Abstract
The evidence linking body mass index (BMI) to severe OA shows a strong association in the knee. There are limited data exploring the effect of BMI on the risk of joint arthroplasty in a healthy population with long periods of follow up. We compared the self-reported BMI at age 20, measured BMI at baseline, year 5 and year 10 with the year 19 risk of total knee arthroplasty (TKA) in a well-described, population based cohort of healthy women. A total of 733 women attended the 19th year visit, of whom 31 underwent TKA and 676 were used as a control group after 26 were removed for having hip arthoplasty.
Using logistic regression, an increase in 1 unit of BMI at baseline was associated with a 10.5% increased risk of TKA (p = 0.017) and at year 5 the increased risk is 8.6% (p = 0.042). When adjusted for baseline age and smoking, baseline BMI was the only significant predictor of TKA at 10.0% with p = 0.024. There was no significant association at 10 years or for change in BMI over time.
In this prospective, population based study, BMI predicted the risk of TKA for OA. The risk was greatest at baseline when the patients were in middle age suggesting that this is the most important time to target weight reduction interventions.
Keywords: Knee, Arthroplasty, Risk, BMI
1. Introduction
Osteoarthritis (OA) of the major joints in the lower limb is a painful and disabling condition that accounts for an enormous healthcare burden [1–3]. The pathogenesis of OA is complex and thus predicting individuals in a healthy population who will progress to develop severe OA remains difficult. Consequently, there is a paramount need to reliably identify high-risk population cohorts who can be targeted for future therapeutic trials, for early intervention of modifiable risk factors and for ongoing research into the complex disease process and natural history of progression of OA.
There are many risk factors associated with end-stage knee OA [4,5], however few are as modifiable as body mass index (BMI) [6]. There is currently a strong level of evidence correlating BMI to future radiographic OA and clinical OA of the knee [7–9] with a similar but weaker association with the hip [10,11]. Whilst the link between BMI and end-stage OA in the knee continues to be scrutinised, it is also of interest to know how temporal changes in weight affect OA risk. Of the previous studies that have investigated this relationship, few have looked at change in BMI and end stage disease in a healthy community-based sample followed for almost 20 years. Additionally, surgically relevant OA endpoints such as total joint arthroplasty (TJA) have seldom been used, with many studies favouring radiographic change and clinical score severity as surrogate endpoints for OA [12–14].
The present study investigated the relationship between BMI measurements at different time points and risk of end-stage OA in the knee, defined by TKA.
2. Patients and methods
In 1989, all women between 45 and 64 years of age who were registered at the same medical practice in Chingford, North-East London were invited to join in this longitudinal prospective study. The primary focus of the study was to investigate the natural history of osteoporosis and osteoarthritis. A total of 1003 women volunteered to join the study at its commencement, representing 78% of the initially invited women. At year 19, 733 of these women were still actively involved in yearly follow-up clinics (104 had died, 78 dropped out, 62 moved away, 23 did not respond and three were too ill to attend). The clinical characteristics of the cohort and selected group are shown in Table 1. At study baseline (year 1), each individual was measured for height and weight. Also, each participant was asked to estimate their body weight from when they were aged 20. These measurements were taken in addition to extensive medical history questionnaires, social history, SF36 forms and radiological and serological investigations. Follow-up clinics were conducted annually up till year 10 of the study and annual postal questionnaires were sent thereafter.
Table 1.
Clinical characteristics.
| Characteristic | Case control respondents at year 19 (n = 707) | Control group respondents at year 19 without TJA (n = 676) | Case group respondents at year 19 with TKA (n = 31) |
|---|---|---|---|
| Baseline age, years, median (IQR) | 53 (48,58) | 53 (48,58) | 53 (51,59) |
| Baseline weight, kg, median (IQR) | 64.8 (58.5,72.3) | 64.4 (58.3,72) | 68.6 (64,74.8)* |
| Height, m, mean (SD) | 1.62 (0.06) | 1.62 (0.06) | 1.62 (0.06) |
| BMI at age 20, kg/m2, median (IQR) | 20.9 (19.3,22.8) n = 705 | 21.4 (19.8,23.5) n = 674 | 21.6 (19.5,23.5) |
| BMI at Year 1 (baseline), kg/m2, median (IQR) | 24.7 (22.6,27.3) | 24.5 (22.6,27.2) | 26.5 (24.1,28.8)* |
| BMI at year 5, kg/m2, median (IQR) | 25.6 (23.1,28.5) n = 650 | 25.5 (23.0,28.3) n = 621 | 27.4 (25.1,29.4)* n = 29 |
| BMI at year 10, kg/m2, median (IQR) | 26.3 (23.6,29.3) n = 679 | 26.1 (23.5,29.3) n = 651 | 27.5 (24.9,31.3) n = 28 |
| Baseline activity score, range = [3,12], median (IQR) | 7 (6,8) n = 702 | 7 (6,8) n = 670 | 7 (6,8) |
| Baseline parity, range = [0,9], median(IQR) | 2 (1,3) | 2 (1,3) | 2 (1,3) |
| Age of menopause, years, median (IQR) | 49 (45,51) n = 519 | 49 (45,51) n = 491 | 47.5 (42,51) n = 28 |
| Smoking,% never, ever | 56.6,43.4 | 56.8,43.2 | 51.6,48.4 |
*p-values < 0.05, for cases verses controls.
During the follow up period, TKA was deemed to be due to OA where OA was listed as the primary indication. Only cases where OA was listed as indication for TKA were included in the study, whereas cases with other indications for TKA (rheumatoid arthritis, trauma, etc.) were excluded. Of the respondents remaining in the study at year 19, 31 had received a TKA due to OA and one had received TKA due to RA. Individuals who underwent more than one TKA during the follow-up period were only counted once. Arthroplasty records were initially gathered via patient testimony at annual follow-up clinics and then validated by verification with each individual's General Practitioner records. Indications for arthroplasty were also verified during this validation process. The TKA cases were compared against a control group comprising those respondents remaining in the study at year 19 who did not have TJA (TKAs and/or THAs were excluded to create a more stringent control group, n = 676).
BMI (defined as weight/height2) was calculated at four time points (recalled BMI at age 20, measured BMI at baseline, year 5 and year 10). Individual BMI measurements were stratified into categories based on the World Health Organization (WHO) classification: underweight (BMI < 18.50), normal (BMI 18.50–24.99), overweight (BMI 25–29.99) and obese (BMI ≥ 30.00) at each time point. The changes in BMI between different time points t1 and t2 (defined as ΔBMIt1,t2 = BMI at t2 − BMI at t1, where t2 > t1) were also calculated. Approval for this study was granted by the Redbridge and Waltham Forest ethics committee and all patients were consenting volunteers who freely donated their time.
3. Statistical analyses
The baseline clinical characteristics of the respondents at year 19 (case and control groups) were compared against the non-respondents using the Mann–Whitney test, two tailed unpaired t-test with unequal variances and the Fisher's exact test for non-normal, normal and categorical data respectively (results discussed but not shown). The same was done when comparing TKA cases against the control group (shown in Table 1).
The correlation of BMI at different time points was assessed using the Spearman correlation matrix. This produced high values (> 80%) between pairs of measured BMI at baseline, year 5, year 10, suggesting that an overall model incorporating multiple time points would be unsuitable. Therefore the value of BMI as a predictor at each individual time point rather than collectively was reviewed.
Logistic regression, adjusted for age at baseline and smoking (“never” or “ever”) was performed using TKA by year 19 as the outcome and BMI (for each time point or ΔBMIt1,t2) as the exposure. Using 90% power at the 5% significance level, the detectable departure from the mean of baseline BMI was 0.65 kg/m2.
Stata SE v10, StatCorp, College Station, TX, USA and Matlab R2010a, The Mathworks, Natick, MA, USA was used for the analyses.
4. Results
The baseline clinical characteristics of case control respondents used in this study (n = 707) were compared to the non-respondents (n = 296). The respondents were younger than than the non-respondents on entry to the study (median age 53 versus 57 years, p < 0.0005), had a lower BMI (median BMI 24.7 versus 25.3 kg/m2, p = 0.026) and had less smokers in their group (43.4% versus 52.7%, p = 0.004). When compared against the controls, the women who underwent TKA (cases group) during the follow-up period had significantly elevated BMI measurements at baseline (median 26.5 versus 24.5 kg/m2, p = 0.005) and year 5 (median 27.4 versus 25.5 kg/m2, p = 0.022) as shown in Table 1. They did not however have an increased self reported BMI at age 20 compared to controls (median 21.6 versus 21.4 kg/m2, p = 0.251). There were no other significant associations.
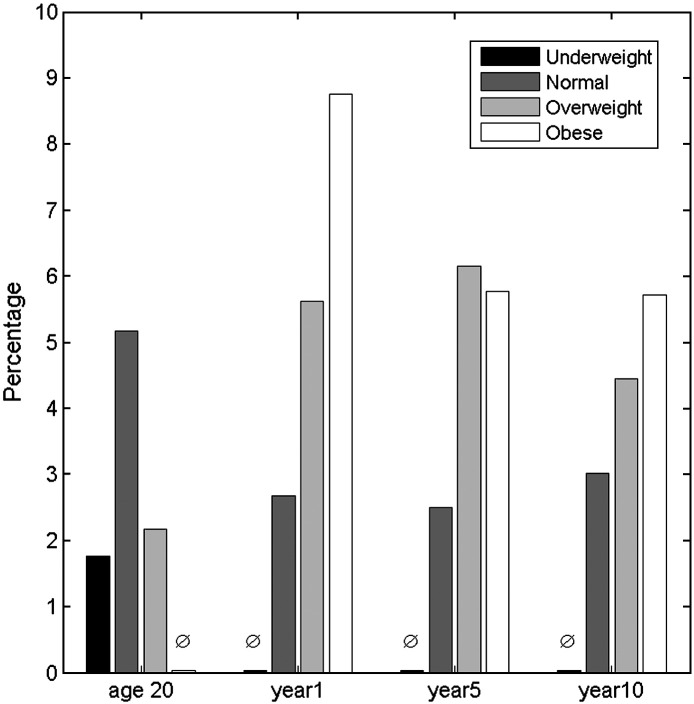
Individual BMI measurements were grouped into the WHO classifications. The risk (percentage of patients) of TKA was calculated for each WHO category at age 20, study baseline, year 5 and 10 (Fig. 1). There was no obvious relationship identified between estimated BMI at age 20 and TKA. There was an increase in risk of TKA with increased BMI at baseline, but this was attenuated at years 5 and 10.
Fig. 1.
Four time points stratified by the WHO categories for BMI, showing the percentage of patients within each group that had TKA by year 19 (based on case control respondents).
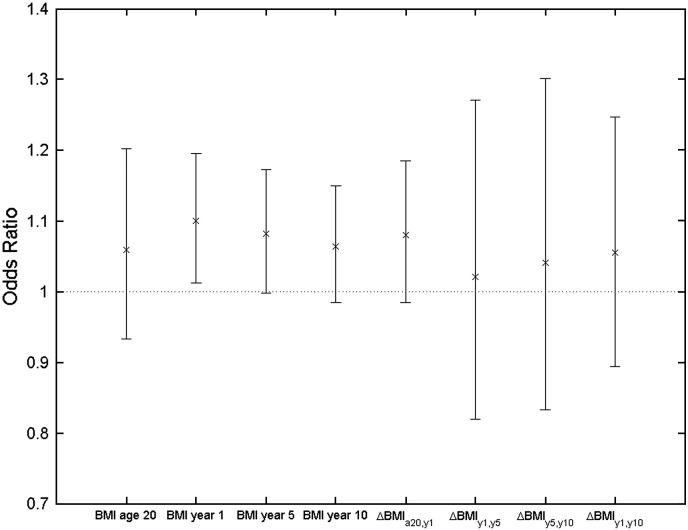
Logistic regression analyses using TKA as the outcome and BMI as the univariate exposure (BMI at age 20, baseline, year 5, year 10 and BMI changes ΔBMIa20,y1, ΔBMIy1,y5, ΔBMIy5,y10, ΔBMIy1,y10) revealed that the significant predictors of TKA were BMI at baseline with an OR(95% CI) of 1.105 (1.018, 1.198), p = 0.017 and BMI at year 5, 1.086 (1.003, 1.175), p = 0.042. When adjusted for baseline age and smoking, the BMI at baseline was the only significant predictor 1.100 (1.013, 1.195), p = 0.024. The association with BMI at year 5 was of borderline statistical significance 1.082 (0.998, 1.173), p = 0.056. No other significant values were found for predicting TKA, although all OR's were greater than 1, as shown in Fig. 2.
Fig. 2.
Odds ratios (95% CI) for case control respondents calculated using logistic regression with TKA (outcome) and BMI (exposure), adjusted for baseline age and smoking.
Repeating the regression analyses using BMI WHO categories (underweight, normal, overweight, obese) as the univariate exposure and adjusting for age and smoking revealed the categories at baseline to be the only significantly predictor of TKA, 1.867 (1.150, 3.030), p = 0.012.
5. Discussion
This longitudinal, population based study of women aged between 45 and 65 years of age at baseline demonstrated that both baseline and year 5 BMI were significantly associated with an elevated risk of TKA over the follow-up period. When adjusted for baseline age and smoking, the results showed a single unit increase in BMI at baseline was associated with a 10.0% rise in the risk of TKA by year 19. Change in BMI between these time points was a less useful predictor of TKA.
These results are consistent with several other large studies that have demonstrated the positive relationship between obesity and risk of radiographic knee OA. Spector et al. [12] initially used radiographic data from a small subgroup (n = 58) of this same population cohort and demonstrated that BMI had a strong effect on the risk of incidence and progression of radiographic OA. Again using this same population, Hart et al. [13] examined paired knee radiographs with a 4 year follow-up between imaging and found those individuals in the upper BMI tertile had an odds ratio of 2.38 for developing radiographic knee OA. These results have been further strengthened by several larger population-based cohort studies that demonstrate the positive relationship between BMI and incidence of radiographic knee OA [7,9,15]. Lohmander et al. [11] recently demonstrated the strong relationship between BMI and knee OA in a study similar to the present investigation using TKA as an outcome measure for severe disease.
This study demonstrates that BMI in middle age is the strongest predictor of the need for TKA with diminishing risks associated with BMI assessed at later time points. Change in BMI between various time points however, showed no significant association with the risk of TKA at year 19. The majority of previous studies have only examined BMI at one time point as an explanatory variable [5,10,16] and thus to our knowledge the present study, by comparing longitudinal changes in BMI to future OA outcome data, is unique. The results suggest that interventions targeting weight loss may have to be targeted prior to middle age, if the risk of severe knee OA and TKA is to be minimised.
This study has several strengths and potential limitations. This is a unique, long-term population based study with multiple measures of BMI and TJA in the same population. TJA is a surrogate measure of severe OA in the knee and hip, however it is a surgical end-point with good clinical relevance to both patient and practitioner. It should be noted that the UK has a taxpayer-funded public health system, and thus all individuals in the present study have more-or-less equal access to TJA with no personal expense. Despite the relative equity in TJA access, there are some disadvantages of using this as a severe OA measurement. Hawker et al. [17] have shown that patient willingness to consider TJA is the primary determinant in the time taken to the receipt of a first TJA. Willingness is in turn affected by level of patient education, meaning that individual's use of a tax payer-funded TJA service is probably still influenced by socioeconomic status.
Other limitations of the data include loss to follow up, the single sex of the cohort, the mono-ethnicity and the subjectively reported weight at age 20. Whilst there does not appear to be a systemic misreporting of data, we acknowledge that this measurement may be under reported.
In conclusion, we found an increasing risk of TKA due to severe OA with BMI at baseline and at year 5. Our data suggest that weight loss interventions for the prevention of severe knee OA may have to be targeted in early middle age for optimal effect.
Conflict of interest statement
All authors declare that they do not have any conflict of interest relating to this paper.
Acknowledgments
Acknowledgments are extended to the following for this piece of research: The 1003 Chingford women for participating; Maxine Daniels for assistance with data collection; the rest of the Chingford team; the NIHR Musculoskeletal Biomedical Research Unit, University of Oxford, and Arthritis Research UK for funding contributions.
References
- 1.Yelin E., Murphy L., Cisternas M.G., Foreman A.J., Pasta D.J., Helmick C.G. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56–5:1397–1407. doi: 10.1002/art.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2008;34–3:515–529. doi: 10.1016/j.rdc.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence R.C., Felson D.T., Helmick C.G., Arnold L.M., Choi H., Deyo R.A. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58–1:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lievense A.M., Koes B.W., Verhaar J.A., Bohnen A.M., Bierma-Zeinstra S.M. Prognosis of hip pain in general practice: a prospective followup study. Arthritis Rheum. 2007;57–8:1368–1374. doi: 10.1002/art.23094. [DOI] [PubMed] [Google Scholar]
- 5.Flugsrud G.B., Nordsletten L., Espehaug B., Havelin L.I., Meyer H.E. Risk factors for total hip replacement due to primary osteoarthritis: a cohort study in 50,034 persons. Arthritis Rheum. 2002;46–3:675–682. doi: 10.1002/art.10115. [DOI] [PubMed] [Google Scholar]
- 6.Felson D.T., Zhang Y., Anthony J.M., Naimark A., Anderson J.J. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116–7:535–539. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- 7.Reijman M., Pols H.A., Bergink A.P., Hazes J.M., Belo J.N., Lievense A.M. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66–2:158–162. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engstrom G., Gerhardsson de Verdier M., Rollof J., Nilsson P.M., Lohmander L.S. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Grotle M., Hagen K.B., Natvig B., Dahl F.A., Kvien T.K. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flugsrud G.B., Nordsletten L., Espehaug B., Havelin L.I., Engeland A., Meyer H.E. The impact of body mass index on later total hip arthroplasty for primary osteoarthritis: a cohort study in 1.2 million persons. Arthritis Rheum. 2006;54–3:802–807. doi: 10.1002/art.21659. [DOI] [PubMed] [Google Scholar]
- 11.Lohmander L.S., Gerhardsson M., Rollof J., Nilsson P.M., Engstrom G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass. A population-based prospective cohort study. Ann Rheum Dis. 2009;68:490–496. doi: 10.1136/ard.2008.089748. [DOI] [PubMed] [Google Scholar]
- 12.Spector T.D., Hart D.J., Doyle D.V. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population: the effect of obesity. Ann Rheum Dis. 1994;53–9:565–568. doi: 10.1136/ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart D.J., Doyle D.V., Spector T.D. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis Rheum. 1999;42–1:17–24. doi: 10.1002/1529-0131(199901)42:1<17::AID-ANR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Oliveria S.A., Felson D.T., Cirillo P.A., Reed J.I., Walker A.M. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999;10–2:161–166. [PubMed] [Google Scholar]
- 15.Felson D.T., Zhang Y., Hannan M.T., Naimark A., Weissman B., Aliabadi P. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40–4:728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 16.Karlson E.W., Mandl L.A., Aweh G.N., Sangha O., Liang M.H., Grodstein F. Total hip replacement due to osteoarthritis: the importance of age, obesity, and other modifiable risk factors. Am J Med. 2003;114–2:93–98. doi: 10.1016/s0002-9343(02)01447-x. [DOI] [PubMed] [Google Scholar]
- 17.Hawker G.A., Guan J., Croxford R., Coyte P.C., Glazier R.H., Harvey B.J. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54–10:3212–3220. doi: 10.1002/art.22146. [DOI] [PubMed] [Google Scholar]