Abstract
PURPOSE
To evaluate the efficacy of sodium hypochlorite (1 : 10) and iodophor disinfectants on alginate impressions along with their effect on the survived bacterium count on the gypsum cast.
MATERIALS AND METHODS
Four alginate impression on each dentate patients were made, of which Group I were not washed or disinfected, Group II impressions were merely washed with water, Group III were disinfected by spraying with sodium hypochlorite (1 : 10), Group IV were disinfected with iodophor (1 : 213). Gypsum cast (type III) were made from all the impression. Impressions and gypsum cast were swabbed in mid palatal region for bacterial culture. Bacterial colony counting done after 3 days of incubation at 37℃ in blood agar media. The data obtained was analyzed by one way ANOVA test at a significant difference level of 0.05.
RESULTS
Group I and Group II showed significantly more bacteria compared to Group III and Group IV. Bacterial colonies on the alginate impression and gypsum cast in group disinfected with Sodium hypochlorite (1 : 10) were 0.18, 0.82 respectively compared to group treated with iodophor (1 : 213). There was an increase in bacterial count on dental cast compared to source alginate impressions.
CONCLUSION
Sodium hypochlorite (1 : 10) was found to be better disinfectant for alginate impression. There was an indication of increase in number of bacteria from alginate impression to making of dental cast. Additional gypsum cast disinfectant procedures need to be encouraged to completely eliminate cross infection to dental laboratory.
Keywords: Dental impression, Infection control, Dental laboratory, Sodium hypochlorite, Iodophor, Aerobic and anaerobic bacteria
INTRODUCTION
Infection control is indispensible part of dental practice. Impression disinfection is an integral part to prevent cross infection between dentists, dental office staff, dental technicians and patients. It is well documented the dental impressions harbors harmful bacteria due to their contact with blood and saliva.1 Some of this bacterium can survive outside oral fluids for long time. Dental cast obtained from these infected impressions can transmit pathogens to dental laboratory, exposing dental laboratory personnel for cross infection.2
Selected disinfectant should not adversely affect the dimensional stability of the impression and physical properties of subsequent dental cast. The practice of impression cross infection control in the general dental practice is a cause of concern.3,4 Many impressions are sent to dental laboratories without proper disinfection, some of which are clearly contaminated with blood and food debris. Studies5 have reported that 67% of the all the dental impression, crown, denture, wax and other materials send to laboratory have harmful bacteria on them.
Irreversible hydrocolloid materials are widely used for both diagnostic and definitive impression procedures. It has been reported6 that irreversible hydrocolloid impression carries two to five times more microorganisms than elastomers. Impressions can be disinfected by immersion or spraying in any compatible disinfectant. Irreversible hydrocolloids are susceptible to dimensional distortion during disinfection procedure because of its hydrophilic nature,7-9 hence disinfection of alginate impression by spraying is preferred.10,11
Although a lot of importance is given to infection control in the dental clinic, it is usually overlooked in the laboratory.12,13 Some of the bacteria do survive even after disinfection of dental impression14 and will eventually be carried on to the gypsum cast made from them. Contaminated dental casts carry the virus, micro-organisms from the oral cavity and some of them survive for longer periods. So it is all more important to know the bacterial count on the resultant dental cast in comparison to the dental impression. Many factors that can affect the bacterial count on the resultant dental cast during cast making procedure. These factors includes setting time for gypsum, exothermic heat of gypsum setting, continued presence of disinfectant traces on the impression during setting time and chemical properties of gypsum itself. Hence, it also important to know the cumulative effect of above factors on bacterial count on the dental cast.
The objective of this study was to evaluate the efficiency of two recommended chemical disinfectants sodium hypochlorite and iodophor15,16 on alginate dental impression and the effect on the survived bacterium on dental cast.
MATERIALS AND METHODS
Eleven dentatu patients with age group of 20 - 30 were randomly selected. Medical history and oral examination were conducted to exclude the presence of local disorders. Four alginate impressions were made for each patient with suitable stock tray. Impressions were made with 24 hours intervals between one another. Impressions made were divided into four categories as follows.
Group I (Control): Alginate impression without water rinsing and no disinfectant solution treatment.
Group II: Alginate impression with only water rinsing. This category was included in the study because some of the clinician still fallows this method.
Group III: Alginate impression disinfected with sodium hypochlorite (1 : 10).
Group IV: Alginate impression disinfected with iodophor (1 : 213).
Disinfection methodology followed for Group III, Group IV was to thoroughly soak the impression by spraying, and later these impressions were stored in the plastic bag for 10 minutes to avoid the evaporation of disinfectant. Bacterial swabs were collected from dry sterile cotton swab for all groups in mid palatal region (Fig. 1). Dental cast were made by pouring the impression with type III gypsum. Dental cast were retrieved from impression after one hour and bacterial swabs were recollected from all dental casts in mid palatal region. These bacterial swabs were used inoculate aerobic and anaerobic bacterial culture in blood agar media for 3 days at 37℃.
Fig. 1.
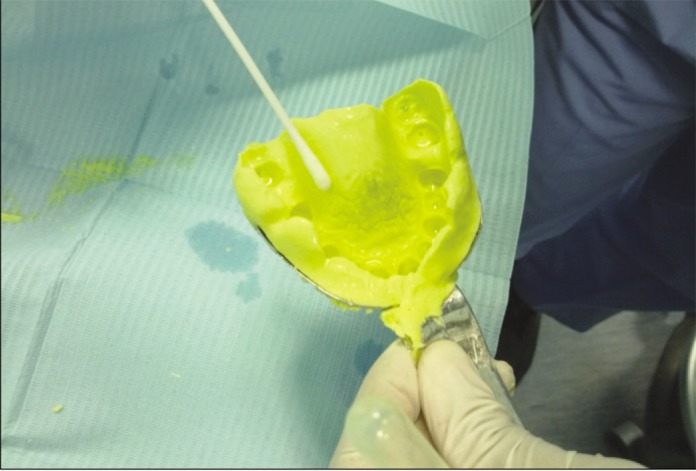
Bacterial swab collection on alginate impression.
Bacterial colonies were counted after 3 days of culturing. Bacterial colonies counted with the aid of light microscope. One colony among this culture was selected for the staining purpose in identification process. The bacteria were stained with Gram stain followed by oxidase and catalase. The light microscope was used for the identification of bacteria after staining process. The resultant data collected was subjected for one way ANOVA test to find the statistically significant difference among the groups at the significance level of 0.05 or less.
RESULTS
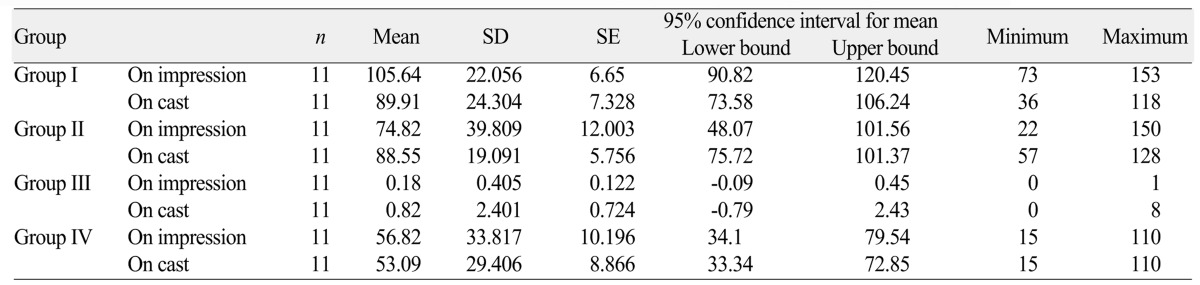
The mean aerobic colony and associated standard deviations found between Group I, Group II, Group III, Group IV are shown in Table 1.The results indicates the presence of higher number of bacterial colony on impression Group I (105.64) and group II (74.82). Impressions disinfected with sodium hypochlorite (1 : 10) had the least amount of bacterial colony both on impression (0.18) and dental cast (0.82).
Table 1.
Shows means and standard deviations of aerobic colony for each group (Group I, Group II, Group III, Group IV)
Group II and Group III dental casts showed increase in number of bacterial colonies from 74.82, 0.18 to 88.55,0.82 compared to the corresponding source impression respectively.
Table 2 depicts the statistical analysis by one-way ANOVA to compare the difference in mean aerobic bacterial colonies across all the groups. It shows the presence of statistically significant difference between all groups with F value of 27.777, and P value of <.00.
Table 2.
Statistical comparison of mean bacterial colony of all samples of different groups by ANOVA
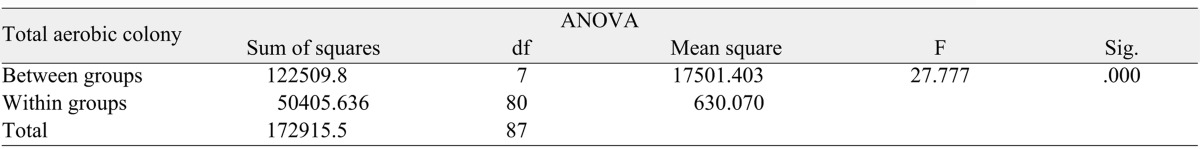
Mean anaerobic colony with respective standard deviation among all the groups shown in Table 3. The results follow the same pattern of aerobic colonies. The highest number of colonies in Group I was 108.45 and 71.55 on alginate impression, dental cast respectively. 0.5% sodium hypochlorite was able to eliminate all anaerobic bacteria's on alginate impression as well as on corresponding cast.
Table 3.
Shows means and standard deviations of aerobic colony for each group (Group I, Group II, Group III, Group IV)
Table 4 depicts the statistical analysis by one-way ANOVA to compare the difference in mean anaerobic bacterial colonies between the Group I, Group II, Group III, Group IV. It shows the statistically significant difference among the means all groups with F value of 21.612, and P value of <.00.
Table 4.
Statistical comparison of mean bacterial colony of all samples of different groups by ANOVA
Anerobic colonies numbers on Group II (72.73) and Group IV (48.27) were increased compared to the corresponding numbers (56.45 and 40.27) on alginate impression.
DISCUSSION
Dentists are responsible for prevention of cross infection in dental clinic, including proper impression disinfection before sending to dental laboratory.4,17 Studies12 have documented the presence of bacteria on the impression and dental prosthesis sent to laboratory. Hence, dental impressions should always be disinfected to prevent infection. Results of the study reconfirm that dental impressions without proper disinfection procedure are contaminated with substantial amount of bacteria. The resultant casts made from these impressions also have significant number of bacteria over them. Alginate impressions are widely used in dentistry for diagnostic impression as well as definitive impression for clinical procedures. Alginate impression materials should be handled carefully to prevent distortion during disinfection procedure. The product with the shortest contact time will allow less distortion to occur during this process. In order to prevent evaporation of the disinfectant during the contact period, impressions were loosely wrapped in a plastic bag for 10 minutes. Studies14 have reported the presence of bacteria on the impression even after disinfection; these bacteria will eventually be carried on to the cast. Hence, it is important to know the bacterial count the subsequent cast to be confident about prevention of cross infection to dental laboratory.
In the present study alginate impressions were disinfected with sodium hypochlorite (1 : 10) and iodophor (1 : 213) solutions by spraying method. The study clearly demonstrates bacterial colony count is substantially less in disinfected impression and corresponding cast.
The mean aerobic colony was the highest on the Group I untreated control dental impression as well as in corresponding dental cast (Fig. 2) with 105.6, 89.91 colonies respectively. The results of study results clearly reemphasizes mere washing of impression results in inadequate disinfection, with Group II had 74.82 and 88.5 colonies on impression and dental cast (Fig. 3) respectively. The least amount of bacterial colonies was found on impression treated with sodium hypochlorite (Group III) with 0.18 on impression and 0.82 colonies on dental cast (Fig. 4). Group treated with iodophor had the mean bacterial colonies 56.82 , 53.09 for impression and dental cast (Fig. 5) respectively.
Fig. 2.
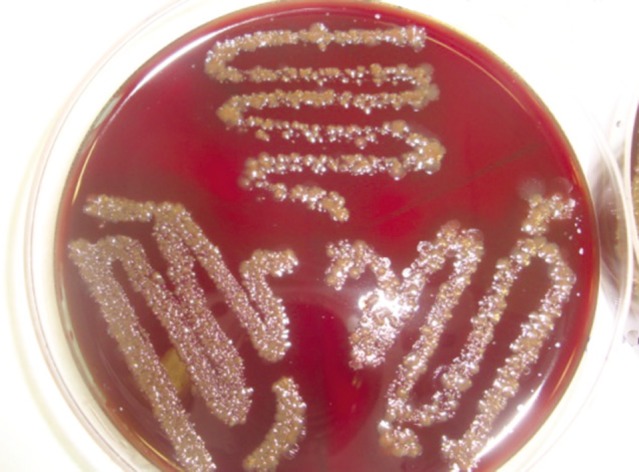
Bacterial colony on blood agar media by the swab collected on dental cast made from Group I alginate impression.
Fig. 3.
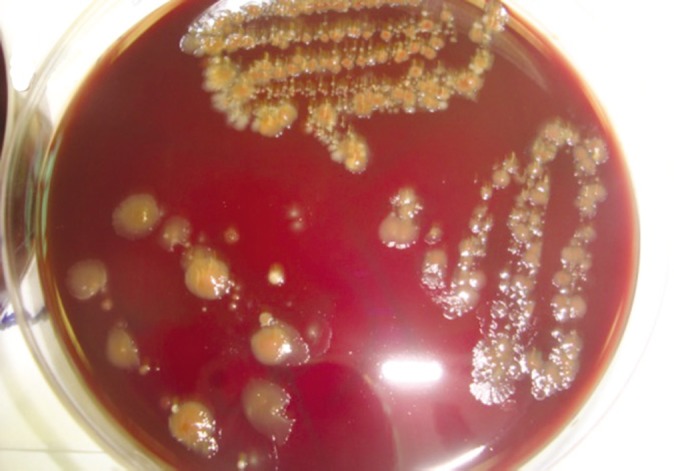
Bacterial colony on blood agar media by the swab collected on dental cast made from Group II alginate impression.
Fig. 4.

Bacterial colony on blood agar media by the swab collected on dental cast made from Group III alginate impression.
Fig. 5.

Bacterial colony on blood agar media by the swab collected on dental cast made from Group IV alginate impression.
Results of disinfection on bacterial count are similar on anaerobic colonies as well. Sodium hypochlorite disinfection was able to completely eradicate the anaerobic bacteria from the alginate impression as well as from subsequent cast. While anaerobic bacterial count colony on the impression disinfected with iodophor was around 40.27, corresponding cast had 48.27 bacterial colonies. The results indicated alginate impression disinfected with sodium hypochlorite found to be highly effective than iodophor against both aerobic and anaerobic bacteria.
Interestingly, the results from the study indicates there were more bacterial colonies on corresponding dental cast compared to the source impression. Mean aerobic bacterial colony count on dental cast for Group II was 88.55 compared to 74.82 on the impression. Same results were found on the Group III with bacterial colony count of 0.82, 018 for impression and cast respectively. The result was repeated with anerobic Group IV with count of 40.27, 48.27 for dental impression and cast respectively. The result indicates gypsum does not have any inherent antibacterial property in them. Though proper impression disinfection procedure is sufficient to control the cross infection from dental clinic to laboratory, additional dental cast disinfection procedures should be encouraged.
Majority of bacteria's found after staining procedure were Staphylococcus, Streptococcus, Enterococcus, Micrococcus, and Acinetobacter.
CONCLUSION
Alginate impression as well as the dental cast without disinfection harbors lots of bacteria over them. Study emphasizes mere washing of impression in water is not an efficient disinfection method. Hence, it is imperative on the part of the clinicians to disinfect the alginate impression before sent to laboratory.
Within the limitation of the study sodium hypochlorite (1 : 10) preceded with water rinsing found to be the best disinfectant for disinfecting alginate impression. The least number of bacterial colonies were found on the dental cast made from these impressions.
Bacterial colonies on the corresponding dental cast are dependent on impression disinfection procedure; some dental cast showed increase in number of bacterial colonies compared to source disinfected impressions. Hence additional disinfection procedure for the dental cast can be justified to completely eliminate the cross infection.
References
- 1.Connor C. Cross-contamination control in prosthodontic practice. Int J Prosthodont. 1991;4:337–344. [PubMed] [Google Scholar]
- 2.Leung RL, Schonfeld SE. Gypsum casts as a potential source of microbial cross-contamination. J Prosthet Dent. 1983;49:210–211. doi: 10.1016/0022-3913(83)90503-6. [DOI] [PubMed] [Google Scholar]
- 3.Kugel G, Perry RD, Ferrari M, Lalicata P. Disinfection and communication practices: a survey of U.S. dental laboratories. J Am Dent Assoc. 2000;131:786–792. doi: 10.14219/jada.archive.2000.0278. [DOI] [PubMed] [Google Scholar]
- 4.Almortadi N, Chadwick RG. Disinfection of dental impressions - compliance to accepted standards. Br Dent J. 2010;209:607–611. doi: 10.1038/sj.bdj.2010.1134. [DOI] [PubMed] [Google Scholar]
- 5.Powell GL, Runnells RD, Saxon BA, Whisenant BK. The presence and identification of organisms transmitted to dental laboratories. J Prosthet Dent. 1990;64:235–237. doi: 10.1016/0022-3913(90)90185-f. [DOI] [PubMed] [Google Scholar]
- 6.Samaranayake LP, Hunjan M, Jennings KJ. Carriage of oral flora on irreversible hydrocolloid and elastomeric impression materials. J Prosthet Dent. 1991;65:244–249. doi: 10.1016/0022-3913(91)90169-w. [DOI] [PubMed] [Google Scholar]
- 7.Jagger DC, Al Jabra O, Harrison A, Vowles RW, McNally L. The effect of a range of disinfectants on the dimensional accuracy of some impression materials. Eur J Prosthodont Restor Dent. 2004;12:154–160. [PubMed] [Google Scholar]
- 8.Martin N, Martin MV, Jedynakiewicz NM. The dimensional stability of dental impression materials following immersion in disinfecting solutions. Dent Mater. 2007;23:760–768. doi: 10.1016/j.dental.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RL, Wright PS, Maryan C. Disinfection procedures: their effect on the dimensional accuracy and surface quality of irreversible hydrocolloid impression materials and gypsum casts. Dent Mater. 2002;18:103–110. doi: 10.1016/s0109-5641(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 10.Westerholm HS, 2nd, Bradley DV, Jr, Schwartz RS. Efficacy of various spray disinfectants on irreversible hydrocolloid impressions. Int J Prosthodont. 1992;5:47–54. [PubMed] [Google Scholar]
- 11.Bergman B. Disinfection of prosthodontic impression materials: a literature review. Int J Prosthodont. 1989;2:537–542. [PubMed] [Google Scholar]
- 12.Wakefield CW. Laboratory contamination of dental prostheses. J Prosthet Dent. 1980;44:143–146. doi: 10.1016/0022-3913(80)90125-0. [DOI] [PubMed] [Google Scholar]
- 13.Jagger DC, Huggett R, Harrison A. Cross-infection control in dental laboratories. Br Dent J. 1995;179:93–96. doi: 10.1038/sj.bdj.4808846. [DOI] [PubMed] [Google Scholar]
- 14.Jennings KJ, Samaranayake LP. The persistence of microorganisms on impression materials following disinfection. Int J Prosthodont. 1991;4:382–387. [PubMed] [Google Scholar]
- 15.Rentzia A, Coleman DC, O'Donnell MJ, Dowling AH, O'Sullivan M. Disinfection procedures: their efficacy and effect on dimensional accuracy and surface quality of an irreversible hydrocolloid impression material. J Dent. 2011;39:133–140. doi: 10.1016/j.jdent.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Look JO, Clay DJ, Gong K, Messer HH. Preliminary results from disinfection of irreversible hydrocolloid impressions. J Prosthet Dent. 1990;63:701–707. doi: 10.1016/0022-3913(90)90329-b. [DOI] [PubMed] [Google Scholar]
- 17.Infection control recommendations for the dental office and the dental laboratory. ADA Council on Scientific Affairs and ADA Council on Dental Practice. J Am Dent Assoc. 1996;127:672–680. doi: 10.14219/jada.archive.1996.0280. [DOI] [PubMed] [Google Scholar]