Abstract
Capsaicin, the pungent ingredient in hot pepper, activates nociceptors to produce pain and inflammation. However, prolonged exposures of capsaicin will cause desensitization to nociceptive stimuli. Hyperpolarization-activated cation currents (Ih) contribute to the maintenance of the resting membrane potential and excitability of neurons. In the cultured dorsal root ganglion (DRG) neurons, we investigated mechanisms underlying capsaicin-mediated modulation of Ih using patch clamp recordings. Capsaicin (1 µM) inhibited Ih only in the capsaicin-sensitive neurons. The capsaicin-induced inhibition of Ih was prevented by preexposing the TRPV1 antagonist, capsazepine (CPZ). Capsaicin-induced inhibition of Ih was dose dependent (IC50= 0.68 µM) and partially abolished by intracellular BAPTA and cyclosporin A, specific calcineurin inhibitor. In summary, the inhibitory effects of capsaicin on Ih are mediated by activation of TRPV1 and Ca2+-triggered cellular responses. Analgesic effects of capsaicin have been thought to be related to desensitization of nociceptive neurons due to depletion of pain-related substances. In addition, capsaicin-induced inhibition of Ih is likely to be important in understanding the analgesic mechanism of capsaicin.
Keywords: capsaicin, DRG neuron, hyperpolarization-activated cation current, rat
INTRODUCTION
The hyperpolarization-activatedcurrent (Ih) is a cation current activated by membrane hyperpolarization. The characteristics of Ih channels are: slow opening at negative potentials, permeability to both Na+ and K+ ions, and sensitivity to blockade with Cs+ ions. Activation of Ih at negative potentials can result in a slow depolarization, such a depolarizing influence could accelerate neuronal firing discharges. Because Ih has been thought to contribute to the maintenance of the resting membrane potential [1], modulation of Ih can affect neuronal excitability. The ion channels underlying Ih have been discovered about a decade ago, these proteins were termed hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. In mammals, the HCN channel family comprises four distinct members (HCN1-4) [2]. HCN1 and HCN2 channels are largely expressed in the DRG neurons [3] that play an important role in the transmission of sensory information from periphery. Especially, Emeryet al. reported that HCN2 ion channels play a critical role in inflammatory and neuropathic pain in mice [4]. Upregulation of Ih expression in the DRG neurons were related to the neuronal hyperexcitability in the neuropathic pain and pharmacological blockade of Ih could relieve the pain [5-8]. There have been many reports that suggest the inhibition of Ih may be the mechanism of opioid analgesics [3, 9] and local anesthetics [3, 5, 10-13]. These reports suggest that inhibition of Ih may be responsible for the peripheral analgesia [9].
Capsaicin, the main pungent ingredient in chili peppers can excite nociceptive sensory neurons and produce transient pain in animals and humans. Capsaicin activates the transient receptor potential vanilloid subtype 1 (TRPV1), a nonselective cation channel with high Ca2+ permeability [14]. The TRPV1 channel is expressed in subsets of primary sensory neurons and nerve terminals and plays an essential role in detecting noxious heat and several other nociceptive stimuli. Based on the reports that TRPV1knockout mice exhibit reduced inflammatory thermal hyperalgesia, TRPV1 appears to be essential for mediating thermal hyperalgesia induced by inflammation [15, 16]. Paradoxically, prolonged or repetitive exposure to capsaicincan desensitize nociceptive sensory neurons and results in long lasting pain relief [17]. Ca2+-dependent desensitization of TRPV1 themselves likely contributes to the analgesic effects of capsaicin [18].
Neuronal excitability is due to the presence of voltage-sensitive ion channels in the plasma membrane [19]. Thus, activation or inhibition of these ion channels may contribute to the excitability of the sensory neurons and capsaicin analgesia. Capsaicin blocks voltage-gated Na+ channels in trigeminal ganglion [20] and DRG neurons [20-22], Ca2+ channels in the sensory neurons [23-25] and K+ channels in the trigeminal ganglion neurons [26]. Such inhibitions of voltage-sensitive channels by capsaicin may result in decrease oft he excitability of neurons and block the transmission of painful signal from peripheral tissues.
As stated above, inhibition of Ih may be related to peripheral analgesia. Thus, we hypothesized that inhibition of Ih in the DRG neurons may be one of the mechanisms underlying analgesic effect of capsaicin. Here, we studied the hypothesis that capsaicin could induce the inhibition of Ih in the cultured DRG neurons using patch clamp technique.
MATERIALS AND METHODS
Cell culture
Primary cultures of DRG neurons dissected from all levels of lower cervical, thoracic, and lumbar spinal cord of two-day-old neonatal rats were prepared. The dissected ganglia were collected in cold culture medium (4℃), which contained Dulbecco's modified Eagle medium/F-12 mixture (DMEM/F-12; Gibco, Invitrogen, Grand Island, NY, USA), 10% fetal bovine serum (FBS; Gibco, Invitrogen), 1 mM sodium pyruvate, and 100 U/ml penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA). The collected ganglia were washed with the culture medium and incubated at 37℃ for 30 min in 1 mg/ml collagenase (Type II; Worthington, Freehold, NJ, USA). The ganglia were then washed three times with Mg2+- and Ca2+-free Hank's balanced salt solution (HBSS; Gibco, Invitrogen) and incubated in 2.5 mg/ml trypsin (Gibco, Invitrogen) at 37℃ for 30 min. Subsequently, the ganglia were centrifuged at 1,000 rpm for 10 min, and the pellet was washed two or three times with the culture medium to inhibit the enzyme. The pellet in turn was suspended in the culture medium by gentle trituration with a Pasteur pipette, and the suspended cells were plated on square glass coverslips coated with poly-L-lysine (Sigma-Aldrich), which were placed in small Petri dishes. Then, 25 ng/ml nerve growth factor (Alomone Labs, Jerusalem, Israel) were added to each Petri dish. Cells were incubated at 37℃ in a 95% air -5% CO2 gas mixture and used 1~3 days after plating.
Electrophysiology
Whole-cell membrane currents were recorded from the somata of DRG neurons. Electrodes were made by pulling borosilicate glass capillaries (Harvard, Kent, UK). Tip resistances were 2~3 MΩ for whole-cell recordings. To record whole-cell current, cellmembrane was ruptured by gentle suction after the formation of a gigaohmseal. Capacitative transients were then cancelled. Whole-cell membrane currents were recorded at -60 mV using an Axopatch 200 Bamplifier (Molecular Devices, Union City, CA, USA). Membrane currents were low pass filtered at 1 kHz and sampled at 2.5 kHz with a Digidata 1,322 data acquisition system (Molecular Devices). Data were analyzed using the pClamp 8.0 software (Molecular Devices) and OriginPro 8.0 (OriginLab, Northampton, MA, USA). All electrophysiological experiments were performed at room temperature.
Solutions and chemicals
Normal Tyrode's (NT) solution contained (in mM) 140 NaCl, 3 KCl, 10 HEPES, 10 glucose, 2.5 CaCl2, 1 MgCl2 (pH was adjusted to 7.4 with NaOH). The recording electrodes were filled with apipette solution containing (in mM) 135 K gluconate, 1 MgCl2, 10 NaCl, 10 HEPES, 2 Mg-ATP, and 0.1 Na-GTPtitrated to pH 7.4 with KOH. For intracellular Ca2+ chelation, 10 mM BAPTA was added to the internal solution. The nominally Ca2+-free bath solution contained (in mM) 140 NaCl, 3 KCl, 10 HEPES, 10 glucose, 1 MgCl2,10 EGTA (pH was adjusted to 7.4 with NaOH). To isolate Ih from other voltage-sensitive currents, 0.5 mM BaCl2, 0.1 µM tetrodotoxin, 10 mM tetraethylammonium chloride, 1 mM 4-aminopyridine and 0.1 mM NiCl2 were added to the bath solution throughout the experiments. Capsaicin was dissolved and stored as a 10 mM stock solution in 100% ethanol. All stock solutions were stored at -20℃ and were diluted with the bath solution to the desired final concentrations at the beginning of each experiment.
Statistical analysis
Data are presented as means±standard error of the mean (SEM). Inhibitory effects capsaicin were given as % inhibition=100×(1-(Ih,cap/Ih,con)), where Ih,con and Ih,cap are the currents measured before and during application of capsaicin, respectively. All comparisons between means were tested for significance using Student's paired t-test and analysis of variance (ANOVA) followed by the post hoc Tukey test. p<0.05 was considered to indicate significant difference.
RESULTS
Effect of capsaicin on Ih
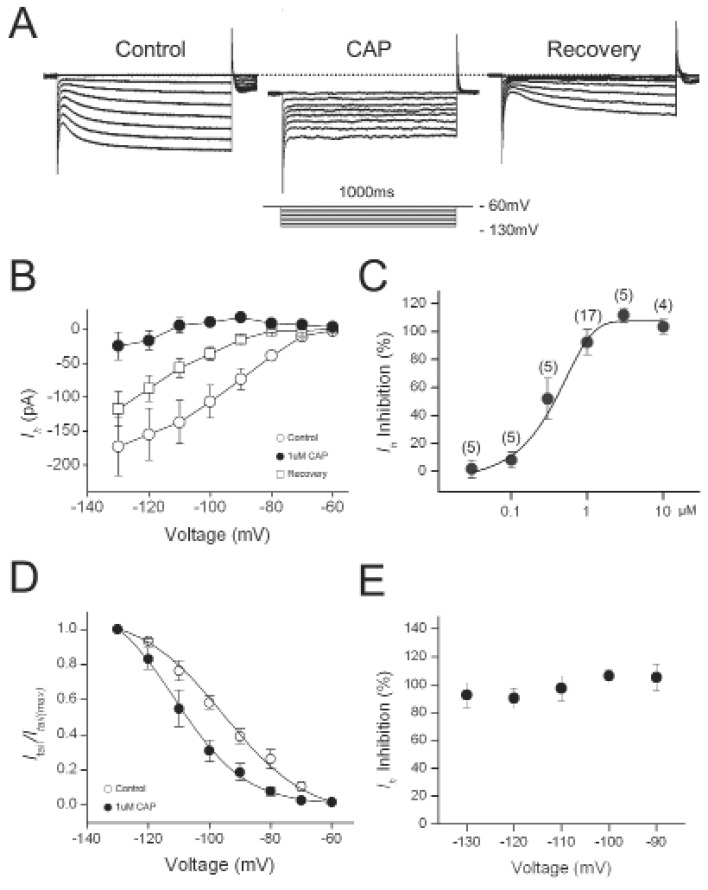
Hyperpolarization of DRG neurons voltage-clamped at -60 mV induced noninactivatinginward current consisting of an instantaneous and a slowly-activating component (Fig. 1A). Ih was calculated as the difference between instantaneous current (Iinst) at the beginning and the steady state current (Iss) at the end of the 1s-voltage step, respectively. Capsaicin (1 µM) evoked inward currents ranging from 0.69±0.15 nA at -60 mV in the DRG neurons. After applying capsaicin continuously for 1~3 min, the capsaicin-evoked currents were desensitized to a near steady state value 0.38±0.05 nA (n=17). At this time, to study the effect of capsaicin on Ih, hyperpolarizing voltage pulses were applied from -130 to -60 mV with 10 mV increments in the presence of capsaicin. Amplitudes of Ih measured at -130 mV in the presence of capsaicin were significantly reduced by 92.5±14.2% (n=16). After washing these cells for another 3~5 min, Ih was partially recovered to their pre-capsaicin levels. Capsaicin blocked Ih in a dose-dependent manner (Fig. 1C). IC50 was 0.68 µM and Hill coefficient was 1.13. The half-activation voltage (V1/2) was -94.1±2.3 mV in control and -109.9±3.1 mV in capsaicin treated cell, a negative shift of about 15 mV (Fig. 1D). Inhibition magnitudes of Ih by capsaicin were not significantly different at various voltages (One-way ANOVA, F=1.57393, p=0.21659, n=19) (Fig. 1E).
Fig. 1.
Capsaicin inhibited Ih in DRG neurons. (A) Representative current responses of DRG neurons to 1-s voltage pulses injected in 10-mV increment under control condition, during application of 1 µM capsaicin (CAP) and after washout (recovery). (B) Current-voltage relationship of Ih. Amplitudes of Ih in the presence were compared with those of control Ih at the same test voltage. (C) Concentration dependence of capsaicin inhibition of Ih. The curve was fitted by Hill equation (IC50=0.68 µM). (D) Effects of capsaicin on the voltage dependence of activation. Normalized tail current was plotted versus various potentials (-60 to -130 mV) and fitted with Boltzmann equation. Estimated V1/2 was shifted to left by CAP. (E) Voltage dependence of capsaicin inhibition of Ih. Amplitudes of capsaicin inhibition of Ih in the presence of capsaicin were normalized to those in control at the same test voltages.
Involvement of TRPV1 in Ih inhibition by capsaicin
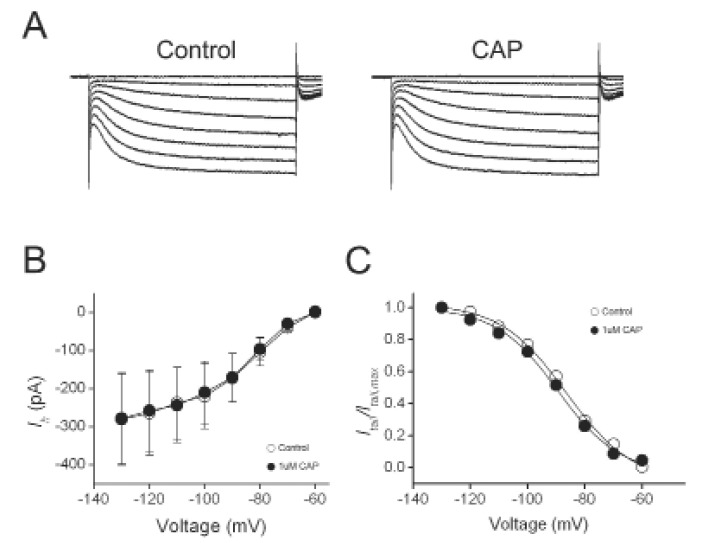
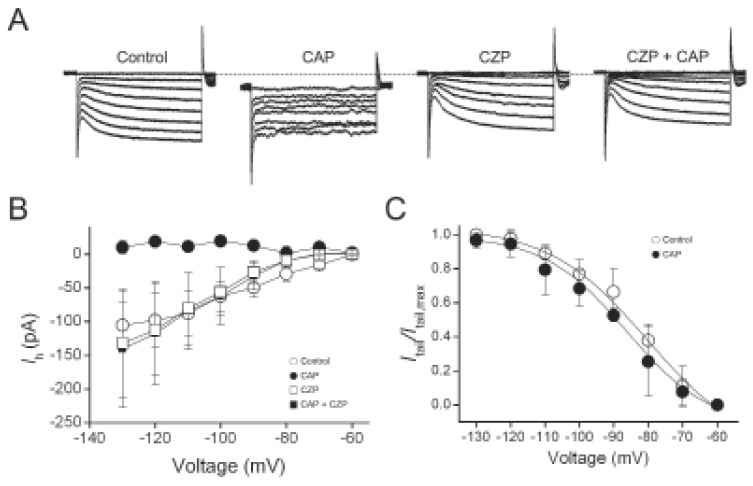
We found that in capsaicin-insensitive cells where capsaicin did not evoke membrane currents, capsaicin had no significant effect on the amplitudes of Ih (n=3) and activation kinetics (Fig. 2). To determine whether the effect of capsaicin on Ih is mediated by TRPV1 activation, we used TRPV1 antagonist, capsazepine. As shown in Fig. 3, capsazepine (10 µM )alone did not produce any effect on Ih. In the presence of capsazepine, 1 µM capsaicin failed to induce any inward currents as well as inhibitory effects on Ih. These results suggest that capsaicin indirectly inhibited Ih through the activation of TRPV1.
Fig. 2.
Capsaicin did not inhibit Ih in the capsaicin-insensitive DRG neurons. (A) Representative current responses of DRG neurons to 1-s voltage pulses injected in 10-mV increment under control condition, during application of 1 µM capsaicin (CAP). (B) Current-voltage relationship of Ih. Amplitudes of Ih in the presence CAP were compared with those of control Ih at the same test voltage. (C) Effects of capsaicin on the voltage dependence of activation. Normalized tail current was plotted versus various potentials (-60 to -130 mV) and fitted with Boltzmann equation. Estimated V1/2 was not significantly changed by CAP.
Fig. 3.
Capsazepineprevented the inhibition of Ih by capsaicin. (A) Representative current responses of DRG neurons to 1-s voltage pulses injected in 10-mV increment under control condition, during application of 1 µM capsaicin (CAP), during application of 10 µM capsazepine (CZP) and subsequent addition of CAP. (B) Current-voltage relationship of Ih. Amplitudes of Ih in the presence of CAP, CZP and CAP+CZP were compared with those of control Ih at the same test voltage. (C) Normalized tail current was plotted versus various potentials (-60 to -130 mV) and fitted with Boltzmann equation. Estimated V1/2 was not significantly changed by CAP+CZP.
Effect of Ca2+ on Ih inhibition by capsaicin
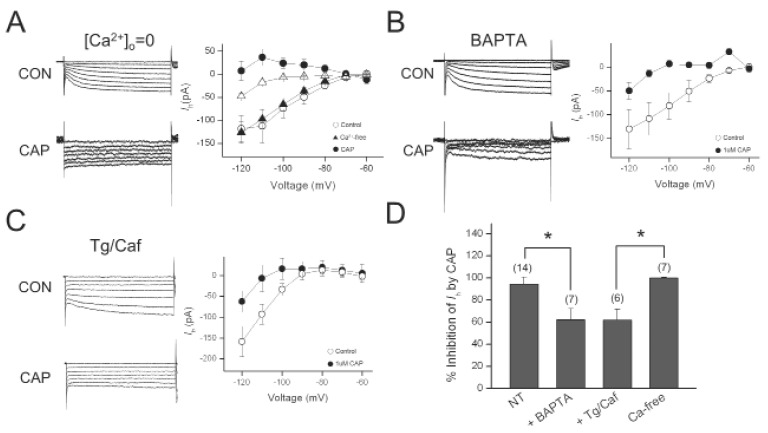
Because TRPV1 is highly permeable to Ca2+ [14], intracellular Ca2+ concentration can be greatly increased when the channel is opened. To determine the role of Ca2+ influx in the inhibitory effect of capsaicin on Ih, we removed extracellular Ca2+ from the bath solution. Amplitudes of Ih were not significantly changed by in Ca2+-free bath solution. Capsaicin inhibition of Ih was not abolished in Ca2+-free bath solution (Fig. 4). In the presence of intracellular BAPTA (10 mM), a rapid Ca2+ chelator, 1 µM capsaicin still inhibited Ih (n=6). However, the magnitude of Ih inhibition by capsaicin (62.1±10.2%) was significantly different from that obtained in pipette solution without BAPTA (n=6) (Fig. 4A).Therefore, intracellular Ca2+ may be required for the inhibition of Ih by capsaicin in the DRG neurons.
Fig. 4.
Effect of Ca2+ on Ih inhibition by capsaicin. (A) Representative current responses of DRG neurons to 1-s voltage pulses under control (CON) condition, during application of 1 µM capsaicin (CAP) in Ca2+-free bath solution (0 Ca2+). Graph shows the current-voltage relationship of Ih under control and during capsaicin application in Ca2+-free bath solution. (B) Representative current responses of DRG neurons to 1-s voltage pulses under control condition, during application of CAP in the presence of BAPTA in the pipette. Graph shows the current-voltage relationship of Ih under control and during capsaicin application in the presence of BAPTA. (C) Representative current responses of DRG neurons to 1-s voltage pulses under control condition, during application of 1 µMCAP after intracellular Ca2+ depletion bythapsigargin (Tg) and caffeine (Caf, 10 mM). Graph shows the current-voltage relationship of Ih under control and during capsaicin application after Tg/Caft retatment. (D) Bar graph summarizes % inhibitions induced by capsaicin. Values at the top of each bar represent the number of experiments. Bars represent the mean±S.E.M. Asterisks indicate a significant difference (*p<0.05).
Rapid rise in intracellular Ca2+ levels may due to either Ca2+ influx or release of Ca2+ from an intracellular store. Thus, we investigated whether the inhibitory effect of capsaicin on Ih was mediated by Ca2+ released from the intracellular stores. To deplete intracellular Ca2+ stores, we pretreated DRG cells with thapsigargin (10 µM) and caffeine (10 mM) for 3 min and washed them out for at least 5 min, before applying capsaicin. The percentile inhibition of Ih by capsaicin at -120 mV were reduced to 61.9±9.9% (n=7, p<0.05). These results demonstrated that increase in intracellular Ca2+ level is partially involved in the inhibition of Ih by capsaicin.
Role of Calcineurin in Ih inhibition by capsaicin
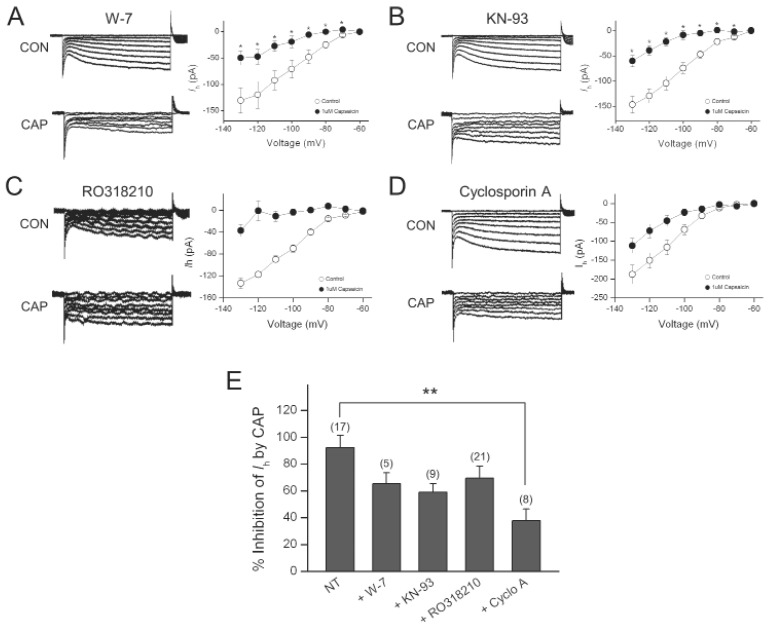
Because the increase in intracellular Ca2+ appeared to be required for the inhibitory effect of capsaicin on Ih, we next determined if calmodulin and Ca2+/calmodulin-dependent protein kinase II are involved in this effect. The specific calmodulin antagonist, W-7 or the selective Ca2+/Calmodulin-dependent protein kinase II inhibitor, KN-93 was included in the pipette solution. In the presence of W-7 (10 µM), capsaicin still inhibited Ih (65.1±8.3%, n=5), which was not significantly different from the effect of capsaicin on Ih recorded using pipette solution without W-7 (92.5±8.9%, n=17) (Fig. 5A). In the presence of KN-93 (10 µM), capsaicin still produced large inhibition on Ih (59.0±6.2%, n=9), which was not significantly different from the effect of capsaicin on Ih recorded using control pipette solution (Fig. 5B).
Fig. 5.
Cyclosporin A partially abolished the Ih inhibition by capsaicin. (A) Representative current responses of DRG neurons to 1-s voltage pulses under control condition, during application of 1 µM capsaicin (CAP) in the presence of W-7 in the pipette. Graph shows the current-voltage relationship of Ih under control and during capsaicin application in the presence of W-7. (B) Representative current responses of DRG neurons to 1-s voltage pulses under control condition, during application of 1 µM capsaicin in the presence of KN-93 in the pipette. Graph shows the current-voltage relationship of Ih under control and during capsaicin application in the presence of KN-93. (C) Representative current responses of DRG neurons to 1-s voltage pulses under control condition, during application of 1 µM capsaicin in the presence of thapsigargin in the pipette. Graph shows the current-voltage relationship of Ih under control and during capsaicin application in the presence of thapsigargin. (D) Representative current responses of DRG neurons to 1-s voltage pulses under control condition, during application of CAP in the presence of cyclosporine A in the pipette. Graph shows the current-voltage relationship of Ih under control and during capsaicin application in the presence of cyclosporine A. (E) Bar graph summarizes % inhibitions induced by capsaicin. Values at the top of each bar represent the number of experiments. Bars represent the mean±S.E.M. Asterisks indicate a significant difference from the first bar (**p<0.01).
Ih can be inhibited by phosphorylation by PKC [27, 28], we determined whether PKC inhibitor can block the inhibitory effect of capsaicin on Ih. When 20 µM RO318210, PKC inhibitorwas included in the pipette solution, capsaicin decreased Ih by 69.3±9.0% (n=21), which was not significantly different from the capsaicin-induced% inhibition obtained with normal pipette solution (Fig. 5C).
Calcineurin (protein phosphatase 2B) is a Ca2+-sensitive protein phosphatase and can be activated by a rise in intracellular Ca2+. To determine whether capsaicin inhibited Ih through dephosphorylationmediated by calcineurin, cyclosporin A, aspecific inhibitor of calcineurin was added to the pipette solution. As shown in Fig. 5D, subsequent application of capsaicin reduced Ih amplitude measured at -130 mV by 37.7±8.7% (n=8), a significantly smaller reduction than the effect of capsaicin in the absence of cyclosporin A (p< 0.01).
DISCUSSION
In the present study, we demonstrate that capsaicin reversibly and voltage-independently inhibited hyperpolarization-activated cation current, Ih, in the culturedrat DRG neurons. We also found that capsaicin did not change Ih in the capsaicin-insensitive DRG neurons and TRPV1 antagonist, capsazepine eliminated the effect of capsaicin on Ih. Therefore, capsaicin may indirectly modulate Ih via TRPV1 activation.
Because TRPV1, capsaicin receptor is highly permeable to Ca2+ [14], extracellular Ca2+ influx through the activated channel can influence on Ih. But, our findings that inhibition of Ih was not abolished by removal of Ca2+ from the bath solution revealed that extracellular Ca2+ influx and Ca2+-induced Ca2+ release (CICR) were not essential for the effect of capsaicin on Ih. We found that intracellular Ca2+ chelation and depletion of intracellular store partially abolished the inhibitory effect of capsaicin on Ih. It has been reported that functional TRPV1 was also expressed on the endoplasmic reticulum membrane of DRG neuron [29, 30] and capsaicin could increase intracellular Ca2+ level in the Ca2+-free condition [31]. Thus, it is likely that release of Ca2+ by the activation of TRPV1 in the endoplasmic reticulum could contribute to the inhibition of Ih by capsaicin to some extent.
As described in the result section, BAPTA, thapsigargin and cyclosporin A partly attenuated the inhibition of Ih by capsaicin. It is possible that additional mechanism underlies in the capsaicin-induced Ih inhibition. Remaining portion of Ih inhibition by capsaicin may be caused by decrease in driving forces that move cations into the DRG neurons. When TRPV1 is activated by capsaicin, Na+ can enter rapidly into the neurons by concentration gradient and negative holding potential (-60 mV). It has been reported that activation of TRPV1 blocked voltage-gated Na+ channels as a result of increase in intracellular Na+ concentration. Ih is a mixed cation current carried by both Na+ and K+ [2]. Therefore, movement of Na+ through the Ih channels might be inhibited by decreased Na+ concentration gradient due to increase of [Na+]i by TRPV1 activation. Therefore, amplitudes of Ih can be decreased by capsaicin as a result of decreased Na+ movement into the DRG neuronsthrough Ih channels in addition to Ca2+-dependent modulation of Ih.
Increase of intracellular Ca2+ concentration can trigger many intracellular events such as protein phosphorylation. Therefore, we were interested in the cellular events following the increase of intracellular Ca2+ level through activated TRPV1 in the modulation of Ih by capsaicin. Ca2+ can bindcalmodulin and desensitize TRPV1 [18]. If TRPV1 desensitization were responsible for the effect of capsaicin on Ih, inhibition of calmodulin could abolish the effect the capsaicin action. From the result thatcalmodulin antagonist, W7 failed to diminish the capsaicin-induced Ih inhibition, Ca2+/calmodulin-dependent TRPV1 desensitization may not be responsible for the Ih inhibition. It has been reported that activation of PKC could inhibit Ih [27, 28], we tested the effect of PKC inhibitor in Ih inhibition by capsaicin. However, PKC was not involved in the inhibition of Ih in our experiment condition. In the present study, we found that calcineurin (protein phosphatase 2B) could play a role in down-regulation of Ih caused by TRPV1 activation in the DRG neurons. Calcineurin is a Ca2+-dependent protein phosphatase and is responsible for the desensitization of TRPV1, either [32]. Since calmodulin antagonist failed to reverse the capsaicin effect on Ih, desensitization of TRPV1 may be irresponsible for thisinhibition of Ih. The target molecule of the dephosphorylarion by calcineurin may be not TRPV1 but Ih channel. From these results, we suggest that capsaicin may increase intracellular Ca2+ followed by modification of the Ih channel.
It has been reported that Ih plays an important role in the control of electrical activity in various neuronal cells. Thus, suppression of Ih by capsaicin may cause depressant effects on neuronal activity and analgesia. Topical application of capsaicin has been used to treat neuropathic pain [33, 34]. It has been thought that capsaicin causes release of substance P from C fiber afferent neurons, and repeated application depletes stores of substance P and therefore reduces pain transmission from peripheral nerve fibers to higher centers [35]. In addition to substance P depletion, inhibition of Ih may explain the analgesic effect of capsaicin. Our electrophysiological data can suggest the possible involvement of Ih inhibition in capsaicin-induced analgesia. Althoughrecent report that showed coexpression of TRPV1 and subtypes of HCNs in the sensory neurons [36] support this possibility, further animal studies will be needed to elucidate it.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MEST) (KRF-2004-202-E00033) to J. Kwak.
References
- 1.Doan TN, Kunze DL. Contribution of the hyperpolarization-activated current to the resting membrane potential of rat nodose sensory neurons. J Physiol. 1999;514:125–138. doi: 10.1111/j.1469-7793.1999.125af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- 3.Vasilyev DV, Shan Q, Lee Y, Mayer SC, Bowlby MR, Strassle BW, Kaftan EJ, Rogers KE, Dunlop J. Direct inhibition of Ih by analgesic loperamide in rat DRG neurons. J Neurophysiol. 2007;97:3713–3721. doi: 10.1152/jn.00841.2006. [DOI] [PubMed] [Google Scholar]
- 4.Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462–1466. doi: 10.1126/science.1206243. [DOI] [PubMed] [Google Scholar]
- 5.Dalle C, Eisenach JC. Peripheral block of the hyperpolarization-activated cation current (Ih) reduces mechanical allodynia in animal models of postoperative and neuropathic pain. Reg Anesth Pain Med. 2005;30:243–248. doi: 10.1016/j.rapm.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169–1178. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Q, Xing GG, Tu HY, Han JS, Wan Y. Inhibition of hyperpolarization-activated current by ZD7288 suppresses ectopic discharges of injured dorsal root ganglion neurons in a rat model of neuropathic pain. Brain Res. 2005;1032:63–69. doi: 10.1016/j.brainres.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Weng X, Smith T, Sathish J, Djouhri L. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Aδ-nociceptors. Pain. 2012;153:900–914. doi: 10.1016/j.pain.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Ingram SL, Williams JT. Opioid inhibition of Ih via adenylyl cyclase. Neuron. 1994;13:179–186. doi: 10.1016/0896-6273(94)90468-5. [DOI] [PubMed] [Google Scholar]
- 10.Funahashi M, Higuchi H, Miyawaki T, Shimada M, Matsuo R. Propofol suppresses a hyperpolarization-activated inward current in rat hippocampal CA1 neurons. Neurosci Lett. 2001;311:177–180. doi: 10.1016/s0304-3940(01)02169-3. [DOI] [PubMed] [Google Scholar]
- 11.Cacheaux LP, Topf N, Tibbs GR, Schaefer UR, Levi R, Harrison NL, Abbott GW, Goldstein PA. Impairment of hyperpolarization-activated, cyclic nucleotide-gated channel function by the intravenous general anesthetic propofol. J Pharmacol Exp Ther. 2005;315:517–525. doi: 10.1124/jpet.105.091801. [DOI] [PubMed] [Google Scholar]
- 12.Putrenko I, Schwarz SK. Lidocaine blocks the hyperpolarization-activated mixed cation current, Ih, in rat thalamocortical neurons. Anesthesiology. 2011;115:822–835. doi: 10.1097/ALN.0b013e31822ddf08. [DOI] [PubMed] [Google Scholar]
- 13.Perkins KL, Wong RK. Intracellular QX-314 blocks the hyperpolarization-activated inward current Iq in hippocampal CA1 pyramidal cells. J Neurophysiol. 1995;73:911–915. doi: 10.1152/jn.1995.73.2.911. [DOI] [PubMed] [Google Scholar]
- 14.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 15.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 16.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 17.Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75:157–168. doi: 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- 18.Vyklický L, Nováková-Tousová K, Benedikt J, Samad A, Touška F, Vlachová V. Calcium-dependent desensitization of vanilloid receptor TRPV1: a mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiol Res. 2008;57:S59–S68. doi: 10.33549/physiolres.931478. [DOI] [PubMed] [Google Scholar]
- 19.Catterall WA. The molecular basis of neuronal excitability. Science. 1984;223:653–661. doi: 10.1126/science.6320365. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Oortgiesen M, Li L, Simon SA. Capsaicin inhibits activation of voltage-gated sodium currents in capsaicin-sensitive trigeminal ganglion neurons. J Neurophysiol. 2001;85:745–758. doi: 10.1152/jn.2001.85.2.745. [DOI] [PubMed] [Google Scholar]
- 21.Onizuka S, Yonaha T, Tamura R, Hosokawa N, Kawasaki Y, Kashiwada M, Shirasaka T, Tsuneyoshi I. Capsaicin indirectly suppresses voltage-gated Na+ currents through TRPV1 in rat dorsal root ganglion neurons. Anesth Analg. 2011;112:703–709. doi: 10.1213/ANE.0b013e318204ea5b. [DOI] [PubMed] [Google Scholar]
- 22.Su X, Wachtel RE, Gebhart GF. Capsaicin sensitivity and voltage-gated sodium currents in colon sensory neurons from rat dorsal root ganglia. Am J Physiol. 1999;277:G1180–G1188. doi: 10.1152/ajpgi.1999.277.6.G1180. [DOI] [PubMed] [Google Scholar]
- 23.Kopanitsa MV, Panchenko VA, Magura EI, Lishko PV, Krishtal OA. Capsaicin blocks Ca2+ channels in isolated rat trigeminal and hippocampal neurones. Neuroreport. 1995;6:2338–2340. doi: 10.1097/00001756-199511270-00016. [DOI] [PubMed] [Google Scholar]
- 24.Hagenacker T, Splettstoesser F, Greffrath W, Treede RD, Büsselberg D. Capsaicin differentially modulates voltage-activated calcium channel currents in dorsal root ganglion neurones of rats. Brain Res. 2005;1062:74–85. doi: 10.1016/j.brainres.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Yueyan L, Min X, Qing H. Capsaicin inhibits voltage-activated calcium channel via calcium signals in sensory neurons. Acta Universitatis Medicinalis Anhui. 2009;44:01. [Google Scholar]
- 26.Liu L, Simon SA. Modulation of IA currents by capsaicin in rat trigeminal ganglion neurons. J Neurophysiol. 2003;89:1387–1401. doi: 10.1152/jn.00210.2002. [DOI] [PubMed] [Google Scholar]
- 27.Cathala L, Paupardin-Tritsch D. Neurotensin inhibition of the hyperpolarization-activated cation current (Ih) in the rat substantia nigra pars compacta implicates the protein kinase C pathway. J Physiol. 1997;503:87–97. doi: 10.1111/j.1469-7793.1997.087bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Bunney EB, Appel SB, Brodie MS. Serotonin reduces the hyperpolarization-activated current (Ih) in ventral tegmental area dopamine neurons: involvement of 5-HT2 receptors and protein kinase C. J Neurophysiol. 2003;90:3201–3212. doi: 10.1152/jn.00281.2003. [DOI] [PubMed] [Google Scholar]
- 29.Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem. 2001;276:11021–11030. doi: 10.1074/jbc.M008392200. [DOI] [PubMed] [Google Scholar]
- 30.Gallego-Sandín S, Rodríguez-García A, Alonso MT, García-Sancho J. The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels. J Biol Chem. 2009;284:32591–32601. doi: 10.1074/jbc.M109.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eun SY, Jung SJ, Park YK, Kwak J, Kim SJ, Kim J. Effects of capsaicin on Ca2+ release from the intracellular Ca2+ stores in the dorsal root ganglion cells of adult rats. Biochem Biophys Res Commun. 2001;285:1114–1120. doi: 10.1006/bbrc.2001.5272. [DOI] [PubMed] [Google Scholar]
- 32.Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 33.Epstein JB, Marcoe JH. Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia. Oral Surg Oral Med Oral Pathol. 1994;77:135–140. doi: 10.1016/0030-4220(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 34.Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- 35.Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317–328. doi: 10.2165/00002512-199507040-00007. [DOI] [PubMed] [Google Scholar]
- 36.Obreja O, Klusch A, Ponelies N, Schmelz M, Petersen M. A subpopulation of capsaicin-sensitive porcine dorsal root ganglion neurons is lacking hyperpolarization-activated cyclic nucleotide-gated channels. Eur J Pain. 2008;12:775–789. doi: 10.1016/j.ejpain.2007.11.010. [DOI] [PubMed] [Google Scholar]