Abstract
Primary dissociated neuronal cultures are widely used research tools to investigate of pathological mechanisms and to treat various central and peripheral nervous system problems including trauma and degenerative neuronal diseases. We introduced a protocol that utilizes hippocampal and cortical neurons from embryonic day 17 or 18 mice. We applied appropriate markers (GAP-43 and synaptophysin) to investigate whether neurite outgrowth and synaptogenesis can be distinguished at a particular period of time. GAP-43 was found along the neural processes in a typical granular pattern, and its expression increased proportionally as neurites lengthened during the early in vitro period. Unlike GAP-43, granular immunoreactive patterns of synaptophysin along the neurites were clearly found from day 2 in vitro with relatively high immunoreactive levels. Expression of synaptic markers from cortical neurons reached peak level earlier than that of hippocampal neurons, although neurite outgrowths of hippocampal neurons were faster than those of cortical neurons. The amount of peak synaptic markers expressed was also higher in cortical neurons than that in hippocampal neurons. These results strongly suggest the usefulness of primary cultured neurons from mice embryos for synaptic function and plasticity studies, because of their clear and typical patterns of morphology that establish synapses. Results from this study also suggest the proper amount of time in vitro according to neuronal types (cortical or hippocampal) when utilized in experiments related with synaptogenesis or synaptic activities.
Keywords: primary neuronal culture, mouse embryo, neurite outgrowth, synaptogenesis, gap-43, synaptophysin
INTRODUCTION
Primary neural cultures allow continuous visual access for morphological studies such as neurite sprouting, connectivity, and variability. These cultures make individual living cells accessible to apply chemical or pharmacological agents and patch clamp recording [1-3], which are difficult tasks to perform in a sectioned slice alone. Individual neuronal activity of multiple neurons can be recorded for several weeks, which is extremely difficult to perform in a sectioned slice. Additionally, the relative proportions of neurons and neuroglial cells can be controlled, and different patterns of neuronal connectivity are beginning to be studied with developments in culture substrates. Thus, primary neuronal culture is an important research tool that can be applied on a cell-by-cell basis to morphological and physiological studies of a variety of brain areas, including the cerebral cortex, hippocampal formation, basal ganglia, and the diencephalon. In primary neuronal cultures, newly formed neurons undergo a series of extensive morphological changes as they mature, including neurite sprouting and outgrowth, neurite branching, and establishment of synaptogenesis. Although insufficient, these morphological changes are necessary for the formation of neural network circuits facilitating neuronal functions [4].
Several researchers have adopted various substrates and methods for primary neuronal or neural stem cell cultures to stimulate and enhance neurite outgrowth. Positively charged hydrogels were used as substrates for nerve cell attachment and neurite outgrowth in rat primary dorsal root ganglion (DRG) cultures [5]. Thermoresponsive hydrogel scaffolds have been introduced into primary embryonic cortical neuron cultures to repair spinal cord injuries [6]. Artificial scaffolds have been investigated as a means of addressing axonal regeneration inhibiting stimuli [7, 8] such as glial scars [9, 10] or inhibiting molecules [11]. Most recently, a three-dimensional microfluidic device showed dissociated cortical neurons cultured in three-dimensional multilayered scaffolds based on an agarose-alginate mixture for the first time [12]. Primary cultures offer an opportunity to visualize neurite outgrowth or synaptogenesis at the level of single cells, and modern immunofluorescent methods are frequently used for this purpose. Because it is inevitable that cells are grown on glass cover slips to prevent quenching or autofluorescence, cover slips must be coated for successful cell attachment and growth. Various compounds, such as poly-l-lysine (PLL), laminin, or poly-l-ornithine have been used for coating [13-15]. Here, we introduce culturing primary cortical and hippocampal neural cells isolated from embryonic mice on cover slips coated with a soluble basement membrane (Matrigel), which contains laminin, collagen IV, heparin sulfate, proteoglycans, entactin, and nidogen [16]. Because dissociated hippocampal or cortical cell cultures are composed of neurons and glial cells, strongly proliferating astrocytes will displace the non-proliferating neurons. Thus, we have added cytostatic drugs, such as cytosine arabinoside (AraC) to growing cells [17-19].
A variety of markers are used widely to detect core matter and core factor during neurite sprouting and outgrowth as well as synaptogenesis. Many researchers who study synapse formation have selected better substances such as axonal membrane protein (GAP-43), synaptophysin, and synapsin [20, 21]. GAP-43 is a phosphoprotein of the nerve terminal membrane that has been linked to the development and restructuring of axons [22]. Recent studies have shown that GAP-43 is not only related to an increase in synapse formation and synaptic plasticity in mice or rat primary neuronal cultures [23], but it is as also a significant substance for neurite outgrowth as shown by a morphological study with primary neuronal cultures from human fetal forebrain [24]. Moreover, GAP-43 reduction is associated with stress and aging in brain [25-27]. Synaptophysin is one of the synaptic vesicular proteins that has recently been characterized for the first time [28] and has four transmembrane domains including synaptogyrin and synaptoporin [29]. It has been suggested that synaptophysin promotes the formation of highly curved membranes such as synaptic vesicles [30]. An ultra-structural study demonstrated that synaptophysin forms a structure similar to connexons [31]. Synaptophysin was recently reported to regulate the kinetics of synaptic vesicular endocytosis in neurons [32].
In this study, we focused on neurite outgrowth and synaptogenic activity using immunofluorescence and Western blotting with synaptogenic markers during the early period of in vitro culture. We aimed to demonstrate whether the patterns of neurite outgrowth were consistent with the expression levels of neurite outgrowth and synaptogenic markers by comparing morphological findings with those of Western blotting. Data from this study will be a useful reference for many primary neuronal culture protocols, because the primary culture system used in this study is a feasible in vitro model for a wide variety of neurophysiological and neuropharmacological applications.
MATERIALS AND METHODS
Primary neural cell culture
We used C57/BL6 mouse strain for primary neural culture. Embryonic day 16 to 18 embryos were obtained from surgically sacrificed pregnant mouse and separated cerebral cortex and hippocampus under surgical stereomicroscope. Separated tissues were trypsinized (5 mg/ml) for 10 min in 37℃. Finally, dissociated neurons were cultured on Matrigel (BD Science) coated 12 mm coverslips (total 12 coverslips per cortex or hippocampus). Number of total plated cell was adjusted at around 100,000 per each coverslip. Culture media was prepared based on MEM (minimum essential media) We added glucose (5 gm/l), transferrin (0.1 gm/l), insulin (0.25 gm/l), glutamine (0.3 gm/l), heat-inactivated FBS (5~10%) and B-27 supplement (2%) to MEM as supplements. Culture media was changed only two times in day 1 and day 4 through all in vitro period. At the time of media change, only half of the media was removed and replaced with same amount of fresh media. To inhibit glial cell outgrowth, cytosine arabinoside (1 µM) was added at the moment of media change.
Immuno-fluorescence staining (GAP-43 & Synaptophysin)
Cover slips those scheduled to be used for immune-fluorescence staining were transferred into 4-well plates in day 2, day 4, and day 8 in vitro respectively. First, cultured neurons were fixed with ice-cold 70% ethanol for 10 min and washed with DPBS 3 times for 5 min. Nonspecific reactivity was blocked by adding 1% Bovine serum albumin (BSA) into the primary antibody diluting solution. We used GAP-43 (1:1,000, ab7462, ABCAM) and synaptophysin (1:1,000, ab14692, ABCAM) as primary antibody of axonal growth marker and incubated for 24 hrs in 4℃. After the termination of primary antibody reaction, cover slips were washed with DPBS 3 times for 5 min and incubated with fluorescence tagged secondary antibody (Alexa 555, goat anti-rabbit IgG, A21424, Invitrogen) for 90 min in RT. Cover slips were washed again 3 times for 5 min after the termination of secondary antibody incubation. Finally, nuclei of cultured neurons were counter-stained with DAPI (ABBOT Molecular) and immediately investigated under laser confocal microscope system.
Confocal microscopy
Confocal microscope (LSM-700 Carl Zeiss, Germany) equipped with associated software of ZEN2009 (version 5,5,0,375 Carl Zeiss, Germany) was used for the analysis of GAP-43 and synaptophysin immunofluorescence staining. We set up the standard master gain of DAPI fixation of 700 (GAP-43 & synaptophysin), master gain of rhodamin fixation of 619 (GAP-43) and 700 (synaptophysin), digital gain below 1 and pinhole below 5.
Western blotting
Cultured neurons were extracted from coverlslips in each wells and homogenized with 5X sample buffer (250 mM Tris-HCl pH 6.8, 30% glycerol, 5% beta-mecaptoethanol, 0.02% bromophenol blue, 10% SDS). After SDS-PAGE, transferred membranes were blocked by 5% skim milk for 30 minutes at RT. For primary antibody reactions, Anti-GAP43 (1:2,000, ab7462, rabbit polyclonal, Abcam) and anti-synaptophysin (1:1,000, ab14692, rabbit polyclonal, Abcam) were added to membranes and stayed overnight at 4℃ on orbital shaker. Anti-beta actin (1:3,000, ab8227, rabbit polyclonal, Abcam) was also added to the membranes as loading controls for 1 hour at RT. After the reaction with goat anti-rabbit (1:3,000, 111-036-003, Jackson) for 1 hour at RT, signals were enhanced by ECL (MC154418, Thermo) solutions. Bands were detected by the image detection system (LAS 4000, GE).
RESULTS
Primary cultured cortical and hippocampal neurons were traced their neurite outgrowth under the phase contrast microscope. Neurons were also tested synaptogenic activities along the time courses of early periods in vitro by using one of the well known neurite outgrowth and synaptogenesis markers and the detailed results are as follows.
Morphological changes of primary cultured neurons along the time courses
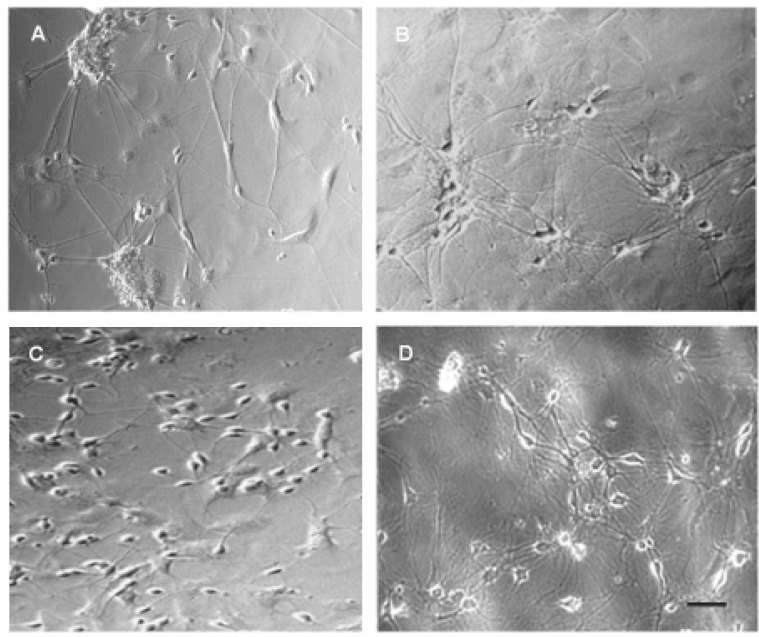
We investigated morphological changes of primary cultured neurons day by day from day 1 in vitro. Hippocampal neurons showed rather fast growing pattern so we could find definite neurite sprouting and outgrowth even from day 1 in vitro. Neurite outgrowth was faster in the areas of relatively high neural populations whereas some isolated neurons in the areas with very low neural populations showed apoptotic features (data not shown). Cultured hippocampal neurons of neighborhood gradually moved to each other showing patterns of aggregating groups and at this stage, neurites of various lengths radiated toward all directions to establish huge meshwork of neurites that were forming numerous synapses to each other (Fig. 1B).
Fig. 1.
Phase contrast microscopy of primary cultured hippocampal (A, B) and cortical (C, D) neurons. (A) Hippocampal neurons of day 4 in vitro. Cultured neurons started neurite sprouting from the early days in vitro. (B) Hippocampal neurons of day 10 in vitro. Neurons are more likely to be aggregated and fully grown neurites shows spider web pattern. (C) Cortical neurons of day 4 in vitro. Neurites are still very short compared to hippocampal neurons of same day in vitro. (D) Cortical neurons of day 13 in vitro. Note that neurites outgrowth of cultured cortical neurons are relatively slow than those of hippocampal neurons. Scale bar is 50 µm.
Neurite sprouting and outgrowth of cortical neurons were slower than hippocampal neurons. In many cases, we could find only limited degree of neurite sprouting from cortical neurons in early days in vitro (Fig. 1C). Furthermore, we could find more frequent apoptotic features in case of cortical neurons than hippocampal neurons (data not shown). Although the neurite sprouting and outgrowth of primary cultured cortical neurons were slow than those of hippocampal neurons, cortical neurons showed almost same morphological features with hippocampal neurons toward the end of second week in vitro (Fig. 1D). Neurite outgrowth of primary cultured cells stopped from the day 14 or 18 in vitro and decayed slowly thereafter in both of cortical and hippocampal neurons.
Immunofluorescence findings
GAP-43 immunoreactivities were gradually increased according to these morphological changes from day 2 to day 8 in vitro. In day 2 in vitro, GAP-43 expressions still remained relatively low and hard to find granular immunofluorescence patterns along the outgrowing neurites. GAP-43 immunoreactivities increased rapidly thereafter as neurites extend and make connections with neighboring neurons. Typical granular patterns of GAP-43 immunofluorescence along the outgrowing neurites (Fig. 2D, E) could be found from day 4 in vitro and the intensity of immunofluorescence already reached maximum during the early stages of culture (day 8 in vitro). Unlike Gap-43, granular immunoreactive patterns of synaptophysin along the outgrowing neurites (Fig. 3A, C, D) were clearly found from day 2 in vitro with relatively high immunoreactive levels (Fig. 3A). These granular findings of GAP-43 and synaptophysin immunoreactivities also resembled synaptic button like structures when considered only in morphology. Patterns of GAP-43 and synaptophysin immunoreactivities were similar in both types of cortical and hippocampal neurons. Morphological synaptic connections among the neighboring neurites could be confirmed more clearly especially under high magnifications.
Fig. 2.
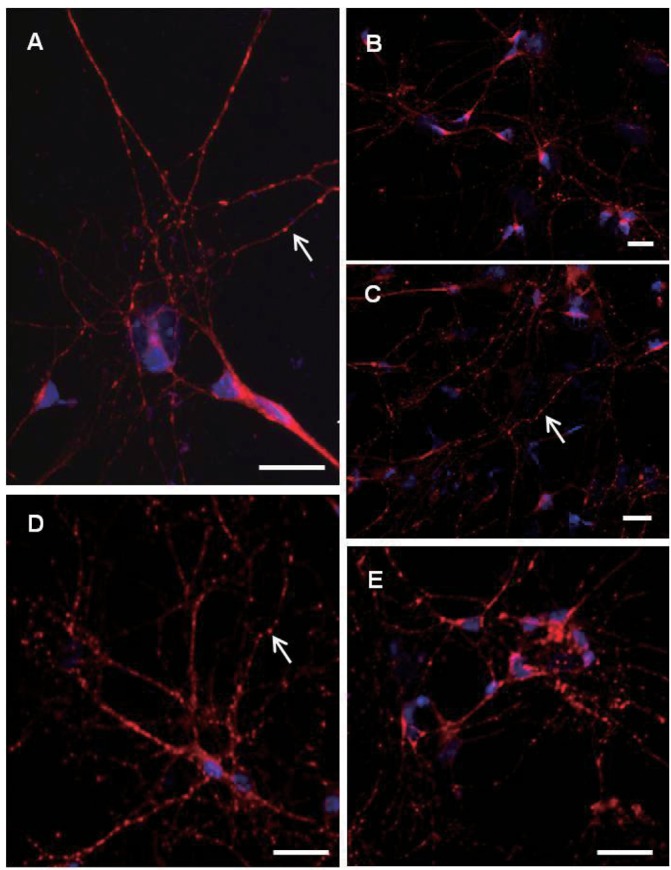
Confocal microscopic deteciton of GAP-43 immunopositive cells in pirmary cultured hippocampal neurons at day 2 (A, B) day 4 (C~E) and day 8 (F, G). Nuclei are counterstained with DAPI (blue color). Note granular pattern of GAP-43 immunofluorescence along the neurites (arrows) and gradual increase of immunoreactivities according to time courses. Scale bars are 20 µm each.
Fig. 3.
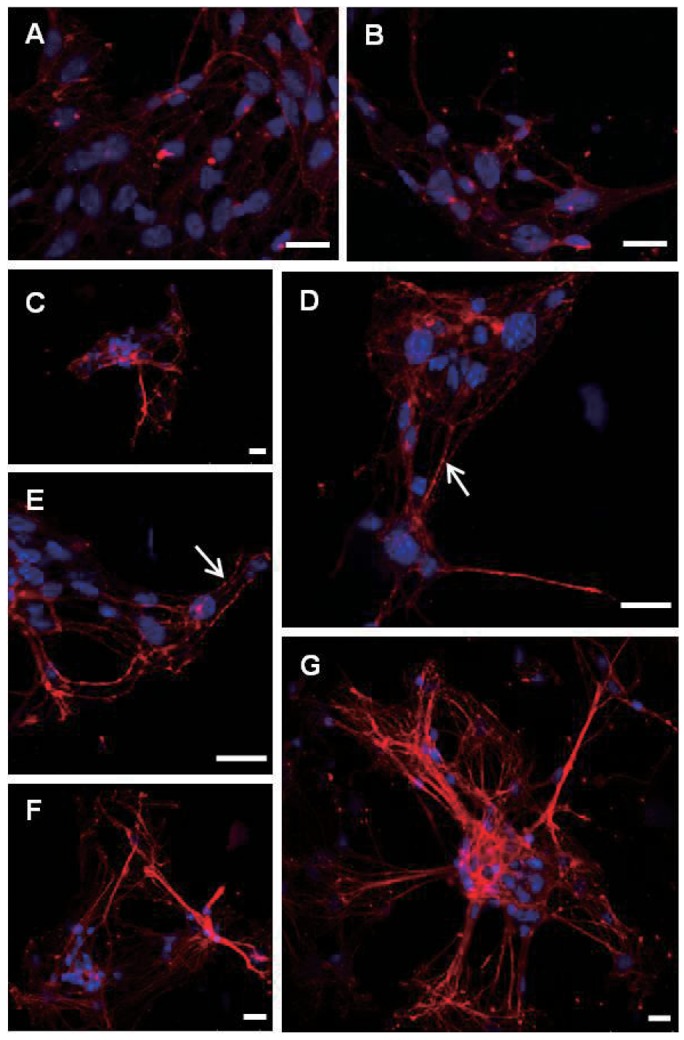
Confocal microscopic deteciton of synaptophysin immunopositive cells in pirmary cultured hippocampal neurons at day 2 (A) day 4 (B, C) and day 8 (D, E). Nuclei are counterstained with DAPI (blue color). Note revelation of granular synaptophysin immunofluorescence along the neurites (arrows) from day 2 in vitro with relatively high immunofluorescence levels. Scale bars are 20 µm each.
Western blotting findings
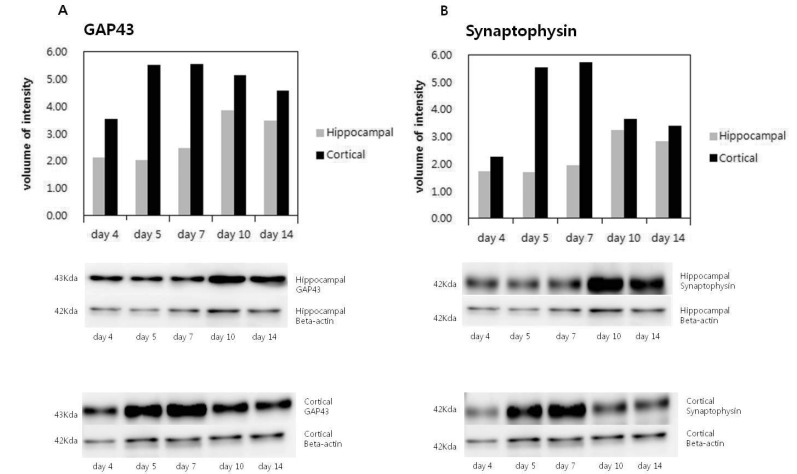
Along with the immunofluorescence, quantification of synaptic marker expressions was examined by western blotting through the wide range of in vitro periods. GAP-43 and synaptophysin were detected from day 4 in vitro but the expression levels between hippocampal and cortical neurons were different with the time courses. In cortical neurons, GAP-43 and synaptophysin expressions reached highest levels within 1 week (day 5) in vitro. However in hippocampal neurons, both GAP-43 and synaptophysin expressions reached highest levels after 1 week (day 10) in vitro. In cortical neurons, the amounts of GAP-43 and synaptophysin expressions were considerably higher when they reached to highest levels than those in hippocampal neurons. The differences between cortical and hippocampal neurons were more prominent in case of synaptophysin (Fig. 4B).
Fig. 4.
Western blotting of GAP43 and synaptophysin in cortical and hippocampal neurons. In this figure, GAP43 and synaptophysin bands are digitized and appeared on the graphs as volules of intensities according to beta actins respectively. Expression levels of GAP43 (A) and synaptophysin (B) according to the time courses of in vitro are shown in hippocampal and cortical neurons.
DISCUSSION
We characterized neurite outgrowth and synaptogenic activities in primary cultured cortical and hippocampal neurons using immunofluorescence and Western blotting. The main features of survival, proliferation, and neurite outgrowth were similar to those cultured in a mixture of MEM and B27-supplemented neurobasal medium from rat embryos [33]. Interestingly, the expression of synaptic markers from cortical neurons reached a peak level earlier (around 5 days in vitro) than that from hippocampal neurons (10 days in vitro), although neurite outgrowth from hippocampal neurons was faster than that of cortical neurons. Furthermore, the peak amounts of expressed synaptic markers were also higher in cortical neurons than those in hippocampal neurons. Mice embryos at embryonic days 16~18 were used, although postnatal new-born rodents could be also used for primary neuronal or neural stem cell cultures [34-36]. The matrigel that we used for coating the glass cover slips creates more optimal conditions for survival and neurite outgrowth than coating with laminin or other substrates alone. The majority of plated neurons did not survive on non-coated cover slips and clumped heavily (data not shown). We found a gradual increase in GAP-43 immunoreactivity that was associated with extension and branching of neurites. Synaptophysin immunoreactivity was already strong enough from day 2 in vitro. Although we are unable to confirm whether they are true synaptic buttons, these findings could be considered important because the more synaptic buttons secured, the better the synaptic functional or morphological study. GAP-43 is a marker of synaptic plasticity that is critical for normal development of serotonergic innervation [37], and synaptophysin is a well-known synaptic vesicular protein. Considering prompt neurite outgrowth and abundant branching, synaptic marker expression levels should be clarified, particularly during the early period in vitro. Our Western blotting results seemed to be a useful reference for experimental designs on synaptic activity and plasticity.
We confirmed delayed neurite sprouting and outgrowth of primary cultured cortical neurons compared to those of hippocampal neurons. However, the expression levels of neurite outgrowth and synaptogenesis markers showed reversed patterns between cortical and hippocampal neurons. These results suggest that primary cultured cortical neurons could be utilized rather earlier (within 1 week in vitro) in functional studies associated with synaptogenesis. Although we are unable to suggest a possible explanation for this differential expression, the expression of synaptic vesicular associated proteins from cortical neurons seemed to be accelerated within the first week in vitro as a preparatory stage for explosive synaptogenic processes thereafter. To explain these discrepancies between neuronal groups during the early in vitro period, we are going to introduce primary cultured neurons that lack synaptic markers or synaptic vesicular associated proteins (synaptobrevin and Munc-18) as a next step. Although we are only showing findings based on morphological changes and synaptic markers expression at this time, we are planning to identify more detailed clues or mechanisms of growth cone formation and synaptogenesis during neuronal growth and differentiation by adding neurotrophic factors such brain derived neurotrophic factor (BDNF). Findings from peripheral nerve cell and neuronal stem cell cultures should also be compared to determine whether they have different mechanisms according to their origin.
ACKNOWLEDGEMENTS
The present research was conducted by the research fund of Dankook University in 2010.
References
- 1.Ito C, Wakamori M, Akaike N. Dual effect of glycine on isolated rat suprachiasmatic neurons. Am J Physiol. 1991;260:C213–C218. doi: 10.1152/ajpcell.1991.260.2.C213. [DOI] [PubMed] [Google Scholar]
- 2.Quintero JE, McMahon DG. Serotonin modulates glutamate responses in isolated suprachiasmatic nucleus neurons. J Neurophysiol. 1999;82:533–539. doi: 10.1152/jn.1999.82.2.533. [DOI] [PubMed] [Google Scholar]
- 3.Walsh IB, van den Berg RJ, Marani E, Rietveld WJ. Spontaneous and stimulated firing in cultured rat suprachiasmatic neurons. Brain Res. 1992;588:120–131. doi: 10.1016/0006-8993(92)91351-e. [DOI] [PubMed] [Google Scholar]
- 4.Sanes DH, Reh TA, Harris WA. Development of the nervous system. London: Elsevier Academic Press; 2006. [Google Scholar]
- 5.Dadsetan M, Knight AM, Lu L, Windebank AJ, Yaszemski MJ. Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials. 2009;30:3874–3881. doi: 10.1016/j.biomaterials.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nisbet DR, Moses D, Gengenbach TR, Forsythe JS, Finkelstein DI, Horne MK. Enhancing neurite outgrowth from primary neurones and neural stem cells using thermoresponsive hydrogel scaffolds for the repair of spinal cord injury. J Biomed Mater Res A. 2009;89:24–35. doi: 10.1002/jbm.a.31962. [DOI] [PubMed] [Google Scholar]
- 7.Tian WM, Hou SP, Ma J, Zhang CL, Xu QY, Lee IS, Li HD, Spector M, Cui FZ. Hyaluronic acid-poly-D-lysine-based three-dimensional hydrogel for traumatic brain injury. Tissue Eng. 2005;11:513–525. doi: 10.1089/ten.2005.11.513. [DOI] [PubMed] [Google Scholar]
- 8.Mahoney MJ, Anseth KS. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 2006;27:2265–2274. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Yu TT, Shoichet MS. Guided cell adhesion and outgrowth in peptide-modified channels for neural tissue engineering. Biomaterials. 2005;26:1507–1514. doi: 10.1016/j.biomaterials.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Schwab ME. Regenerative nerve fiber growth in the adult central nervous system. News Physiol Sci. 1998;13:294–298. doi: 10.1152/physiologyonline.1998.13.6.294. [DOI] [PubMed] [Google Scholar]
- 11.Stabenfeldt SE, García AJ, LaPlaca MC. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J Biomed Mater Res A. 2006;77:718–725. doi: 10.1002/jbm.a.30638. [DOI] [PubMed] [Google Scholar]
- 12.Kunze A, Giugliano M, Valero A, Renaud P. Micropatterning neural cell cultures in 3D with a multi-layered scaffold. Biomaterials. 2011;32:2088–2098. doi: 10.1016/j.biomaterials.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 13.Kondratyev AD, Zotova EE, Loginov BV, Tsirenina ML, Melnik EI, Severin ES. Production and immunohistochemical characterization of primary cultures of human embryo brain cells. Neuroscience. 1989;32:261–268. doi: 10.1016/0306-4522(89)90125-5. [DOI] [PubMed] [Google Scholar]
- 14.Pons S, Trejo JL, Martínez-Morales JR, Martí E. Vitronectin regulates Sonic hedgehog activity during cerebellum development through CREB phosphorylation. Development. 2001;128:1481–1492. doi: 10.1242/dev.128.9.1481. [DOI] [PubMed] [Google Scholar]
- 15.Rubin JB, Choi Y, Segal RA. Cerebellar proteoglycans regulate sonic hedgehog responses during development. Development. 2002;129:2223–2232. doi: 10.1242/dev.129.9.2223. [DOI] [PubMed] [Google Scholar]
- 16.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 17.Hertz L, Juurlink BHJ, Fosmark H, Schousboe A. Astrocytes in primary cultures. In: Pfeiffer SE, editor. Neuroscience approached through cell culture. Boca Raton, FL: CRC Press; 1982. pp. 175–186. [Google Scholar]
- 18.Hertz E, Yu ACH, Hertz L, Juurlink BHJ, Schousboe A. Preparation of primary cultures of mouse cortical neurons. In: Shahar A, de Vellis J, Vernadakis A, Haber B, editors. A dissection and tissue culture manual of the nervous system. New York: Liss; 1989. pp. 183–186. [Google Scholar]
- 19.Waagepetersen HS, Bakken IJ, Larsson OM, Sonnewald U, Schousboe A. Comparison of lactate and glucose metabolism in cultured neocortical neurons and astrocytes using 13C-NMR spectroscopy. Dev Neurosci. 1998;20:310–320. doi: 10.1159/000017326. [DOI] [PubMed] [Google Scholar]
- 20.Eastwood SL, Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull. 2001;55:569–578. doi: 10.1016/s0361-9230(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 21.Jin Y. Synaptogenesis. WormBook. 2005:1–11. doi: 10.1895/wormbook.1.44.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dani JW, Armstrong DM, Benowitz LI. Mapping the development of the rat brain by GAP-43 immunocytochemistry. Neuroscience. 1991;40:277–287. doi: 10.1016/0306-4522(91)90190-y. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 24.Hammond RR, Iskander S, Achim CL, Hearn S, Nassif J, Wiley CA. A reliable primary human CNS culture protocol for morphological studies of dendritic and synaptic elements. J Neurosci Methods. 2002;118:189–198. doi: 10.1016/s0165-0270(02)00126-7. [DOI] [PubMed] [Google Scholar]
- 25.Chen B, Wang JF, Sun X, Young LT. Regulation of GAP-43 expression by chronic desipramine treatment in rat cultured hippocampal cells. Biol Psychiatry. 2003;53:530–537. doi: 10.1016/s0006-3223(02)01551-2. [DOI] [PubMed] [Google Scholar]
- 26.Mladenovic Djordjevic A, Perovic M, Tesic V, Tanic N, Rakic L, Ruzdijic S, Kanazir S. Long-term dietary restriction modulates the level of presynaptic proteins in the cortex and hippocampus of the aging rat. Neurochem Int. 2010;56:250–255. doi: 10.1016/j.neuint.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Afadlal S, Polaboon N, Surakul P, Govitrapong P, Jutapakdeegul N. Prenatal stress alters presynaptic marker proteins in the hippocampus of rat pups. Neurosci Lett. 2010;470:24–27. doi: 10.1016/j.neulet.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 28.Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- 29.Südhof TC, Lottspeich F, Greengard P, Mehl E, Jahn R. A synaptic vesicle protein with a novel cytoplasmic domain and four transmembrane regions. Science. 1987;238:1142–1144. doi: 10.1126/science.3120313. [DOI] [PubMed] [Google Scholar]
- 30.Leube RE. The topogenic fate of the polytopic transmembrane proteins, synaptophysin and connexin, is determined by their membrane-spanning domains. J Cell Sci. 1995;108:883–894. doi: 10.1242/jcs.108.3.883. [DOI] [PubMed] [Google Scholar]
- 31.Arthur CP, Stowell MH. Structure of synaptophysin: a hexameric MARVEL-domain channel protein. Structure. 2007;15:707–714. doi: 10.1016/j.str.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon SE, Chapman ER. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 2011;70:847–854. doi: 10.1016/j.neuron.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie C, Markesbery WR, Lovell MA. Survival of hippocampal and cortical neurons in a mixture of MEM+ and B27-supplemented neurobasal medium. Free Radic Biol Med. 2000;28:665–672. doi: 10.1016/s0891-5849(99)00268-3. [DOI] [PubMed] [Google Scholar]
- 34.Tao K, Chen J, Wang G, Shu X. Culture and identification of monoclonal neural stem cells derived from cerebral cortex. J Huazhong Univ Sci Technolog Med Sci. 2006;26:451–454. doi: 10.1007/s11596-006-0419-5. [DOI] [PubMed] [Google Scholar]
- 35.Xie Z, Zheng Q, Guo X, Yi C, Wu Y. Isolation, culture and identification of neural stem cells in new-born rats. J Huazhong Univ Sci Technolog Med Sci. 2004;24:75–78. doi: 10.1007/BF02830712. [DOI] [PubMed] [Google Scholar]
- 36.Ternaux JP, Portalier P. Culture of hypoglossal cells, dissociated from foetal and new-born rats. J Neurosci Methods. 1993;49:33–47. doi: 10.1016/0165-0270(93)90107-3. [DOI] [PubMed] [Google Scholar]
- 37.Donovan SL, Mamounas LA, Andrews AM, Blue ME, McCasland JS. GAP-43 is critical for normal development of the serotonergic innervation in forebrain. J Neurosci. 2002;22:3543–3552. doi: 10.1523/JNEUROSCI.22-09-03543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]