Abstract
Rationale: Cognitive and psychiatric morbidity is common and potentially modifiable after acute lung injury (ALI). However, practical measures of neuropsychological function for use in multicenter trials are lacking.
Objectives: To determine whether a validated telephone-based neuropsychological test battery is feasible in a multicenter trial. To determine the frequency and risk factors for long-term neuropsychological impairment.
Methods: As an adjunct study to the Acute Respiratory Distress Syndrome Clinical Trials Network Fluid and Catheter Treatment Trial, we assessed neuropsychological function at 2 and 12 months post–hospital discharge.
Measurements and Main Results: Of 406 eligible survivors, we approached 261 to participate and 213 consented. We tested 122 subjects at least once, including 102 subjects at 12 months. Memory, verbal fluency, and executive function were impaired in 13% (12 of 92), 16% (15 of 96), and 49% (37 of 76) of long-term survivors. Long-term cognitive impairment was present in 41 of the 75 (55%) survivors who completed cognitive testing. Depression, post-traumatic stress disorder, or anxiety was present in 36% (37 of 102), 39% (40 of 102), and 62% (63 of 102) of long-term survivors. Enrollment in a conservative fluid-management strategy (P = 0.005) was associated with cognitive impairment and lower partial pressure of arterial oxygen during the trial was associated with cognitive (P = 0.02) and psychiatric impairment (P = 0.02).
Conclusions: Neuropsychological function can be assessed by telephone in a multicenter trial. Long-term neuropsychological impairment is common in survivors of ALI. Hypoxemia is a risk factor for long-term neuropsychological impairment. Fluid management strategy is a potential risk factor for long-term cognitive impairment; however, given the select population studied and an unclear mechanism, this finding requires confirmation.
Keywords: acute respiratory distress syndrome, acute lung injury, cognitive function, critical illness
At a Glance Commentary
Scientific Knowledge on the Subject
Cognitive and psychiatric morbidities are prevalent, long-lasting, and potentially modifiable in survivors of critical illness. Whether neuropsychological function can be assessed in a multicenter trial is unknown. Furthermore, the frequency and risk factors for long-term neuropsychological impairment in survivors of acute lung injury have not been studied in a multicenter trial.
What This Study Adds to the Field
We found that neuropsychological function in survivors from a multicenter trial could be assessed using a validated telephone battery. Our findings show that most survivors of acute lung injury experienced long-term cognitive and psychiatric morbidity, and we detected that hypoxemia is a risk factor for the development of long-term cognitive and psychiatric impairment.
In the United States, approximately 200,000 patients develop acute lung injury (ALI) each year (1). Advances in ALI management have decreased mortality to 25–40% (1–4), resulting in an expanding population of survivors who have been ravaged by their acute illness. Survivorship, it has been put forth, will be the defining challenge of modern day critical care (5). Beyond the physical ailments that survivors endure, neuropsychological impairment is increasingly recognized as a prevalent, important, and potentially modifiable outcome among survivors of ALI (6–21) and critical illness in general (22–29).
Traditionally, neuropsychological (cognitive and psychiatric) function is assessed in-person by an expert, a constraint that has limited the ability to study the frequency and determinants of neuropsychological impairment in critically ill populations and to measure the effects of an intervention on long-term neuropsychological function. Neuropsychological function has not been studied in survivors from a multicenter trial. Consequently, the generalizability of observations from single-center cohort studies is unknown. Furthermore, evidence regarding the etiology of neuropsychological impairment in survivors of ALI is extremely limited.
As a practical approach to assess the neuropsychological function of survivors from a multicenter trial, we developed a telephone battery of standardized neuropsychological tests which could be administered by a nonexpert (10). We then validated the telephone battery against standard in-person assessments (10, 30) and in survivors of ALI specifically (10, 11). Here, we administered the battery to a subset of survivors from the Acute Respiratory Distress Syndrome Clinical Trials Network (ARDSNet) Fluid and Catheter Treatment Trial (FACTT) (4, 31). FACTT tested the hypothesis that a conservative fluid-management strategy targeted to lower intravascular pressures as measured by either a pulmonary artery catheter (PAC) or central venous catheter improves outcomes in ALI. Our goals were to determine whether a telephone-based neuropsychological test battery was feasible in a multicenter trial, to determine the frequency of long-term neuropsychological morbidity in survivors from a multicenter trial, and to identify potential risk factors for the development of long-term cognitive and psychiatric impairment. Results of this study were previously reported in abstract form (32).
Methods
An expanded Methods section is available in the online supplement. The study was approved by the institutional review board of participating hospitals and as a substudy of FACTT by the ARDSNet.
Study Design
The ARDS Cognitive Outcomes Study (ACOS) is a prospective, multicenter cohort study of a subset of survivors from FACTT. FACTT enrolled patients between June 2000 and October 2005. Between July 2002 and July 2003, FACTT was halted and new regulatory approval related to the study was prohibited. The regulatory process for ACOS began before the halt and testing was conducted between March 2003 and September 2006 in concert with the Economic Analysis of the Pulmonary-Artery Catheter (EA-PAC) study, a long-term follow-up study of FACTT (33).
To be eligible for ACOS, subjects had to be enrolled in FACTT and EA-PAC (33) and ACOS-specific regulatory approval had to be in place. FACTT enrolled mechanically ventilated adult patients with ALI (34). Subjects not consented before discharge for ACOS were recruited by the telephone and through written communication when telephone contact failed.
Neuropsychological Function and Quality of Life
We used a validated telephone battery of standardized neuropsychological tests (10, 11, 30). The telephone battery was administered from the University of Pittsburgh to consenting, English-speaking subjects at 2 and 12 months post–hospital discharge. Subjects were not required to undergo 2 month testing to be tested at 12 months.
The instruments used to assess cognition, anxiety, depression, and post-traumatic stress disorder (PTSD) symptoms are presented in Table E1 in the online supplement (35–42). Quality of life (43) was assessed at 12 months as part of EA-PAC (33). We focused our study on survivors tested at 12 months to assess long-term neuropsychological function.
Data Analysis
The cognitive battery yielded scaled scores for each domain that were normalized to allow for comparisons across tests and subjects (44, 45). We report the median and interquartile range of results as percentiles. We defined impairment in a single domain as a score greater than 2 SD below the population normative data (6, 7, 9, 44–46). Cognitive impairment at the subject level was defined as impairment in memory, verbal fluency, or executive function in subjects who completed tests in each of these domains (6, 9–11, 27).
We a priori hypothesized that long-term cognitive impairment would be associated with duration of mechanical ventilation, either conservative or liberal fluid-management strategy (4), hypotension (9), hypoxemia (6), PAC use (19, 47, 48), sepsis as the cause of ALI (49, 50), and severity of illness (26). The prespecified potential confounding variables (6–12, 51–53) are detailed in the online supplement.
Psychiatric impairment was defined as impairment in any of the three psychiatric measures (anxiety, depression, and PTSD) in subjects completing each measure. We tested the following candidate risk factors for the development of long-term psychiatric morbidity: age (18, 27, 28); sex (18, 27–29); race and ethnicity; level of education (19, 27); Acute Physiologic and Chronic Health Evaluation III scores; trial interventions (fluid-management strategy, PAC); hypotension; hypoxemia (18, 27); any episode of hypoglycemia (glucose <60 mg/dl) during the hospitalization (20, 27); corticosteroid administration (54); ICU length of stay (17, 21, 27); and duration of mechanical ventilation (17, 18, 27).
Statistical Analysis
Multivariable logistic regression was used to investigate the relationship between candidate risk factors and long-term neuropsychological (cognitive or psychiatric) impairment. We adjusted for each candidate risk factor and potential covariates with an α level of significance less than 0.20 in univariate analyses (55). To avoid over-fitting, adjustment was performed one covariate at a time (56). A P value of less than or equal to 0.05 was used to signify statistical significance.
The cognitive impairment risk factor analysis was limited to 75 survivors who completed memory, verbal fluency, and executive function testing at 12 months. The Hayling Sentence Completion Test (HSCT) assesses executive function with an error score and response latency component to produce an overall score (35, 36). The HSCT response times were not measured before February 2005 because of an error in the administration of the timing of the test. The timing error resulted in 21 survivors tested at 12 months having incomplete testing. Secondary analyses were performed in 90 survivors who completed assessments in memory, verbal fluency, and the error score of the HSCT at 12 months.
We tested for associations between identified risk factors and memory, verbal fluency, and executive function scores as continuous variables using Spearman rank-correlation coefficients. Finally, we performed sensitivity analyses to determine the effects of missing data on the observed associations. Statistical analyses were performed using Stata 10.0 software (Stata Datacorp, College Station, TX).
Results
Enrollment
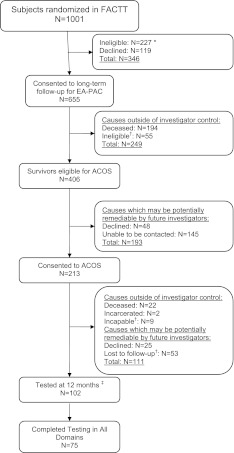
Of 1,001 patients randomized in FACTT, 406 survivors were eligible for ACOS (Figure 1) (see Figure E1 for enrollment by fluid-management strategy). Of 406 eligible survivors, we approached 261 to participate and 213 consented. Consent rates did not differ by age, sex, race and ethnicity, or geographic region after adjusting for mortality and excluding ineligible subjects in whom the time window to be tested had elapsed (see Table E2). Of 213 consenting subjects, 14 died before initial testing, and we were able to contact and test 122 of 199 alive and consenting subjects (61%) at least once. The 122 subjects were drawn from 28 hospitals and constitute the ACOS cohort.
Figure 1.
Enrollment and outcomes. ACOS = Adult Respiratory Distress Syndrome Cognitive Outcomes Study. *Of 1,001 Fluid and Catheter Treatment Trial (FACTT) subjects who underwent randomization, 227 were ineligible for long-term follow-up as part of Economic Analysis of the Pulmonary-Artery Catheter (EA-PAC) because no regulatory approval was in place and 119 declined consent (33). †Subjects were categorized as ineligible if the time window to be tested had elapsed because of the regulatory halt, as incapable if self-determined or determined by a surrogate to be physically or mentally incapable of telephone-based neuropsychological testing, and as lost to follow-up if consent was obtained but the subject was not tested. Of 53 subjects categorized as lost to follow-up, no contact information was available for 1 subject, no telephone service was available for 1 subject, no explanation was provided for 1 subject, and the remaining 50 subjects were recorded as “lost to follow-up.” ‡Of 52 subjects tested at 2 months, 32 were retested at 12 months. The reasons for 20 subjects being tested at 2 months but not at 12 months were that 11 patients were lost to follow-up, 5 declined, 3 died, and 1 was incarcerated. Subjects were not required to undergo testing at 2 months to be tested at 12 months.
Compared with non-ACOS FACTT participants who survived 60 days (n = 609), the ACOS group was more likely to be female, more likely to be non-Hispanic whites, less likely to have HIV-AIDS, and less likely to have pneumonia as the cause of ALI (Table 1). The number of ACOS subjects enrolled from each hospital ranged from 1 to 17 (median = 3; interquartile range [IQR], 1–5).
TABLE 1.
COMPARISON OF BASELINE CHARACTERISTICS BETWEEN FACTT 60-DAY SURVIVORS NOT ENROLLED IN ACOS AND ACOS PARTICIPANTS
| Variable | FACTT Survivors Not Enrolled in ACOS (n = 609) | ACOS Cohort (n = 122) | P Value |
| Age, yr | 47 (37–57) | 49 (40–58) | 0.23 |
| Male sex, % | 54 | 43 | 0.04 |
| Race or ethnic group, % | <0.001 | ||
| White non-Hispanic | 64 | 86 | |
| Black non-Hispanic | 21 | 11 | |
| Hispanic | 7 | 3 | |
| Other | 8 | 0 | |
| Primary lung injury, % | 0.005 | ||
| Pneumonia | 49 | 36 | |
| Sepsis | 20 | 25 | |
| Aspiration | 16 | 16 | |
| Trauma | 9 | 8 | |
| Multiple transfusions | 1 | 2 | |
| Other | 5 | 13 | |
| Coexisting conditions, % | |||
| None | 71 | 75 | 0.39 |
| Diabetes | 16 | 18 | 0.65 |
| HIV infection or AIDS | 7 | 0 | 0.001 |
| Cirrhosis | 3 | 2 | 0.78 |
| Solid tumors | 1 | 1 | 1.00 |
| Leukemia | 2 | 1 | 1.00 |
| Lymphoma | 0 | 2 | 0.13 |
| Immunosuppression | 6 | 7 | 0.84 |
| APACHE III score, mean (SD) | 85 (66–105) | 85 (63–102) | 0.32 |
| Medical intensive care unit | 65 | 56 | 0.06 |
| Glasgow Coma Scale | 8 (6–12) | 8 (4–11) | 0.38 |
| Mean arterial pressure, mm Hg | 76 (68–87) | 77 (67–85) | 0.49 |
| Vasopressor use, % | 34 | 33 | 0.81 |
| PaO2:FiO2 | 122 (83–168) | 122 (86–165) | 0.73 |
| Conservative strategy, % | 51 | 55 | 0.38 |
| Pulmonary artery catheter, % | 51 | 52 | 0.70 |
Definition of abbreviations: ACOS = Adult Respiratory Distress Syndrome Cognitive Outcomes Study; APACHE III = Acute Physiologic and Chronic Health Evaluation III scores; FACTT = Fluid and Catheter Treatment Trial.
Values expressed as a percentage or median (interquartile range).
By 12 months, 22 consenting subjects were deceased, 2 were incarcerated, and 9 were deemed physically or mentally incapable of telephone-based neuropsychological testing. Of the remaining 180 subjects who were alive and able to be tested, 102 (57%) were tested at 12 months. These 102 long-term survivors completed individual cognitive domain testing to various degrees (see Table E1), and 74% (75 of 102) completed testing in all cognitive domains. Long-term survivors were tested on average 12 months post–hospital discharge (IQR, 11–13 mo). None of these long-term survivors had a history of preexisting dementia.
Neuropsychological Test Battery Administration
The test battery required 45–60 minutes to complete. During the first several tests, subject fatigue was noted to be significant when the battery was administered during the same telephone call as the EAPAC questionnaire, which required 15–20 minutes (33). Subsequently, the battery was administered during a separate call. None of the initial tests were included in the primary analyses of long-term cognitive impairment.
Using the separate testing session, survivors occasionally expressed frustration and requested to end testing. Despite encouragement, explanation of the reasons for the tests, and reassurance that the tests were meant to be challenging, eight long-term survivors declined to complete cognitive testing.
Baseline Characteristics and Effects of Fluid-Management Strategy
Acute Physiologic and Chronic Health Evaluation III score was higher (P = 0.04) in the liberal-fluid strategy group (see Table E3). No other baseline characteristic differed between fluid-management strategies. The ACOS patients in the conservative-strategy group received more furosemide (P < 0.001), resulting in a more negative 7-day cumulative fluid balance (P = 0.02), lower central venous pressures (P < 0.001), and a trend toward lower cardiac index (P = 0.06) (see Table E4). As in the larger FACTT study, in ACOS the conservative fluid-management strategy resulted in shorter duration of mechanical ventilation (median difference of 2.5 d; P = 0.04) and a trend to shorter ICU stays (median difference of 3 ICU d; P = 0.07).
Long-Term Cognitive Impairment and Psychiatric Morbidity
Cognitive function was below normal population mean scores in each domain tested (see Table E1). Cognitive impairment was observed in 41 of 75 survivors (55%) at 12 months. Using the error score of the HSCT to assess executive function rather than the overall score, cognitive impairment was observed in 54 of 90 survivors (60%).
Vocabulary and reasoning, domains hypothesized to be resilient to acquired brain injury, were impaired in 3 of 98 survivors (3%). Among the domains hypothesized to be susceptible to impairment after ALI, memory was impaired in 12 of 92 (13%), verbal fluency in 15 of 96 (16%), and executive function in 37 of 76 (49%) survivors at 12 months. Executive function was impaired in 57 of 100 (57%) survivors when assessed using the error score of the HSCT.
Symptoms of moderate or severe depression occurred in 37 of 102 (36%) long-term survivors. PTSD screening was positive in 40 of 102 (39%) survivors. Symptoms of moderate or severe anxiety occurred in 63 of 102 (62%) survivors. Psychiatric symptoms were present in depression, anxiety, or PTSD in 67 of 102 (66%) survivors. Symptoms in two or more psychiatric domains occurred in 43 of 102 (42%) survivors. Cognitive impairment was significantly associated with the presence of psychiatric symptoms (P = 0.04) at 12 months. Specifically, cognitive impairment was associated with anxiety (P = 0.04), but not depression (P = 0.20) or PTSD (P = 0.33).
Long-Term Cognitive Impairment, Psychiatric Morbidity, and Quality of Life
Quality of life was low in ACOS survivors (median utility = 0.67; IQR, 0.37, 0.90) at 12 months. There were no significant differences in quality of life between cognitively impaired and nonimpaired survivors (median utility in impaired = 0.66; IQR, 0.45, 0.9; nonimpaired = 0.69; IQR, 0.42, 0.82; P = 0.85). In survivors with psychiatric symptoms, quality of life was significantly worse (median utility in 59 survivors with psychiatric symptoms = 0.57; IQR, 0.26, 0.76; utility in 35 survivors without symptoms = 0.90; IQR, 0.53, 0.95; P < 0.001).
Risk Factors for Long-Term Cognitive Impairment
Lower PaO2 during FACTT was associated with cognitive impairment at 12 months (P = 0.02), as were enrollment in the conservative fluid-management strategy (P = 0.004) and lower central venous pressure (P = 0.04) (Table 2, Figure 2; see Table E5). As detailed in the online supplement, lower PaO2 values (P = 0.05), lower central venous pressures (P = 0.02), and enrollment in the conservative fluid-strategy group (P < 0.001) correlated with worse executive function. After adjustment for potential covariates, lower PaO2 and enrollment in the conservative fluid-management strategy were associated independently with cognitive impairment at 12 months (Table 2; see Table E6).
TABLE 2.
ASSOCIATIONS WITH LONG-TERM COGNITIVE IMPAIRMENT IN SURVIVORS OF ALI
| Long-Term Cognitive Impairment |
|||||
| Not Impaired (n = 34) | Impaired (n = 41) | P Value | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)* | |
| Candidate risk factors | |||||
| Duration of mechanical ventilation | 8 (4–11) | 6 (4–9) | 0.43 | ||
| Conservative fluid-management strategy, % | 32 | 66 | 0.004 | 4.03 (1.53–10.59) | 3.35 (1.16–9.70)–5.46 (1.92–15.53) |
| Hypotension (hemodynamic data on-study)† | |||||
| Systolic blood pressure (mm Hg) | 108 (102–113) | 104 (96–112) | 0.32 | ||
| Cardiac index (L/min/m2)‡ | 4.5 (3.8–5.3) | 4.5 (3.7–5) | 0.49 | ||
| Shock, %† | 32 | 29 | 0.77 | ||
| Vasopressor use | 26 | 24 | 0.84 | ||
| Hypoxemia (respiratory variables on-study) | |||||
| PaO2 | 86 (70–98) | 71 (67–80) | 0.02 | 1.56 (1.09–2.24)§ | 1.51 (1.01–2.26)–1.68 (1.14–2.49)§ |
| PaO2:FiO2 | 152 (132–192) | 157 (133–190) | 0.63 | ||
| Oxygenation index | 7.38 (4.55–10.42) | 7.67 (5.97–10.02) | 0.57 | ||
| Oxygen saturation (%) | 95.1 (93.3–96.8) | 94.2 (92.6–95.8) | 0.10 | ||
| Pulmonary artery catheter, % | 53 | 61 | 0.48 | ||
| Primary lung injury, % | 0.10 | ||||
| Pneumonia | 24 | 46 | |||
| Sepsis | 29 | 20 | |||
| Aspiration | 9 | 17 | |||
| Trauma | 12 | 10 | |||
| Multiple transfusions | 6 | 2 | |||
| Other | 21 | 5 | |||
| Severity of illness | |||||
| APACHE III | 87 (60–106) | 72 (60–94) | 0.42 | ||
| ICU length of stay | 11 (8–18) | 10 (7–16) | 0.65 | ||
| Potential covariates | |||||
| Age, yr | 50 (43–58) | 51 (45–60) | 0.51 | ||
| Male sex, % | 32 | 51 | 0.10 | ||
| Race or ethnic group, % | 0.53 | ||||
| White | 91 | 90 | |||
| Black | 6 | 10 | |||
| Hispanic | 3 | 0 | |||
| Level of education, yr | 13 (12–16) | 12 (12–15) | 0.36 | ||
| Coexisting conditions, % | |||||
| Heavy alcohol use | 3 | 13 | 0.21 | ||
| Cerebrovascular disease | 0 | 2 | 1.00 | ||
| Psychiatric impairment, 1 yr, % | 59 | 80 | 0.04 | ||
| Hospital length of stay before enrollment, d | 2 (1–3) | 3 (1–4) | 0.22 | ||
| Time from discharge to testing, mo | 12 (11–13) | 12 (11–13) | 0.51 | ||
Definition of abbreviations: ALI = acute lung injury; APACHE III = Acute Physiologic and Chronic Health Evaluation III scores; CI = confidence interval; CVP = central venous pressure; FACTT = Fluid and Catheter Treatment Trial; ICU = intensive care unit.
Cognitive impairment at the subject level was defined as impairment in memory, verbal fluency, or executive function in the 75 subjects who completed tests in each of these domains at the 12-month follow-up. Values expressed as a frequency (percent) or median (interquartile range).
On-study variables were summarized at the subject level as means to account for influential observations. With the exception of systolic blood pressure, in which the worst values over the preceding 24 hours were recorded to derive the cardiovascular component of the Brussels organ failure score, values were measured and recorded daily (measure closest to 8:00 a.m.) during the study.
Potential covariates were included in the multivariable logistic regression models one covariate at a time. The adjusted odds ratios presented represent the range of odds ratios observed in multivariable logistic regression models. For additional details, see Table E6.
Shock defined as mean arterial pressure less than 60 or vasoactive agent use (assignment to FACTT protocol cells 1 or 2) at any time during FACTT (4).
Cardiac index was measured in 39 survivors (20 in the liberal-strategy group and 19 in the conservative-strategy group).
For each 10-unit decrease in PaO2, odds ratio for the development of cognitive impairment increased by 1.56.
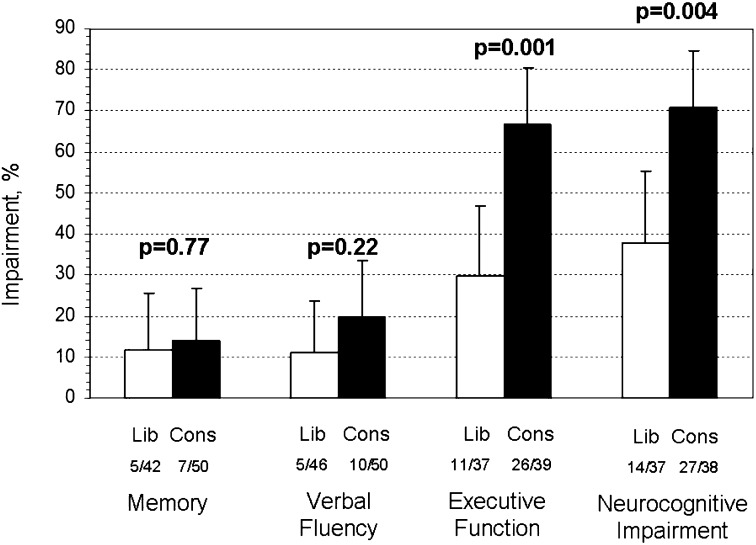
Figure 2.
Long-term cognitive impairment according to fluid management strategy. Impairment in a domain was defined as a score greater than 2 SD below the population norm. Cognitive impairment was defined as impairment in memory, verbal fluency, or executive function in the 75 survivors completing testing in each of these cognitive domains. Memory, verbal fluency, or executive function was assessed in 92, 96, and 76 subjects, respectively. Proportions of impaired subjects are specified by domain, according to fluid-management strategy. Error bars reflect upper bound of 95% confidence interval. Cons = conservative fluid strategy; Lib = liberal fluid strategy.
In secondary analyses, using the untimed error score of the HSCT for the executive function assessment, the conservative fluid-management strategy remained associated with cognitive impairment at 12 months (71% [34 of 48] vs. 48% [20 of 42] in the liberal group; P = 0.02). The association between PaO2 values and cognitive impairment was no longer significant in these analyses (P = 0.32). As detailed in the supplement, we performed multiple sensitivity analyses to determine the effects of missing data and incomplete cognitive testing. These analyses may suggest that a relationship exists between hypoxemia and fluid-management strategy and long-term cognitive impairment.
Risk Factors for Long-Term Psychiatric Morbidity
Survivors with psychiatric morbidity at 12 months had lower PaO2 values (P = 0.02), lower systolic blood pressures (P = 0.05), and were more likely to have had an episode of hypoglycemia (P = 0.03) (Table 3). As detailed in the online supplement, lower PaO2 values (P = 0.05), lower systolic blood pressures (P = 0.04), and an episode of hypoglycemia (P = 0.01) were associated with anxiety. After adjustment for potential covariates, lower PaO2 was associated independently with long-term psychiatric morbidity.
TABLE 3.
ASSOCIATIONS WITH LONG-TERM PSYCHIATRIC MORBIDITY IN SURVIVORS OF ALI
| Psychiatric Morbidity |
|||
| Not Present (n = 35) | Present (n = 67) | P Value | |
| Baseline characteristics | |||
| Age, yr | 51 (38–64) | 50 (44–57) | 0.58 |
| Male sex, % | 40 | 45 | 0.64 |
| Race or ethnic group, % | 0.19 | ||
| White | 94 | 81 | |
| Black | 6 | 15 | |
| Hispanic | 0 | 4 | |
| Level of education, yr | 14 (12–16) | 12 (12–15) | 0.15 |
| Medical ICU, % | 49 | 58 | 0.44 |
| APACHE III | 88 (63–108) | 77 (60–99) | 0.31 |
| Conservative strategy, % | 46 | 60 | 0.18 |
| Pulmonary artery catheter, % | 57 | 52 | 0.64 |
| On-study variables* | |||
| Hemodynamic variables† | |||
| Systolic blood pressure (mm Hg) | 111 (102–116) | 104 (98–111) | 0.05 |
| Shock, % | 37 | 30 | 0.46 |
| Respiratory variables | |||
| PaO2‡ | 86 (72–98) | 72 (68–90) | 0.02 |
| Metabolic variables | |||
| Glucose, mg/dl | 123 (107–140) | 119 (109–136) | 0.99 |
| Hypoglycemia‡, <60 mg/dl, % | 0 | 13 | 0.03 |
| Hyperglycemia‡, >180 mg/dl, % | 34 | 30 | 0.65 |
| Corticosteroid therapy§ | |||
| Corticosteroids (mg of methylprednisolone, cumulative) | 0 (0–60) | 0 (0–140) | 0.58 |
| Corticosteroids received, % | 29 | 33 | 0.66 |
| ICU length of stay (d) | 11 (7–20) | 12 (8–16) | 0.92 |
| Duration of mechanical ventilation (d) | 7 (4–15) | 7 (4–10) | 0.94 |
Definition of abbreviations: ALI = acute lung injury; APACHE III = Acute Physiologic and Chronic Health Evaluation III scores; FACTT = Fluid and Catheter Treatment Trial; ICU = intensive care unit.
Values expressed as a frequency (percent) or median (interquartile range).
Psychiatric morbidity was defined as present if symptoms of anxiety, depression, or post-traumatic stress disorder were identified at the 12-month follow-up.
On-study variables were summarized at the subject level as means to account for influential observations. With the exception of systolic blood pressure, in which the worst values over the preceding 24 hours were recorded to derive the cardiovascular component of the Brussels organ failure score, values were measured and recorded daily (measure closest to 8:00 a.m.) during the study.
Shock defined as mean arterial pressure less than 60 or vasoactive agent use (assignment to FACTT protocol cells 1 or 2) at any time during FACTT (4). The association between systolic blood pressure and psychiatric impairment was nonsignificant after adjustment for potential covariates (P = 0.14).
For each 10-unit decrease in PaO2, odds ratio for the development of psychiatric morbidity increased by 1.35 (95% confidence interval, 1.02–1.78) and the association remained after adjustment for potential covariates. Hypoglycemia and hyperglycemia were defined as any episode of serum glucose less than 60 mg/dl or greater than 180 mg/dl, respectively, during the hospitalization. We were unable to adjust for potential covariates in the association between hypoglycemia and psychiatric morbidity because psychiatric symptoms were present in each subject who had a hypoglycemia episode.
Corticosteroid therapy was neither associated with PTSD (11 of 32 subjects [34%] who received corticosteroids experienced symptoms of PTSD compared with 29 of 70 subjects [41%] who did not receive corticosteroids; P = 0.50), nor associated with psychiatric morbidity in the subgroup of 27 sepsis-associated subjects with ALI (6 of 12 subjects [50%] who received corticosteroids experienced long-term psychiatric morbidity compared with 11 of 15 subjects [73%] who did not receive corticosteroids; P = 0.26).
Discussion
We found that neuropsychological function in survivors from a multicenter trial can be assessed using a validated telephone battery. Similar to previous investigations, we found most survivors of ALI experience long-term cognitive and psychiatric morbidity. Furthermore, our findings demonstrate a relationship between cognitive impairment, psychiatric symptoms, and adverse quality of life in long-term survivors. Finally, we confirmed prior associations that hypoxemia is a potential risk factor for the development of long-term cognitive and psychiatric impairment.
We found that neuropsychological function can be assessed over the telephone in a multicenter trial. This has the potential to be an important development for future critical care trials, because neuropsychological impairment is increasingly recognized as a common, long-lasting, and potentially modifiable outcome. However, our experience highlights potential limitations of measuring neuropsychological function in multicenter trials.
First, attrition was significant. Attrition is a recognized barrier to conducting long-term outcomes studies in critical care trials (24, 25, 57–59), caused in part by the mortality experienced by critically ill patients. After accounting for mortal losses, the requirement that subjects be enrolled in EA-PAC, and the delay in study initiation, only 406 of the 1,001 subjects randomized in FACTT were alive and eligible for ACOS.
However, because 12% of eligible survivors declined and 36% were unable to be contacted to obtain consent for ACOS after discharge, the opportunity to increase subject participation in future studies exists. Potential solutions to increase consent rate in future studies include enrolling subjects prospectively and prioritizing in-person consent before discharge, and using multiple strategies and repeated attempts to contact potential subjects (60, 61). Furthermore, because 12% of subjects who consented to long-term neuropsychological assessment subsequently declined and 25% were lost to follow-up, the opportunity to improve retention also exists. Although it is unclear why subjects declined to be tested, potential explanations include that subjects were asked to complete multiple follow-up interviews as part of EA-PAC and ACOS and neuropsychological assessments were lengthy. Finally, subjects completed cognitive testing by the telephone to varying degrees. Fatigue or frustration appeared to play a role, as did an error in test administration. Future studies need to balance the merits of administering a more comprehensive battery with the potential burden imposed on study participants.
Potential solutions to improve retention and test completion in future studies include increasing the flexibility of scheduling by centralizing test administration in different time-zones to minimize potential conflicts with work or healthcare visits; increasing the number of trained staff to administer the neuropsychological battery and identifying and using the most effective strategies to ensure test completion; selecting an intermediate testing time point (e.g., 3 mo), which minimizes mortal losses yet ensures adequate time for survivors to transition through the healthcare system to home (24, 25, 62, 63); using a shorter test battery (64) or limiting the domains to be assessed (62); and using multiple strategies in a systematic fashion to retain participants (60, 61). Through the effective use of many of these strategies, more recent studies are demonstrating the ability to recruit and retain survivors of ALI successfully (61, 65, 66). Finally, compared with nonparticipants, ACOS subjects were more likely to be female and white; as such, our findings may not generalize to other populations.
In conclusion, we found that we were able to assess long-term neuropsychological function in survivors from a multicenter trial using a telephone battery. However, we encountered significant challenges in regards to recruitment, retention, and test completion. We acknowledge that these challenges pose a potential threat to the internal and external validity of such an approach. To improve the performance of this practical approach in future studies, we have highlighted potential solutions to improve recruitment, retain study participants, and ensure complete neuropsychological assessments.
The subjects with ALI in our study had long-term cognitive and psychiatric morbidity consistent with prior studies where data were obtained in person (6–9, 24, 25). Coupled with our prior work (10, 11, 30), our findings validate that many survivors of ALI experience clinically important long-term cognitive impairment. Approximately half of our survivors of ALI had executive dysfunction. Although cognitive impairment was not associated with decreased quality of life in survivors of ALI enrolled in ACOS, the evidence supports that cognitive impairment has a considerable adverse impact on individuals and society (6–21, 67, 68).
Survivors of ALI enrolled in ACOS experienced anxiety, depression, and PTSD symptoms. The presence of long-term psychiatric symptoms, and symptoms of anxiety in particular, was found to be associated with lower PaO2 values and hypoglycemia during the hospitalization. Our findings validate the recent work of Hopkins and coworkers (18), wherein hypoxemia was associated with anxiety at 1 year in ARDS survivors; augment the recent work of Dowdy and coworkers (20), which found that hypoglycemia was associated with depression at 3 months; and support the notion that psychiatric symptoms may result from brain injury sustained during the hospitalization. We found that psychiatric morbidity was associated with cognitive impairment and significantly worse quality of life. Because cognitive impairment may lead to development of psychiatric symptoms (18), and psychiatric symptoms may lead to impaired physical function (66), our findings support the idea that cognitive impairment, psychiatric symptoms, and quality of life are interrelated and impact on each other in survivors of critical illness.
We found that hypoxemia is a potential risk factor for the development of long-term cognitive impairment. Consistent with the work of Hopkins and colleagues (6), we found lower PaO2 values were associated with cognitive impairment in general and executive dysfunction specifically. Enrollment in the conservative fluid-management strategy was identified as a potential risk factor for the development of long-term cognitive impairment and executive dysfunction. To potentially explain this finding, we demonstrated that within the ACOS population lower central venous pressures, the explicit target of the conservative fluid-management strategy, were associated with cognitive impairment and executive dysfunction. However, there was no indirect evidence for reduced cerebral perfusion (e.g., cardiac index, systolic blood pressure) as the mediator for the observed association, thus it is unclear how conservative fluid management may have caused cognitive impairment. Given the highly selected population that enrolled in and completed ACOS testing, it is unclear if this finding generalizes to the entire FACTT cohort. This finding needs to be validated.
Our study sample size limits the generalizability of our findings and is a major limitation. We also acknowledge the potential for uncontrolled confounding. Specifically, we were unable to adjust for potential covariates simultaneously due to our sample size, we were unable to adjust for sedation or delirium (25, 69), and we did not screen for preexisting dementia formally (70) or preexisting psychiatric disease. There exists the potential for informative censoring, and although some subjects were determined to be incapable of telephone-based neuropsychological testing, we did not assess formally for hearing loss before testing. Although furosemide has been associated with hearing loss, there was no association between furosemide dose administered and cognitive impairment (see Table E5). We used conservative criteria to define cognitive impairment and limited the probability of a Type I error (46); however, our multiple comparisons could result in falsely rejecting the null hypothesis. If we used a Bonferroni correction factor, only the conservative fluid-management strategy would remain associated with cognitive impairment. Regardless, our risk factor assessment requires confirmation. Finally, depression, anxiety, and PTSD were identified using self-report measures, which may overdiagnose these disorders. These psychiatric instruments, although reliable and valid, are not the gold standard for clinical diagnosis.
In conclusion, we found that neuropsychological function can be assessed in a multicenter trial, but it was challenging and opportunities to improve the performance of this strategy exist. Our findings validate that cognitive and psychiatric morbidity are common in survivors of ALI, findings that have significant implications for these patients’ long-term functioning. Executive dysfunction, specifically, was a common morbidity. We found evidence that hypoxemia is a risk factor for long-term cognitive and psychiatric impairment and a signal that fluid management strategy is a potential risk factor for long-term cognitive impairment. Further studies are necessary to confirm this latter observation given the select population studied and an unclear mechanism. Future clinical trials in critically ill populations should include assessment of long-term outcomes, including neuropsychological outcomes. We need to be cautious in our interpretation of such studies and acknowledge that confirmatory studies may be required. Nevertheless, to improve the lives of critically ill survivors, we should assess the short- and long-term effects of an intervention.
Supplementary Material
Acknowledgments
The authors thank the subjects for their participation in follow-up interviews and their family members for their support to better understand long-term neuropsychological effects of critical illness. They acknowledge the contributions of Gilles Clermont, Lan Kong, and Kim Fusko of the University of Pittsburgh for project and data management and for recruiting, coordinating, and conducting interviews and to investigators and study coordinators at participating ARDS Clinical Trials Network sites for recruitment, data collection, and support.
Footnotes
Author Contributions: Conception and design, D.C.A., R.C.B., J.D.C., R.O.H., P.N.L., and B.T.T.; data collection, D.C.A., J.D.C., E.D., R.O.H., P.N.L., M.E.M., and B.T.T.; analysis and interpretation of the data, D.C.A., S.L.B., R.C.B., J.D.C., R.O.H., A.R.L., M.E.M., and B.T.T.; drafting of the manuscript, D.C.A., S.L.B., R.C.B., J.D.C., E.D., R.O.H., P.N.L., A.R.L., M.E.M., and B.T.T.; and critical revision of the article for important intellectual content, D.C.A., J.D.C., R.O.H., M.E.M., and B.T.T.
Supported in part by N01-HR-46058, N01-HR-46046-64, N01-HR-16146-54, and T32 HL07891 training grant, National Institutes of Health, and National Heart, Lung and Blood Institute.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201111-2025OC on April 6, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693 [DOI] [PubMed] [Google Scholar]
- 2.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 3.The Acute Respiratory Distress Syndrome Network High versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327–336 [DOI] [PubMed] [Google Scholar]
- 4.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Comparison of two fluid management strategies in acute lung injury. N Engl J Med 2006;354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 5.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med 2010;153:204–205 [DOI] [PubMed] [Google Scholar]
- 6.Hopkins R, Weaver L, Pope D, Orme J, Bigler E, Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med 1999;160:50–56 [DOI] [PubMed] [Google Scholar]
- 7.Hopkins RO, Weaver LK, Chan KJ, Orme JF. Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc 2004;10:1005–1017 [DOI] [PubMed] [Google Scholar]
- 8.Rothenhausler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001;23:90–96 [DOI] [PubMed] [Google Scholar]
- 9.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 2005;171:340–347 [DOI] [PubMed] [Google Scholar]
- 10.Christie JD, Biester R, Taichman DB, Shull WH, Hansen-Flaschen J, Shea JA, Hopkins RO. Formation and validation of a telephone battery to assess cognitive function in acute respiratory distress syndrome survivors. J Crit Care 2006;21:125–132 [DOI] [PubMed] [Google Scholar]
- 11.Mikkelsen ME, Shull WH, Biester RC, Taichman DB, Lynch S, Demissie E, Hansen-Flaschen J, Christie JD. Cognitive, mood and quality of life impairments in a select population of ARDS survivors. Respirology 2009;14:76–82 [DOI] [PubMed] [Google Scholar]
- 12.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. , for the Canadian Critical Care Trials Group One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003;348:683–693 [DOI] [PubMed] [Google Scholar]
- 13.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. , for the Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–1304 [DOI] [PubMed] [Google Scholar]
- 14.Adhikari NK, McAndrews MP, Tansey CM, Matté A, Pinto R, Cheung AM, Diaz-Granados N, Barr A, Herridge MS. Self-reported symptoms of depression and memory dysfunction in survivors of ARDS. Chest 2009;135:678–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adhikari NK, Tansey CM, McAndrews MP, Matté A, Pinto R, Cheung AM, Diaz-Granados N, Herridge MS. Self-reported depressive symptoms and memory complaints in survivors five years after ARDS. Chest 2011;140:1484–1493 [DOI] [PubMed] [Google Scholar]
- 16.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med 2008;70:512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson BJ, Weinert CR, Burly CL, Marinelli WA, Gross CR. Intensive care unit drug use and subsequent quality of life in acute lung injury patients. Crit Care Med 2000;28:3626–3630 [DOI] [PubMed] [Google Scholar]
- 18.Hopkins RO, Key CW, Suchyta DO, Weaver LK, Orme JF. Risk factors for depression and anxiety in survivors of acute respiratory distress syndrome. Gen Hosp Psychiatry 2010;32:147–155 [DOI] [PubMed] [Google Scholar]
- 19.Dowdy DW, Bienvenu OJ, Dinglas V, Mendez-Tellez PA, Sevransky J, Shanholtz C, Needham DM. Are intensive care factors associated with depressive symptoms six months after acute lung injury? Crit Care Med 2009;37:1702–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowdy DW, Dinglas V, Mendez-Tellez PA, Bienvenu OJ, Sevransky J, Dennison CR, Shanholtz C, Needham DM. Intensive care unit hypoglycemia predicts depression during early recovery from acute lung injury. Crit Care Med 2008;36:2726–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapfhammer HP, Rothenhausler HB, Krauseneck T, Stoll C, Schelling G. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychol 2004;161:45–52 [DOI] [PubMed] [Google Scholar]
- 22.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJPA, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA 2010;303:763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010;304:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson JC, Girard TD, Gordon SM, Thompson JL, Shintani AK, Thomason JWW, Pun BT, Canonico AE, Dunn JG, Bernard GR, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med 2010;182:183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 2010;38:1513–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones C, Griffiths RD, Slater T, Benjamin KS, Wilson S. Significant cognitive dysfunction in non-delirious patients identified during and persisting following critical illness. Intensive Care Med 2006;32:923–926 [DOI] [PubMed] [Google Scholar]
- 27.Desai S, Lawa TJ, Needham DM. Long-term complications of critical care. Crit Care Med 2011;39:371–379 [DOI] [PubMed] [Google Scholar]
- 28.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry 2008;30:421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med 2009;35:796–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taichman DB, Christie J, Biester R, Mortensen J, White J, Kaplan S, Hansen-Flaschen J, Palevsky HI, Elliott CG, Hopkins RO. Validation of a brief telephone battery for cognitive assessment of patients with pulmonary arterial hypertension. Respir Res 2005;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006;354:2213–2224 [DOI] [PubMed] [Google Scholar]
- 32.Mikkelsen ME, Lanken PN, Biester R, Gallop R, Bellamy S, Localio AR, Hopkins RO, Angus DC. Christie JD, for the ARDSNet. Conservative fluid strategy is associated with neurocognitive deficits in survivors of acute lung injury [abstract]. Am J Respir Crit Care Med 2008;177:A819 [Google Scholar]
- 33.Clermont G, Kong L, Weissfeld LA, Lave JR, Rubenfeld GD, Roberts MS, Connors AF, Bernard GR, Thompson BT, Wheeler AP, et al. The effect of pulmonary artery catheter use on costs and long-term outcomes of acute lung injury. PLoS ONE 2011;6:e22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824 [DOI] [PubMed] [Google Scholar]
- 35.Burgess PW, Shallice T. The Hayling and Brixton tests. London, England: Thames Valley Test Company Limited; 2007 [Google Scholar]
- 36.Sukantarat KT, Burgess PW, Williamson RC, Brett SJ. Prolonged cognitive dysfunction in survivors of critical illness. Anaesthesia 2005;60:847–853 [DOI] [PubMed] [Google Scholar]
- 37.Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung self-rating depression scale. Br J Psychol 1978;132:381–385 [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1998;56:893–897 [DOI] [PubMed] [Google Scholar]
- 39.Weisaeth L. Torture of a Norwegian ship’s crew: the torture, stress reactions and psychiatric aftereffects. Acta Psychiatr Scand Suppl 1989;355:63–72 [PubMed] [Google Scholar]
- 40.Wechsler D, editor Wechsler Adult Intelligence Scale, 3rd edition. San Antonio: The Psychological Corporation; 1997 [Google Scholar]
- 41.Wechsler D. Wechsler Memory Scale. San Antonio: The Psychology Corporation; 1997 [Google Scholar]
- 42.Lezak MD. Neuropsychological assessment. New York: Oxford University Press; 1995 [Google Scholar]
- 43.Torrance GW, Feeny DH, Furlong WJ, Barr RD, Zhang Y, Wang Q. Multi-attribute preference functions for a comprehensive health status classification system: health utilities index Mark 2. Med Care 1996;34:702–722 [DOI] [PubMed] [Google Scholar]
- 44.Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan battery: demographic corrections, research findings and clinical applications. Odessa: Psychological Assessment Resources, Inc; 1991 [Google Scholar]
- 45.Heaton RK. Comprehensive norms for an expanded Halstead-Reitan battery: a supplement for the WAIS-R. Odessa: Psychological Assessment Resources, Inc; 1994 [Google Scholar]
- 46.Ingraham LJ, Aiken CB. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology 1996;10:120–124 [Google Scholar]
- 47.Doblar DD, Hinckle JC, Fay ML, Condon BF. Air embolism associated with pulmonary artery catheter introducer kit. Anesthesiology 1982;56:307–309 [DOI] [PubMed] [Google Scholar]
- 48.Boyd KD, Thomas SJ, Boyd AD. A prospective study of complications of pulmonary artery catheterizations in 500 consecutive patients. Chest 1983;84:245–249 [DOI] [PubMed] [Google Scholar]
- 49.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 2001;58:445–452 [DOI] [PubMed] [Google Scholar]
- 50.Patarca-Montero R, Antoni M, Fletcher MA, Klimas NG. Cytokine and other immunologic markers in chronic fatigue syndrome and their relation to neuropsychological factors. Appl Neuropsychol 2001;8:51–64 [DOI] [PubMed] [Google Scholar]
- 51.Ngandu T, von Strauss E, Helkala EL, Winblad B, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M. Education and dementia: what lies behind the association? Neurology 2007;69:1442–1450 [DOI] [PubMed] [Google Scholar]
- 52.Richards PM, Ruff RM. Motivational effects on neuropsychological functioning: comparison of depressed versus nondepressed individuals. J Consult Clin Psychol 1989;57:396–402 [DOI] [PubMed] [Google Scholar]
- 53.Buckelew SP, Hannay HJ. Relationships among anxiety, defensiveness, sex, task difficulty and performance on various neuropsychological tasks. Percept Mot Skills 1986;63:711–718 [DOI] [PubMed] [Google Scholar]
- 54.Schelling G, Stoll C, Kapfhammer HP, Rothenhausler HB, Krauseneck T, Durst K, Haller M, Briegel J. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder and health-related quality of life in survivors. Crit Care Med 1999;27:2678–2683 [DOI] [PubMed] [Google Scholar]
- 55.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923–936 [DOI] [PubMed] [Google Scholar]
- 56.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–1379 [DOI] [PubMed] [Google Scholar]
- 57.Rubenfeld GD. Improving clinical trials of long-term outcomes. Crit Care Med 2009;37:S112–S116 [DOI] [PubMed] [Google Scholar]
- 58.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med 2003;168:1457–1461 [DOI] [PubMed] [Google Scholar]
- 59.Strom T, Stylsvig M, Toft P. Long-term psychological effects of a no-sedation protocol in critically ill patients. Crit Care 2011;15:R293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson KA, Dennison CR, Wayman DM, Pronovost PJ, Needham DM. Systematic review identified number of strategies important to retaining study participants. J Clin Epidemiol 2007;60:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen KF, Colantuoni E, Siddiqi F, Dinglas VD, Sepulveda KA, Fan E, Pronovost PJ, Needham DM. Repeated attempts using different strategies are important for timely contact with study participants. J Clin Epidemiol 2011;64:1144–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson J, Ely EW, Morey MC, Anderson VM, Siebert CS, Denne LB, Clune J, Archer KR, Torres R, Janz D, et al. Cognitive and physical rehabilitation of intensive care unit survivors: results of the RETURN randomized controlled pilot investigation. Crit Care Med 2012;40:1088–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, Clay AS, Chia J, Gray A, Tulsky JA, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med 2010;153:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699 [DOI] [PubMed] [Google Scholar]
- 65.National Heart, Lung, and Blood Institute; Johns Hopkins University. Evaluating health outcomes and quality of life after acute lung injury among participants of the ALTA, OMEGA, and EDEN (ARDS Network) studies (The ALTOS Study). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000–2012. Available from: http://clinicaltrials.gov/show/ NCT00719446 NLM Identifier: NCT00719446
- 66.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, Dennison CR, Herridge MS, Pronovost PJ, Needham DM. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med 2012;185:517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rozzini L, Chilovi BV, Trabucchi M, Padovani A, Marson D, Griffith R. Impaired financial abilities in mild cognitive impairment: a direct assessment approach. Neurology 2003;60:2021. [DOI] [PubMed] [Google Scholar]
- 68.Jonsson L, Lindgren P, Wimo A, Jonsson B, Winblad B. Costs of mini mental state examination-related cognitive impairment. Pharmacoeconomics 1999;16:409–416 [DOI] [PubMed] [Google Scholar]
- 69.Van den Boogard M, Schoonhoven L, Evers AW, van der Hoeven JG, van Achterberg TV, Pickkers P. Delirium in critically ill patients: impact on long-term health-related quality of life and cognitive functioning. Crit Care Med 2012;40:112–118 [DOI] [PubMed] [Google Scholar]
- 70.Pisani MA, Inouye SK, McNicoll L, Redlich CA. Screening for preexisting cognitive impairment in older intensive care unit patients: use of proxy assessment. J Am Geriatr Soc 2003;51:689–693 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.