Abstract
Rationale: The 2009 H1N1 flu appeared to cause more severe cold symptoms during the 2009–2010 flu season.
Objectives: We evaluated H1N1 infections during peak viral season in children with and without asthma to determine whether the H1N1 infectivity rate and illness severity were greater in subjects with asthma.
Methods: One hundred and eighty children, 4–12 years of age, provided eight consecutive weekly nasal mucus samples from September 5 through October 24, 2009, and scored cold and asthma symptoms daily. Viral diagnostics were performed for all nasal samples.
Measurements and Main Results: One hundred and sixty-one children (95 with asthma, 66 without asthma) completed at least 6 of the 8 nasal samples. The incidence of H1N1 infection was significantly higher in children with asthma (41%) than in children without asthma (24%; odds ratio, 4; 95% confidence interval, 1.8–9; P < 0.001), but rates of human rhinovirus infection (90% each) and other viral infections (47 vs. 41%) were similar. In children with asthma, there was a nonsignificant trend for increased loss of asthma control during H1N1 infections compared with human rhinovirus infections (38 vs. 21%; odds ratio, 2.6; 95% confidence interval, 0.9–7.2; P = 0.07).
Conclusions: During peak 2009 H1N1 flu season, children with asthma were infected almost twice as often with H1N1 compared with other respiratory viruses. H1N1 infection also caused increased severity of cold symptoms compared with other viral infections. Given the increased susceptibility of children with asthma to infection, these findings reinforce the need for yearly influenza vaccination to prevent infection, and raise new questions about the mechanism for enhanced susceptibility to influenza infection in asthma.
Keywords: asthma, viral infection, influenza
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Previous studies show that subjects with asthma have severe symptoms with H1N1 illness, with asthma being the highest comorbidity for hospitalization.
What This Study Adds to the Field
This prospective study shows that children with asthma have increased susceptibility for H1N1 infection. Understanding the mechanism for this enhanced susceptibility could lead to new approaches for the prevention and treatment of influenza in children with asthma.
2009 H1N1 Flu was first reported in the United States in the spring of 2009, and eventually affected 61 million people (1), one-third of them children. Morbidity was high, with 87,000 children requiring hospitalization. The most common comorbidity for patients hospitalized as a result of H1N1 infection was asthma (2), and the percentage of children with asthma hospitalized because of the 2009 H1N1 virus was two- to fivefold higher than previous reports of hospitalization due to seasonal influenza (3, 4). Despite the observation that subjects with asthma suffer H1N1 illness with increased severity, it remains unclear at what rate they are infected and whether their symptom severity is greater than those of their nonasthmatic counterparts. Previous studies of H1N1 infectivity reflect symptomatic infections with limited reporting of mild or asymptomatic infection (5–7).
A study by our group demonstrated that 90% of children with asthma are infected with human rhinoviruses (HRVs) during the month of September, and that the severity of clinical illness varies from no symptoms to severe wheezing illnesses (8). This study provided the foundation on which we designed a larger cohort to prospectively examine the relationship between illness symptoms and viral infectivity. Coincidentally, the H1N1 pandemic occurred during our study, peaking during the week of October 24–31 (9), and so an extension was designed to capture further data on H1N1 infection and illness in the fall of 2009. We hypothesized that children with asthma were infected with H1N1 influenza at the same rate as children without asthma but experienced more severe symptoms when infected. To test this hypothesis, we prospectively obtained weekly specimens of nasal secretions from children with and without asthma during the fall of 2009 to determine whether asthma was associated with more frequent or severe H1N1 infections, and to compare the spectrum caused by H1N1 with that of other respiratory viruses.
Methods
Study Subjects and Design
Children included in this analysis were enrolled in a larger study to determine asthma-specific rates of HRV infections and illness (“RhinoGen”). Of the 193 children participating in RhinoGen in September 2009, 180 children (age, 4–12 yr) agreed to extend participation during the H1N1 pandemic through the end of October 2009. Because admission to RhinoGen was on a rolling basis between 2007 and 2010, we aimed to enroll all subjects participating in the Fall 2009 arm of RhinoGen; thus a sample size was not calculated. From September 5 to October 24, 2009, nasal samples were collected weekly for a total of 8 weeks, concluding on H1N1 vaccine release in Wisconsin, to avoid confounding with immunological effects of the virus and false positive test results from the live virus vaccine. Children, with the help of their parents, were instructed to record upper respiratory infection and asthma symptoms, morning peak expiratory flow, and albuterol use on daily diary cards between August 29, 2009 and October 31, 2009. See the online supplement for additional details about recruitment and inclusion criteria. This study was approved by the University of Wisconsin Human Subjects Committee, and written informed consent was obtained from the parents.
Procedures and Definitions
At the first study visit, subjects were taught to collect samples of their own nasal mucus, using a nose-blowing technique as previously described (10, 11). These samples were analyzed for common respiratory viruses by multiplex polymerase chain reaction (respiratory MultiCode assay; EraGen Biosciences, Madison, WI) (12) and by seminested polymerase chain reaction specific for the hemagglutinin gene of the 2009 H1N1 influenza virus (see the online supplement for details). Partial genetic sequencing was used to identify HRV types (13).
Children scored cold and asthma symptom severity based on a four-point scoring system (see Table E1 in the online supplement) (8). Asthma control was defined according to National Heart, Lung, and Blood Institute (Bethesda, MD) guidelines (14), with criteria for loss of asthma control consisting of at least moderate asthma symptoms and either a decrease in peak expiratory flow of at least 20% or use of albuterol for at least 2 days/week. Current asthma was diagnosed at study completion on the basis of previously reported criteria (15).
Total and allergen-specific IgE levels in plasma (15) were determined on enrollment by skin prick testing and fluoroenzyme immunoassays (UniCAP 100; Phadia, Uppsala, Sweden). Peripheral blood mononuclear cell cytokine responses (IFN-α, IFN-γ, tumor necrosis factor-α, and IL-10) to respiratory syncytial virus (RSV) and HRV were measured as previously described, using blood collected at study enrollment (16).
Children who collected six of eight weekly nasal specimens and were missing less than 20% of diary card data were included in the analysis. Each week was designated as virus positive or negative on the basis of viral detection. If two or more consecutive days of cold or asthma symptoms occurred within 3 days of a positive viral sample, the infection was designated an illness (8). If the same virus was detected in multiple weeks, it was considered a single infection.
Statistical Analysis
Age, race, sex, and sensitization were compared by asthma status, using the two-sample t test and chi-square test for association. Viral infection rates were compared by asthma status, using logistic regression models, both univariate and adjusting for sex, race, and sensitization. Total symptom burden for each illness was calculated as the area under the curve for symptom scores recorded in daily diaries. Generalized linear mixed-effect models with a random effect for subject were used to compare symptom burdens and occurrences of loss of asthma control among viral illnesses, and to assess whether infection with a given virus increased or decreased susceptibility to another virus 1 week later. The Wilcoxon rank-sum test was used to detect differences in cytokine response profiles. A two-sided P value less than 0.05 was regarded as statistically significant.
Results
Subject Characteristics
Of the 180 children enrolled, 16 subjects failed to complete at least 51 days (80%) of symptom diaries and 3 subjects did not submit at least six (75%) of the eight scheduled nasal samples; thus 161 (89.4%) were included in the final analysis (see Figure E1).
Of the 161 subjects enrolled, 95 (59%) had current asthma. In comparing the groups, there were no significant differences in age or sex, but the asthma group had a higher rate of allergic sensitization and contained fewer white individuals (Table 1).
TABLE 1.
STUDY SUBJECTS
| Asthma (n = 95) | No Asthma (n = 66) | P Value | |
| Age, yr | 8.9 ± 2.5 | 9.4 ± 2.1 | 0.14 |
| Sex | 63% male | 55% male | 0.27 |
| Race | 68% white | 92% white | <0.001 |
| Allergen-specific IgE | 73% | 48% | <0.001 |
Identification of Respiratory Viruses
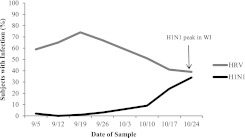
Overall, 346 infections were detected in the 161 subjects who completed the study. Of these infections, the majority were HRV infections (62%). The remaining viruses detected consisted of enterovirus (12%), H1N1 (10%), adenovirus (2%), and multiple virus infection (13%). Other viruses (influenza B, bocavirus, and parainfluenza type 1) were detected at a frequency of less than 1%. When multiple viruses were detected in a single sample, HRV (80%) and H1N1 (51%) were most common. The prevalence of H1N1 infections was low at the beginning of the monitoring period, and was highest in mid- to late October (Figure 1). In contrast, HRV infections were most prevalent in early to mid-September, and were less frequent in October. It has been suggested that HRV infections may reduce the likelihood of H1N1 infection (17, 18). In our study, HRV infection did not reduce the risk of H1N1 infection the following week (odds ratio [OR], 0.68; 95% confidence interval [CI], 0.37–1.2; P = 0.21). In contrast, H1N1 infection reduced the risk of HRV infection the following week (OR, 0.19; 95% CI, 0.07–0.54; P = 0.02).
Figure 1.
Human rhinovirus (HRV) and 2009 H1N1 influenza (H1N1) weekly infection rate (Fall 2009). WI = Wisconsin.
Increased H1N1 Infection Rate in Children with Asthma
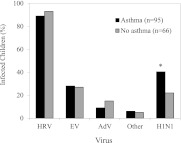
Overall, 34% of children were infected with H1N1 during the 8-week study. Infection rates were significantly higher in children with asthma (Figure 2); 41% of children with asthma were infected with H1N1 whereas only 24% of children without asthma were infected (OR, 2.2; 95% CI, 1.1–4.4; P = 0.03). This analysis was also performed on the basis of asthma diagnosis at study entry, with similar results (OR, 2.4; 95% CI, 1.1–4.9; P = 0.02). Multivariate analysis adjusting for sex, race, and allergic sensitization demonstrated an increased risk for infection in children with asthma (OR, 4.0; 95% CI, 1.8–9.0; P < 0.001), and in white children (OR, 2.8; 95% CI, 1.1–7.1; P = 0.03) (Table 2). Use of inhaled corticosteroid medications and body mass index were similar in those infected with H1N1 and those not infected (data not shown). Asthma did not affect rates of infection with HRV (90% each group), enterovirus (30 vs. 24%), adenovirus (11 vs. 12%), or other viruses (6 vs. 5%) (Figure 2).
Figure 2.
Infection rates according to asthma status. The infection rates for specific respiratory viruses during the 8-week monitoring period were compared for children with versus without asthma. AdV = adenoviruses; EV = enteroviruses; H1N1 = 2009 H1N1 influenza; HRV = human rhinoviruses. *P < 0.001 versus subjects without asthma when adjusted for race, sex, and allergic sensitization.
TABLE 2.
H1N1 INFECTION RATES
| Univariate |
Multivariate |
||||||
| H1N1 Infection (%) | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| No asthma | 24 | 1.0 | — | — | 1.0 | — | — |
| Asthma | 41 | 2.2 | 1.1, 4.4 | 0.03 | 4.0 | 1.8, 9.0 | 0.0008 |
| Nonsensitized | 33 | 1.0 | — | — | 1.0 | — | — |
| Sensitized | 34 | 1.0 | 0.52, 2.1 | 0.90 | 0.78 | 0.36, 1.7 | 0.51 |
| Female | 35 | 1.0 | — | — | 1.0 | — | — |
| Male | 33 | 0.91 | 0.47, 1.8 | 0.79 | 1.0 | 0.51, 2.2 | 0.90 |
| Nonwhite | 23 | 1.0 | — | — | 1.0 | — | — |
| White | 37 | 2.0 | 0.84, 4.8 | 0.11 | 2.8 | 1.1, 7.1 | 0.03 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Viral Effects on Illness Severity
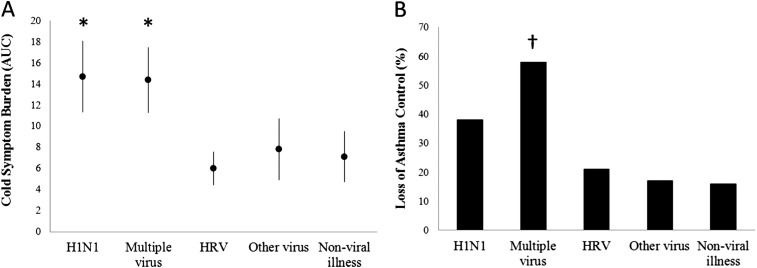
The effects of viral etiology on cold symptoms for all subjects were compared, assessing areas under the curve for symptom scores for each illness. Cold symptoms were significantly worse (P < 0.005) with H1N1 infection (mean, 14.7; 95% CI, 11.3–18.1) or mixed viruses (mean, 14.4; 95% CI, 11.3–17.5) compared with illnesses caused by HRV (mean, 6.0; 95% CI, 4.4–7.6), other viruses (mean, 7.8; 95% CI, 4.9–10.7; P < 0.005), or no virus (mean, 7.1; 95% CI, 4.7–9.5) (Figure 3A). When a subject was infected with more than one virus during one illness, the cold symptom severity was similar to that caused by H1N1 alone (mean, 14.69; 95% CI, 11.31–18.07; vs. mean, 14.38; 95% CI, 11.3–17.46; P = 0.89). Interestingly, asthma was not associated with increased cold symptom severity for any category of viral infection.
Figure 3.
Effects of viral etiology on (A) cold symptoms and (B) asthma control, based on episodes of infection. AUC = area under the curve; H1N1 = 2009 H1N1 influenza; HRV = human rhinoviruses. *P < 0.005 versus HRV, other virus, and nonviral illness; †P < 0.005 versus other virus and P < 0.001 versus HRV and nonviral illness.
Viral Effects on Loss of Asthma Control
We next tested whether infection with H1N1 was more likely to cause loss of asthma control compared with HRV infection. Rates of loss of asthma control per infection were as follows: H1N1, 38% (9 of 24); HRV, 21% (27 of 127); and the combination of HRV and H1N1, 44% (4 of 9) (Figure 3B). There was a nonsignificant trend for increased loss of asthma control with H1N1 infection compared with HRV infection (OR, 2.6; 95% CI, 0.9–7.2; P = 0.07). Infection with multiple viruses was more likely to be associated with loss of asthma control compared with infection with HRV alone (OR, 6.7; 95% CI, 2.5–17; P < 0.001). Infection with a combination of H1N1 plus HRV was associated with loss of asthma control more often than solitary HRV infection (OR, 4.6; 95% CI, 1–20.8; P = 0.05).
We next used a generalized linear mixed-effect model to determine how many episodes of loss of asthma control were attributable to H1N1, either alone or in combination with another virus. Overall, H1N1 infection contributed to 23% of all episodes of loss of asthma control.
Cytokine Responses and H1N1 Infection
We next tested whether individual differences in virus-induced peripheral blood mononuclear cell cytokine responses were related to H1N1 infection rates. There was no difference in HRV- or RSV-induced IFN-α, IFN-γ, tumor necrosis factor-α, or IL-10 observed between the infected and noninfected children (data not shown).
Discussion
Individuals with asthma experienced significant morbidity during the 2009 H1N1 outbreak (2). Although this has been shown in multiple retrospective studies, we had the unique opportunity to prospectively examine infectivity rates in an H1N1-naive population and demonstrated that children with asthma had an increased susceptibility for H1N1 infection. Although increased infectivity among children with asthma is an unexpected finding, it may explain why children with asthma fared worse during the H1N1 pandemic; not only were they experiencing increased symptoms (compared with other viral illness) once infected, they were being infected at a greater rate than their nonasthmatic counterparts. Given the increased susceptibility to H1N1 infection and greater hospitalization rates for those with asthma, these findings raise new questions about the mechanism for enhanced susceptibility to infection with influenza (and not other respiratory viruses) in asthma. In addition, we found that H1N1 accounted for 23% of episodes of loss of asthma control during the peak fall viral season, suggesting that administration of the H1N1 vaccine may have prevented a significant number of asthma exacerbations.
Compared with other viruses, H1N1 caused significantly more severe illness, and there was also a nonsignificant trend between H1N1 infection and increased loss of asthma control. Numerically, most episodes of loss of asthma control were associated with HRV infection, which was much more common than H1N1 infection. However, the proportion of infections leading to loss of asthma control was almost twice as high with H1N1 compared with HRV.
Studies suggest that HRV infection may protect an individual from H1N1 infectivity (17, 18). Overall, we observed that HRV infections peaked earlier than H1N1 infections, and HRV infections were diminishing as H1N1 infections were on the rise. However, in contrast to these previous studies, the time trend analysis was not consistent with a protective effect with HRV, but rather found those who were infected with HRV were just as likely to become infected with H1N1 the following week.
Only influenza infections were increased in asthma, suggesting that the mechanism may be related to specific infective properties of the influenza virus or associated receptors on airway epithelial cells. Previous studies have shown that influenza uses sialic acid on epithelial cells to cleave hemagglutinin and infect the cells (19). Perhaps individuals with asthma have increased protease activation leading to increased susceptibility to infection. Alternatively, several studies have provided evidence that asthma is associated with impaired innate antiviral responses (20, 21). In our study, peripheral blood mononuclear cell innate responses to HRV or RSV between children with and without asthma were similar, suggesting that increased H1N1 infectivity in individuals with asthma is not the result of a defect in innate immunity. It is possible that bronchial epithelial cells have impaired responses to influenza, and additional studies are needed to test this hypothesis.
This study has a number of unique advantages, and some limitations that should be considered in interpreting these data. The prospective study design allowed us to monitor children for rates of both viral infection and associated cold and asthma symptoms, and a wide range of viruses was tested using sensitive polymerase chain reaction–based diagnostics during the peak of the H1N1 pandemic in Wisconsin (9). Furthermore, this was a unique opportunity to observe how the H1N1 pandemic affected a naive population of children. In most years, many patients have partial protection from seasonal influenza because of prior vaccines or infections with related strains. In contrast, children and young adults were especially susceptible to 2009 H1N1 given their lack of prior exposure and the unusually early onset of the influenza season before the H1N1 vaccine was available. In the main RhinoGen study, we monitored subject asthma symptoms and treatment for 1 year, allowing us to confirm the entry diagnosis of asthma. Although our main analysis was run on asthma diagnosis at study completion, the analysis of H1N1 infection rates was similar when using asthma diagnosis at study entry. In fact, only four subjects had a change in asthma diagnosis; three moved to the asthma group and one moved to the nonasthmatic group. One limitation of our study is that children were enrolled after the onset of H1N1 infections in Wisconsin the previous April, although screening in our community indicated that the number of cases was low until the fall season (9, 22). Also, we used a simple scoring system to encourage daily score reporting, and as a result more than 90% of the children turned in satisfactory symptom diaries. The scoring system has proven to be informative in previous studies (8, 23), but has not been validated. Other common cold instruments are available (e.g., Jackson score, WURSS-21), but none have been formally validated in children.
In summary, our findings indicate that children with asthma were more likely to contract infections with 2009 H1N1, but not other respiratory viruses. These findings support recommendations for influenza vaccination in children with asthma and, in addition, suggest that additional studies are warranted to identify mechanisms for this effect. Understanding why children with asthma are uniquely more susceptible to influenza infection could lead to new strategies for prevention and treatment.
Supplementary Material
Acknowledgments
The authors greatly appreciate the efforts put forth by the clinical coordinators in patient recruitment and retention, and the procurement of all the biological specimens used in these analyses. The authors also acknowledge the cooperation of many health care professionals within our surrounding communities and the enthusiastic participation of the RhinoGen families.
Footnotes
Supported by RhinoGen (U19 AI070503-01); COAST (P01 HL070831); clinical research unit: grant 1 (UL1RR025011) from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health; and T32AI007635 (training grant).
Author Contributions: Concept and design—J.E.G., R.F.L., K.A.R.; analysis and interpretation—K.M.K., W.M.L., G.L., M.D.E., R.E.G., R.F.V., J.P.O., J.E.G.; drafting of manuscript for important intellectual content—K.M.K., J.E.G.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201109-1635OC on February 23, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention Seasonal influenza vaccination coverage among children aged 6 months-18 years—eight immunization information system sentinel sites, United States, 2009–10 influenza season. MMWR Morb Mortal Wkly Rep 2010;59:1266–1269 [PubMed] [Google Scholar]
- 2.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 2009;361:1935–1944 [DOI] [PubMed] [Google Scholar]
- 3.Bloom B, Cohen RA, Freeman G. Summary health statistics for US children: National Health Interview Survey, 2008. Vital Health Stat 10 2009;1–81 [PubMed] [Google Scholar]
- 4.Miller EK, Griffin MR, Edwards KM, Weinberg GA, Szilagyi PG, Staat MA, Iwane MK, Zhu Y, Hall CB, Fairbrother G, et al. Influenza burden for children with asthma. Pediatrics 2008;121:1–8 [DOI] [PubMed] [Google Scholar]
- 5.Desmoulins C, Michard-Lenoir AP, Naud J, Claudet I, Nouyrigat V, Chéron G. Clinical features and outcome of 2009 H1N1 influenza in the pediatric setting: multicenter prospective study in the ED [in French]. Arch Pediatr (In press) [DOI] [PubMed] [Google Scholar]
- 6.Mahut B, Refabert L, Marchac V, Iniguez JL, Aubertin G, Tamalet A, Lebras MN, Troadec C, Chatellier G, Delclaux C. Influenza-like illness responsible for severe exacerbations in asthmatic children during H1N1 pandemic: a survey before vaccination. J Asthma 2011;48:224–227 [DOI] [PubMed] [Google Scholar]
- 7.Dawood FS, Kamimoto L, D'Mello TA, Reingold A, Gershman K, Meek J, Arnold KE, Farley M, Ryan P, Lynfield R, et al. Children with asthma hospitalized with seasonal or pandemic influenza, 2003–2009. Pediatrics 2011;128:e27–e32 [DOI] [PubMed] [Google Scholar]
- 8.Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, Evans MD, Bork J, Roberg K, Lemanske RF Jr, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol 2010;125:1001–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisconsin Department of Health Services, Division of Public Health, and Bureau of Communicable Diseases and Emergency Response. Wisconsin's pandemic flu resource [updated 2001 Jan 24; accessed 2012 Feb 29]. Available from: http://pandemic.wisconsin.gov/
- 10.Hayden FG, Herrington DT, Coats TL, Kim K, Cooper EC, Villano SA, Liu S, Hudson S, Pevear DC, Collett M, et al. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis 2003;36:1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell KR, Shorr R, Cherry JD, Hendley JO. Improved method for collection of nasal mucus. J Infect Dis 1977;136:109–111 [DOI] [PubMed] [Google Scholar]
- 12.Lee WM, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, Shult PA, Prudent JR, Gern JE. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol 2007;45:2626–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, Jakiela B, Lemanske RF, Shult PA, Gern JE. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE 2007;2:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol 2007;120:S94–S138 [DOI] [PubMed] [Google Scholar]
- 15.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008;178:667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copenhaver CC, Gern JE, Li Z, Shult PA, Rosenthal LA, Mikus LD, Kirk CJ, Roberg KA, Anderson EL, Tisler CJ, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med 2004;170:175–180 [DOI] [PubMed] [Google Scholar]
- 17.Casalegno JS, Ottmann M, Duchamp MB, Escuret V, Billaud G, Frobert E, Morfin F, Lina B. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin Microbiol Infect 2010;16:326–329 [DOI] [PubMed] [Google Scholar]
- 18.Greer RM, McErlean P, Arden KE, Faux CE, Nitsche A, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J Clin Virol 2009;45:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pappas C, Aguilar PV, Basler CF, Solorzano A, Zeng H, Perrone LA, Palese P, Garcia-Sastre A, Katz JM, Tumpey TM. Single gene reassortants identify a critical role for PB1, HA, and NA in the high virulence of the 1918 pandemic influenza virus. Proc Natl Acad Sci USA 2008;105:3064–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks GD, Buchta KA, Swenson CA, Gern JE, Busse WW. Rhinovirus-induced interferon-gamma and airway responsiveness in asthma. Am J Respir Crit Care Med 2003;168:1091–1094 [DOI] [PubMed] [Google Scholar]
- 21.Gern JE, Brooks GD, Meyer P, Chang A, Shen K, Evans MD, Tisler C, Dasilva D, Roberg KA, Mikus LD, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol 2006;117:72–78 [DOI] [PubMed] [Google Scholar]
- 22.Belongia EA, Irving SA, Waring SC, Coleman LA, Meece JK, Vandermause M, Lindstrom S, Kempf D, Shay DK. Clinical characteristics and 30-day outcomes for influenza A 2009 (H1N1), 2008–2009 (H1N1), and 2007–2008 (H3N2) infections. JAMA 2010;304:1091–1098 [DOI] [PubMed] [Google Scholar]
- 23.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O'Toole S, Myint SH, Tyrrell DA, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995;310:1225–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.