and cytoplasmic nature of localized mRNPs. Semin. Cell Dev. Biol. 18, 186–193 [DOI] [PubMed] [Google Scholar]
- 43. Giorgi C., Yeo G. W., Stone M. E., Katz D. B., Burge C., Turrigiano G., Moore M. J. (2007) The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell 130, 179–191 [DOI] [PubMed] [Google Scholar]
- 44. Panja D., Dagyte G., Bidinosti M., Wibrand K., Kristiansen A. M., Sonenberg N., Bramham C. R. (2009) Novel translational control in Arc-dependent long term potentiation consolidation in vivo. J. Biol. Chem. 284, 31498–31511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teber I., Köhling R., Speckmann E. J., Barnekow A., Kremerskothen J. (2004) Muscarinic acetylcholine receptor stimulation induces expression of the activity-regulated cytoskeleton-associated gene (ARC). Brain Res. Mol. Brain Res. 121, 131–136 [DOI] [PubMed] [Google Scholar]
- 46. Ma L., Seager M. A., Seager M., Wittmann M., Jacobson M., Bickel D., Burno M., Jones K., Graufelds V. K., Xu G., Pearson M., McCampbell A., Gaspar R., Shughrue P., Danziger A., Regan C., Flick R., Pascarella D., Garson S., Doran S., Kreatsoulas C., Veng L., Lindsley C. W., Shipe W., Kuduk S., Sur C., Kinney G., Seabrook G. R., Ray W. J. (2009) Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc. Natl. Acad. Sci. U.S.A. 106, 15950–15955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gil-Bea F. J., Solas M., Mateos L., Winblad B., Ramirez M. J., Cedazo-Minguez A. (2011) Cholinergic hypofunction impairs memory acquisition possibly through hippocampal Arc and BDNF down-regulation. Hippocampus 21, 999–1009 [DOI] [PubMed] [Google Scholar]
- 48. Miyashita T., Kubik S., Haghighi N., Steward O., Guzowski J. F. (2009) Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. J. Neurosci. 29, 898–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fletcher B. R., Baxter M. G., Guzowski J. F., Shapiro M. L., Rapp P. R. (2007) Selective cholinergic depletion of the hippocampus spares both behaviorally induced Arc transcription and spatial learning and memory. Hippocampus 17, 227–234 [DOI] [PubMed] [Google Scholar]
- 50. Marrs G. S., Green S. H., Dailey M. E. (2001) Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nat. Neurosci. 4, 1006–1013 [DOI] [PubMed] [Google Scholar]
- 51. Sadée W., Yu V. C., Richards M. L., Preis P. N., Schwab M. R., Brodsky F. M., Biedler J. L. (1987) Expression of neurotransmitter receptors and myc proto-oncogenes in subclones of a human neuroblastoma cell line. Cancer Res. 47, 5207–5212 [PubMed] [Google Scholar]
- 52. Matesic D. F., Luthin G. R. (1991) Atropine dissociates complexes of muscarinic acetylcholine receptor and guanine nucleotide-binding protein in heart membranes. FEBS Lett. 284, 184–186 [DOI] [PubMed] [Google Scholar]
- 53. Rosenblum K., Futter M., Jones M., Hulme E. C., Bliss T. V. (2000) ERKI/II regulation by the muscarinic acetylcholine receptors in neurons. J. Neurosci. 20, 977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim J. Y., Yang M. S., Oh C. D., Kim K. T., Ha M. J., Kang S. S., Chun J. S. (1999) Signaling pathway leading to an activation of mitogen-activated protein kinase by stimulating M3 muscarinic receptor. Biochem. J. 337, 275–280 [PMC free article] [PubMed] [Google Scholar]
- 55. Wylie P. G., Challiss R. A., Blank J. L. (1999) Regulation of extracellular signal-regulated kinase and c-Jun N-terminal kinase by G-protein-linked muscarinic acetylcholine receptors. Biochem. J. 338, 619–628 [PMC free article] [PubMed] [Google Scholar]
- 56. Comte J. C., Ravassard P., Salin P. A. (2006) Sleep dynamics. A self-organized critical system. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 73, 056127 [DOI] [PubMed] [Google Scholar]
- 57. Vivaldi E. A., Ocampo A., Wyneken U., Roncagliolo M., Zapata A. M. (1994) Short term homeostasis of active sleep and the architecture of sleep in the rat. J. Neurophysiol. 72, 1745–1755 [DOI] [PubMed] [Google Scholar]
- 58. Garavito-Aguilar Z. V., Recio-Pinto E., Corrales A. V., Zhang J., Blanck T. J., Xu F. (2004) Differential thapsigargin sensitivities and interaction of Ca2+ stores in human SH-SY5Y neuroblastoma cells. Brain Res. 1011, 177–186 [DOI] [PubMed] [Google Scholar]
- 59. Ebihara T., Guo F., Zhang L., Kim J. Y., Saffen D. (2006) Muscarinic acetylcholine receptors stimulate Ca2+ influx in PC12D cells predominantly via activation of Ca2+ store-operated channels. J. Biochem. 139, 449–458 [DOI] [PubMed] [Google Scholar]
- 60. Mathes C., Thompson S. H. (1994) Calcium current activated by muscarinic receptors and thapsigargin in neuronal cells. J. Gen. Physiol. 104, 107–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mathes C., Thompson S. H. (1995) The relationship between depletion of intracellular Ca2+ stores and activation of Ca2+ current by muscarinic receptors in neuroblastoma cells. J. Gen. Physiol. 106, 975–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Waltereit R., Dammermann B., Wulff P., Scafidi J., Staubli U., Kauselmann G., Bundman M., Kuhl D. (2001) Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J. Neurosci. 21, 5484–5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brogna S., Wen J. (2009) Nonsense-mediated mRNA decay (NMD) mechanisms. Nat. Struct. Mol. Biol. 16, 107–113 [DOI] [PubMed] [Google Scholar]
- 64. Denning G., Jamieson L., Maquat L. E., Thompson E. A., Fields A. P. (2001) Cloning of a novel phosphatidylinositol kinase-related kinase. Characterization of the human SMG-1 RNA surveillance protein. J. Biol. Chem. 276, 22709–22714 [DOI] [PubMed] [Google Scholar]
- 65. Offermanns S., Bombien E., Schultz G. (1993) Stimulation of tyrosine phosphorylation and mitogen-activated protein (MAP) kinase activity in human SH-SY5Y neuroblastoma cells by carbachol. Biochem. J. 294, 545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pintchovski S. A., Peebles C. L., Kim H. J., Verdin E., Finkbeiner S. (2009) The serum response factor and a putative novel transcription factor regulate expression of the immediate-early gene Arc/Arg3.1 in neurons. J. Neurosci. 29, 1525–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kawashima T., Okuno H., Nonaka M., Adachi-Morishima A., Kyo N., Okamura M., Takemoto-Kimura S., Worley P. F., Bito H. (2009) Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc. Natl. Acad. Sci. U.S.A. 106, 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Maquat L. E., Tarn W. Y., Isken O. (2010) The pioneer round of translation. Features and functions. Cell 142, 368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Conti E., Izaurralde E. (2005) Nonsense-mediated mRNA decay. Molecular insights and mechanistic variations across species. Curr. Opin. Cell Biol. 17, 316–325 [DOI] [PubMed] [Google Scholar]
- 70. Ichikawa H., Fujimoto T., Taira E., Miki N. (2003) The accumulation of arc (an immediate-early gene) mRNA by the inhibition of protein synthesis. J. Pharmacol. Sci. 91, 247–254 [DOI] [PubMed] [Google Scholar]
- 71. Rao V. R., Pintchovski S. A., Chin J., Peebles C. L., Mitra S., Finkbeiner S. (2006) AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat. Neurosci. 9, 887–895 [DOI] [PubMed] [Google Scholar]
- 72. Ying S. W., Futter M., Rosenblum K., Webber M. J., Hunt S. P., Bliss T. V., Bramham C. R. (2002) Brain-derived neurotrophic factor induces long term potentiation in intact adult hippocampus. Requirement for ERK activation coupled to CREB and up-regulation of Arc synthesis. J. Neurosci. 22, 1532–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chotiner J. K., Nielson J., Farris S., Lewandowski G., Huang F., Banos K., de Leon R., Steward O. (2010) Assessment of the role of MAP kinase in mediating activity-dependent transcriptional activation of the immediate-early gene Arc/Arg3.1 in the dentate gyrus in vivo. Learn. Mem. 17, 117–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Peng F., Yao H., Bai X., Zhu X., Reiner B. C., Beazely M., Funa K., Xiong H., Buch S. (2010) Platelet-derived growth factor-mediated induction of the synaptic plasticity gene Arc/Arg3.1. J. Biol. Chem. 285, 21615–21624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Best J., Diniz Behn C., Poe G. R., Booth V. (2007) Neuronal models for sleep-wake regulation and synaptic reorganization in the sleeping hippocampus. J. Biol. Rhythms 22, 220–232 [DOI] [PubMed] [Google Scholar]
- 76. Bramham C. R., Srebro B. (1989) Synaptic plasticity in the hippocampus is modulated by behavioral state. Brain Res. 493, 74–86 [DOI] [PubMed] [Google Scholar]
- 77. Wang G., Grone B., Colas D., Appelbaum L., Mourrain P. (2011) Synaptic plasticity in sleep. Learning, homeostasis, and disease. Trends Neurosci. 34, 452–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Louie K., Wilson M. A. (2001) Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron 29, 145–156 [DOI] [PubMed] [Google Scholar]
- 79. Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 87, 2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Samali A., FitzGerald U., Deegan S., Gupta S. (2010) Methods for monitoring endoplasmic reticulum stress and the unfolded protein response (2010) Int. J. Cell Biol. doi: 10.1155/2010/830307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vembar S. S., Brodsky J. L. (2008) One step at a time. Endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 9, 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hong M., Li M., Mao C., Lee A. S. (2004) Endoplasmic reticulum stress triggers an acute proteasome-dependent degradation of ATF6. J. Cell. Biochem. 92, 723–732 [DOI] [PubMed] [Google Scholar]
- 83. Guo X., Shen S., Song S., He S., Cui Y., Xing G., Wang J., Yin Y., Fan L., He F., Zhang L. (2011) The e3 ligase smurf1 regulates Wolfram syndrome protein stability at the endoplasmic reticulum. J. Biol. Chem. 286, 18037–18047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Toresson H., Grant S. G. (2005) Dynamic distribution of endoplasmic reticulum in hippocampal neuron dendritic spines. Eur. J. Neurosci. 22, 1793–1798 [DOI] [PubMed] [Google Scholar]
- 85. Holbro N., Grunditz A., Oertner T. G. (2009) Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc. Natl. Acad. Sci. U.S.A. 106, 15055–15060 [DOI] [PMC free article] [PubMed] [Google Scholar]