Abstract
The storage of soil samples for PLFA analysis can lead to shifts in the microbial community composition. We show here that conserving samples in RNAlater, which is already widely used to store samples for DNA and RNA analysis, proved to be as sufficient as freezing at −20 °C and preferable over storage at 4 °C for temperate mountain grassland soil. The total amount of extracted PLFAs was not changed by any storage treatment. Storage at 4 °C led to an alteration of seven out of thirty individual biomarkers, while freezing and storage in RNAlater caused changes in the amount of fungal biomarkers but had no effect on any other microbial group. We therefore suggest that RNAlater could be used to preserve soil samples for PLFA analysis when immediate extraction or freezing of samples is not possible, for example during sampling campaigns in remote areas or during transport and shipping.
Keywords: Phospholipid fatty acids, PLFA, RNAlater, Sample storage, Field method, Freezing
Highlights
► We tested storage of soils in RNAlater for analysis of phospholipid fatty acids. ► Storage in RNAlater proofed to be comparable to freezing at −20 °C. ► Freezing and RNAlater led to reduced fungal biomarkers compared to controls. ► Storage in RNAlater is suggested as a novel approach for sampling at remote areas.
In the last decades the use of phospholipid fatty acids (PLFAs) as microbial biomarkers has proven to be a reliable tool to analyse microbial community structure in soils (Ruess and Chamberlain, 2010; Frostegård et al., 2011). Moreover, PLFA analysis together with 13C labelling allows to trace carbon fluxes, e.g. from plants to soil and between microbial groups (Pelz et al., 2005; Paterson et al., 2007). Although a relatively robust method in general, storage before the extraction of the PLFAs tends to alter the resulting microbial community profiles. Several studies have compared the effects of drying and rewetting, freezing or sample storage at different temperatures above 0 °C (Petersen and Klug, 1994; Schutter and Dick, 2000; Trabue et al., 2006; Lee et al., 2007). Most of these studies conclude that it is best to use fresh soil, but if storage is unavoidable the most widely used method is to freeze soils (Högberg et al., 2006; Liu et al., 2008; Schindlbacher et al., 2011). Soils should be frozen continuously and PLFAs should be extracted in a frozen state to avoid nutrient release by freeze-thaw events and associated shifts in microbial community composition (Mannisto et al., 2009). However, when working in remote areas such as alpine or arctic regions it is often neither possible to process samples immediately nor can the samples be kept frozen continuously. We therefore tested the use of a commercial product, RNAlater (Sigma–Aldrich, Austria), to store samples for subsequent PLFA analysis. RNAlater is designed to preserve biological samples for later DNA or RNA analysis. The solution is a mixture of sodium ethylene diamine tetraacetic acid (EDTA), ammonium sulphate and a citrate buffer at pH 5.2 and is designed to readily infiltrate into biological samples and deactivate RNA degrading enzymes (Lader, 2001). The goal of the present study therefore was to test whether storage of soil samples in RNAlater also preserves PLFAs for later analysis. Towards this end we compared PLFA patterns of soil samples stored in RNAlater with those of fresh samples extracted immediately after sampling and samples which were either stored at 4 °C or frozen at −20 °C for 40 days prior to extraction.
Soil samples were taken in October 2010 from a typical temperate mountain grassland near Neustift, Stubai Valley (1900 m a.s.l). The soil (pH 6.8) has been classified as Dystric Cambisol (Bahn et al., 2006, 2008). Samples were taken from the A horizon, sieved (2 mm) and living fine roots were carefully removed to reduce the contribution of plant-derived PLFA markers (Kaiser et al., 2010a). Four independent treatments in five replicates with 1 g soil each were established as follows: Soil samples were transported from the field to the laboratory within 48 h and all treatments were initiated at the same time: Fresh soil was directly extracted (control, C). Samples were either stored at −20 °C (freezing treatment, F) or at 4 °C (cool treatment, S) or treated with RNAlater (R) for 40 days. In the latter case, 1 g of soil was mixed with 2 mL of RNAlater in 3-mL polypropylene vials and kept at 4 °C. These samples were then transferred to 30-mL glass vials and the original vials were washed twice with 1 ml RNAlater. After centrifugation (1000 g for 10 min) the supernatant was pipetted off and discarded. The remaining pellet was used for extraction. In addition, blanks (RNAlater only) were also routinely processed, but did not show any interfering substances. Frozen samples were extracted in a frozen state to avoid thawing effects.
Regardless of treatment all samples were extracted according to Kaiser et al. (2010b). Total lipids were extracted with a mixture of chloroform, methanol and 0.15 M citric acid buffer, at pH 4 (1:2:0.8, v/v/v). Neutral lipids and phospholipids were separated on silica columns (Supelco, LC-Si SPE, Austria) using chloroform, acetone and methanol as eluents. After addition of methyl-nonadecanoate (19:0) as an internal standard and conversion to fatty acid methyl esters (FAMEs) by alkaline methanolysis, samples were dried and re-dissolved in isooctane and analysed with gas chromatography with flame ionisation detection (Trace GC Ultra, Thermo) on a DB23 column (Agilent 60 m × 0.25 mm × 0.25 μm). Bacterial and fungal FAME mixtures (bacterial acid methyl ester mix and 37 Comp. FAME Mix, Supelco) were used as qualitative standards. The internal standard 19:0 was used to calculate the concentration of FAMEs. The assignment of individual PLFAs to microbial groups is shown in Table 1.
Table 1.
Assignment of individual phospholipid fatty acids to microbial groups and mean concentrations and standard errors of individual biomarkers. Actinobacteria (actino), Gram-positive bacteria (gram +), Gram-negative bacteria (gram −), general bacterial (bacteria) and fungi are calculated as the sum of the respective individual biomarkers. The amount of bacteria was calculated as a sum of gram +, gram −, and general bacterial markers. The two sample comparison tests (Student's t-tests, Welch's t-test or Mann–Whitney-U tests) were performed with the statistical software R version 2.12.1 (R Development Core Team, 2010). The statistical significances are presented as following: ***, p-value < 0.001; **, p-values < 0.01; *, p-values < 0.05; n.s., p-values > 0.05.
| PLFA | Marker | Mean concentration of PLFA markers (nmol g−1 dry-weight) |
Two sample comparison tests |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (C) | −20 °C (F) | RNAlater (R) | 4 °C (S) | C vs F | C vs R | C vs S | F vs R | ||
| i14:0 | general | 6.33 ± 0.94 | 10.16 ± 1.61 | 8.87 ± 0.88 | 10.12 ± 0.87 | n.s. | n.s. | * | n.s. |
| 14:0 | general | 5.11 ± 0.71 | 7.21 ± 0.85 | 6.45 ± 0.61 | 7.40 ± 0.42 | n.s. | n.s. | * | n.s. |
| i15:0 | gram + | 36.41 ± 3.02 | 39.77 ± 3.35 | 40.28 ± 3.15 | 41.76 ± 1.60 | n.s. | n.s. | n.s. | n.s. |
| a15:0 | gram + | 32.63 ± 2.69 | 35.43 ± 2.93 | 36.17 ± 2.64 | 40.18 ± 1.71 | n.s. | n.s. | * | n.s. |
| 15:0 | bacteria | 3.97 ± 0.34 | 4.19 ± 0.28 | 3.91 ± 0.32 | 4.30 ± 0.14 | n.s. | n.s. | n.s. | n.s. |
| i16:0 | gram + | 18.30 ± 0.84 | 18.85 ± 0.84 | 18.56 ± 1.52 | 18.36 ± 0.38 | n.s. | n.s. | n.s. | n.s. |
| 16:0 | general | 85.23 ± 3.26 | 85.75 ± 2.16 | 81.95 ± 5.01 | 83.01 ± 2.17 | n.s. | n.s. | n.s. | n.s. |
| 16:1ω11 | general | 7.95 ± 0.45 | 8.57 ± 0.48 | 8.93 ± 0.59 | 10.49 ± 0.52 | n.s. | n.s. | ** | n.s. |
| 16:1ω9 | gram − | 7.58 ± 0.63 | 7.64 ± 0.43 | 7.63 ± 0.24 | 6.28 ± 0.38 | n.s. | n.s. | n.s. | n.s. |
| 10Me16:0 | actino | 32.72 ± 1.00 | 35.74 ± 1.42 | 33.07 ± 1.58 | 36.85 ± 2.11 | n.s. | n.s. | n.s. | n.s. |
| 16:1ω7 | gram − | 42.73 ± 3.22 | 40.36 ± 2.74 | 41.04 ± 3.88 | 50.62 ± 1.33 | n.s. | n.s. | n.s. | n.s. |
| 16:1ω6 | general | 5.04 ± 0.55 | 5.32 ± 0.49 | 4.55 ± 0.58 | 6.45 ± 0.19 | n.s. | n.s. | * | n.s. |
| 16:1ω5 | gram − | 27.48 ± 1.29 | 28.22 ± 1.15 | 27.59 ± 1.60 | 31.50 ± 1.15 | n.s. | n.s. | * | n.s. |
| i17:0 | gram + | 10.35 ± 0.44 | 10.13 ± 0.41 | 9.95 ± 0.85 | 9.65 ± 0.25 | n.s. | n.s. | n.s. | n.s. |
| a17:0 | gram + | 9.54 ± 0.39 | 9.69 ± 0.33 | 9.37 ± 0.76 | 9.57 ± 0.31 | n.s. | n.s. | n.s. | n.s. |
| cy18:0 | gram − | 3.19 ± 0.15 | 3.36 ± 0.11 | 3.37 ± 0.21 | 3.78 ± 0.14 | n.s. | n.s. | * | n.s. |
| 17:0 | bacteria | 2.57 ± 0.12 | 2.63 ± 0.10 | 2.43 ± 0.19 | 2.40 ± 0.07 | n.s. | n.s. | n.s. | n.s. |
| cy17:0 | gram − | 21.03 ± 0.58 | 20.87 ± 0.53 | 20.46 ± 1.17 | 21.55 ± 0.44 | n.s. | n.s. | n.s. | n.s. |
| 17:1ω6 | bacteria | 2.22 ± 0.10 | 2.36 ± 0.08 | 2.30 ± 0.17 | 2.48 ± 0.11 | n.s. | n.s. | n.s. | n.s. |
| 18:0 | general | 15.86 ± 0.73 | 15.10 ± 0.89 | 13.31 ± 0.68 | 14.48 ± 0.80 | n.s. | n.s. | n.s. | n.s. |
| 18:1ω9 | fungi | 29.23 ± 1.42 | 28.66 ± 2.44 | 24.83 ± 2.89 | 26.98 ± 2.07 | n.s. | n.s. | n.s. | n.s. |
| 18:1ω7 | gram - | 77.73 ± 3.01 | 87.99 ± 3.82 | 81.66 ± 4.96 | 88.19 ± 8.57 | n.s. | n.s. | n.s. | n.s. |
| 18:1ω5 | bacteria | 6.97 ± 0.18 | 7.43 ± 0.27 | 6.82 ± 0.33 | 7.48 ± 0.59 | n.s. | n.s. | n.s. | n.s. |
| 18:2ω6,9 | fungi | 28.95 ± 1.04 | 13.51 ± 0.58 | 12.37 ± 0.40 | 27.57 ± 2.49 | *** | *** | n.s. | n.s. |
| cy19:0 | gram − | 3.95 ± 0.16 | 4.28 ± 0.16 | 4.11 ± 0.21 | 3.81 ± 0.19 | n.s. | n.s. | n.s. | n.s. |
| 19:1ω8 | general | 22.24 ± 1.66 | 27.77 ± 2.12 | 23.47 ± 1.72 | 24.57 ± 3.58 | n.s. | n.s. | n.s. | n.s. |
| 18:3ω3,6,9 | fungi | 2.68 ± 0.22 | 2.32 ± 0.21 | 1.65 ± 0.10 | 2.36 ± 0.36 | n.s. | ** | n.s. | * |
| 20:0 | general | 3.27 ± 0.11 | 2.91 ± 0.02 | 3.25 ± 0.12 | 3.32 ± 0.16 | * | n.s. | n.s. | n.s. |
| 20:1ω9 | general | 1.72 ± 0.04 | 1.72 ± 0.04 | 1.63 ± 0.06 | 1.88 ± 0.10 | n.s. | n.s. | n.s. | n.s. |
| Fungi | 60.86 ± 1.72 | 44.49 ± 2.20 | 38.85 ± 3.27 | 56.90 ± 3.44 | *** | *** | n.s. | n.s. | |
| Bacteria | 306.64 ± 13.65 | 323.2 ± 12.93 | 315.64 ± 20.76 | 341.90 ± 14.86 | n.s. | n.s. | n.s. | n.s. | |
| Gram + bacteria | 107.22 ± 7.28 | 113.86 ± 7.48 | 114.33 ± 8.77 | 119.52 ± 3.97 | n.s. | n.s. | n.s. | n.s. | |
| Gram − bacteria | 183.70 ± 6.45 | 192.72 ± 6.34 | 185.84 ± 11.32 | 205.73 ± 11.32 | n.s. | n.s. | n.s. | n.s. | |
| Actinobacteria | 32.72 ± 1.00 | 35.74 ± 1.42 | 33.07 ± 1.58 | 36.85 ± 2.11 | n.s. | n.s. | n.s. | n.s. | |
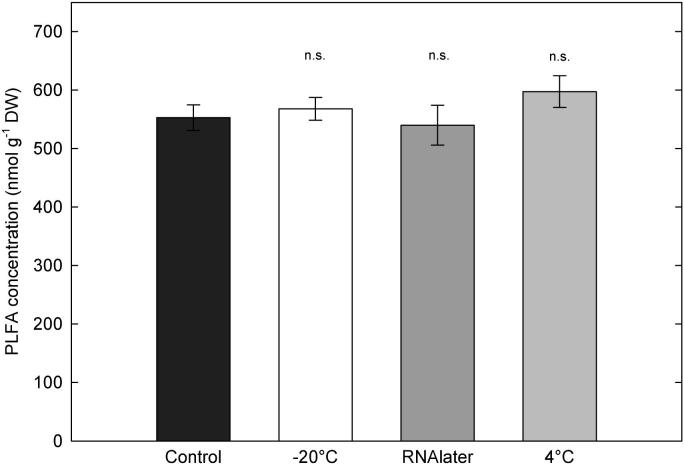
The different storage treatments had no statistically significant effect on the total yield of PLFAs and therefore on the estimates of microbial biomass (Fig. 1). Several other studies, however, found significantly higher total fatty acid methyl esters (FAMEs) content in samples stored at 4 °C or −20 °C compared to controls (Schutter and Dick, 2000; Lee et al., 2007). Freezing as well as the RNAlater treatment led to significantly lower fungal biomarker amounts than the controls, mainly because of the biomarker 18:2ω6,9 (Table 1). The RNAlater treatment also reduced the amount of the 18:3ω3,6,9 fungal marker compared to control and freeze treatment but the contribution of this biomarker was relatively small compared to the other two fungal biomarkers (18:2ω6,9 and 18:1ω9). Lee et al. (2007) also reported changes in the relative amount of fungal biomarkers in one out of three tested soils caused by freezing, but other than in our study the amount of fungi was increased despite a reduction of total FAMEs. Except the fungal PLFAs, no other biomarker that we analysed was significantly different between control and RNAlater treatment. In contrast, storage at 4 °C increased the amount of seven out of the thirty PLFAs significantly, indicating a shift in the microbial community composition (Table 1).
Fig. 1.
Effect of sample storage on the total phospholipid fatty acid content. The total amount of PLFAs was calculated as the sum of all biomarkers presented in Table 1. None of the storage treatments were significantly different from controls (n = 5; p < 0.05, Student's t-tests). Control, extracted immediately; −20 °C, stored frozen for 40 days; RNAlater, stored in RNAlater for 40 days; 4 °C, stored at 4 °C for 40 days.
In conclusion, we were able to demonstrate that storage of soils in RNAlater for later PLFA analysis provides an alternative to freezing or storage at low temperatures under the conditions tested. Despite the fact that storage in RNAlater and at −20 °C led to an underestimation of the contribution of fungal biomarkers to the microbial community compared to freshly extracted control soils, they are preferable to storage at low temperatures, which led to strong alterations of the PLFA pattern. Storage in RNAlater reduces the risk of freeze–thaw effects and eliminates the need of keeping the samples at a constant temperature and may thus be especially applicable as a field method to conserve soils from remote areas during transport and shipping to the laboratory. However, caution is necessary in the interpretation of fungal biomarkers after storage in RNAlater, which should only be directly compared to results from soils stored frozen. As we have tested the method for temperate mountain grassland soil only, we suggest that the suitability of storage in RNAlater shall be tested for other soil types before use.
Acknowledgements
The presented work was financially supported by the Austrian Science Fund (FWF; P 22214 and I 370-B17). We thank Robert Mikutta, Leibniz University Hannover, for comments on an earlier version of the manuscript.
References
- Bahn M., Knapp M., Garajova Z., Pfahringer N., Cernusca A. Root respiration in temperate mountain grasslands differing in land use. Global Change Biology. 2006;12:995–1006. [Google Scholar]
- Bahn M., Rodeghiero M., Anderson-Dunn M., Dore S., Gimeno C., Drosler M., Williams M., Ammann C., Berninger F., Flechard C., Jones S., Balzarolo M., Kumar S., Newesely C., Priwitzer T., Raschi A., Siegwolf R., Susiluoto S., Tenhunen J., Wohlfahrt G., Cernusca A. Soil respiration in European grasslands in relation to climate and assimilate supply. Ecosystems. 2008;11:1352–1367. doi: 10.1007/s10021-008-9198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegård Å., Tunlid A., Bååth E. Use and misuse of PLFA measurements in soils. Soil Biology and Biochemistry. 2011;43:1621–1625. [Google Scholar]
- Högberg M.N., Högberg P., Myrold D.D. Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia. 2006;150:590–601. doi: 10.1007/s00442-006-0562-5. [DOI] [PubMed] [Google Scholar]
- Kaiser C., Frank A., Wild B., Koranda M., Richter A. Negligible contribution from roots to soil-borne phospholipid fatty acid fungal biomarkers 18:2 omega 6,9 and 18:1 omega 9. Soil Biology & Biochemistry. 2010;42:1650–1652. doi: 10.1016/j.soilbio.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C., Koranda M., Kitzler B., Fuchslueger L., Schnecker J., Schweiger P., Rasche F., Zechmeister-Boltenstern S., Sessitsch A., Richter A. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytologist. 2010;187:843–858. doi: 10.1111/j.1469-8137.2010.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lader E.S. Ambion, Inc; 2001. Methods and Reagents for Preserving RNA in Cell and Tissue Samples. [Google Scholar]
- Lee Y.B., Lorenz N., Dick L.K., Dick R.P. Cold storage and pretreatment incubation effects on soil microbial properties. Soil Science Society of America Journal. 2007;71:1299. [Google Scholar]
- Liu Y., Yao H., Huang C. Assessing the effect of air-drying and storage on microbial biomass and community structure in paddy soils. Plant and Soil. 2008;317:213–221. [Google Scholar]
- Mannisto M.K., Tiirola M., Haggblom M.M. Effect of freeze–thaw cycles on bacterial communities of arctic Tundra soil. Microbial Ecology. 2009;58:621–631. doi: 10.1007/s00248-009-9516-x. [DOI] [PubMed] [Google Scholar]
- Paterson E., Gebbing T., Abel C., Sim A., Telfer G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytologist. 2007;173:600–610. doi: 10.1111/j.1469-8137.2006.01931.x. [DOI] [PubMed] [Google Scholar]
- Pelz O., Abraham W.R., Saurer M., Siegwolf R., Zeyer J. Microbial assimilation of plant-derived carbon in soil traced by isotope analysis. Biology and Fertility of Soils. 2005;41:153–162. [Google Scholar]
- Petersen S.O., Klug M.J. Effects of sieving, storage, and incubation temperature on the phospholipid fatty acid profile of a soil microbial community. Applied and Environmental Microbiology. 1994;60:2421–2430. doi: 10.1128/aem.60.7.2421-2430.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team, 2010. R: a language and environment for statisticalcomputing. In: R Foundation for Statistical Computing. Austria, Vienna.
- Ruess L., Chamberlain P.M. The fat that matters: soil food web analysis using fatty acids and their carbon stable isotope signature. Soil Biology & Biochemistry. 2010;42:1898–1910. [Google Scholar]
- Schindlbacher A., Rodler A., Kuffner M., Kitzler B., Sessitsch A., Zechmeister-Boltenstern S. Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biology & Biochemistry. 2011;43:1417–1425. doi: 10.1016/j.soilbio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter M.E., Dick R.P. Comparison of fatty acid methyl ester (FAME) methods for characterizing microbial communities. Soil Science Society of America Journal. 2000;64:1659–1668. [Google Scholar]
- Trabue S.L., Palmquist D.E., Lydick T.M., Singles S.K. Effects of soil storage on the microbial community and degradation of metsulfuron-methyl. Journal of Agricultural and Food Chemistry. 2006;54:142–151. doi: 10.1021/jf0512048. [DOI] [PubMed] [Google Scholar]