Abstract
PURPOSE
To report a case of acute retinal necrosis (ARN) after combined cataract surgery and intracameral triamcinolone acetonide injection in a healthy elderly patient.
METHODS
Clinical examination including fundus photographs, fluorescein angiography, and serologic testing.
RESULTS
A 75-year-old healthy Caucasian woman undergoing cataract extraction received an injection of intracameral triamcinolone acetonide (TA) as a substitute for post-operative topical steroids. Two weeks later, the patient developed ARN, which responded well to systemic antiviral therapy.
CONCLUSION
ARN is a rare, but potentially devastating complication that may be associated with intraocular TA injection.
Keywords: acute retinal necrosis, intraocular injection, triamcinolone acetonide
Intravitreal triamcinolone acetonide (TA) has gained increasing acceptance as a treatment for ocular diseases. It is used for macular edema, exudative age-related macular degeneration, anterior and posterior segment neovascularization, uveitis, and proliferative vitreoretinopathy.1 Intracameral TA has been employed as an alternative to topical steroids after cataract surgery for post-operative inflammation.2 Reports have demonstrated that intravitreal TA is moderately safe, with complications primarily due to intraocular pressure elevation and cataract progression.3 Herein, we describe a case of intracameral TA injection associated with acute retinal necrosis (ARN), a potentially vision-threatening disease.
Case Report
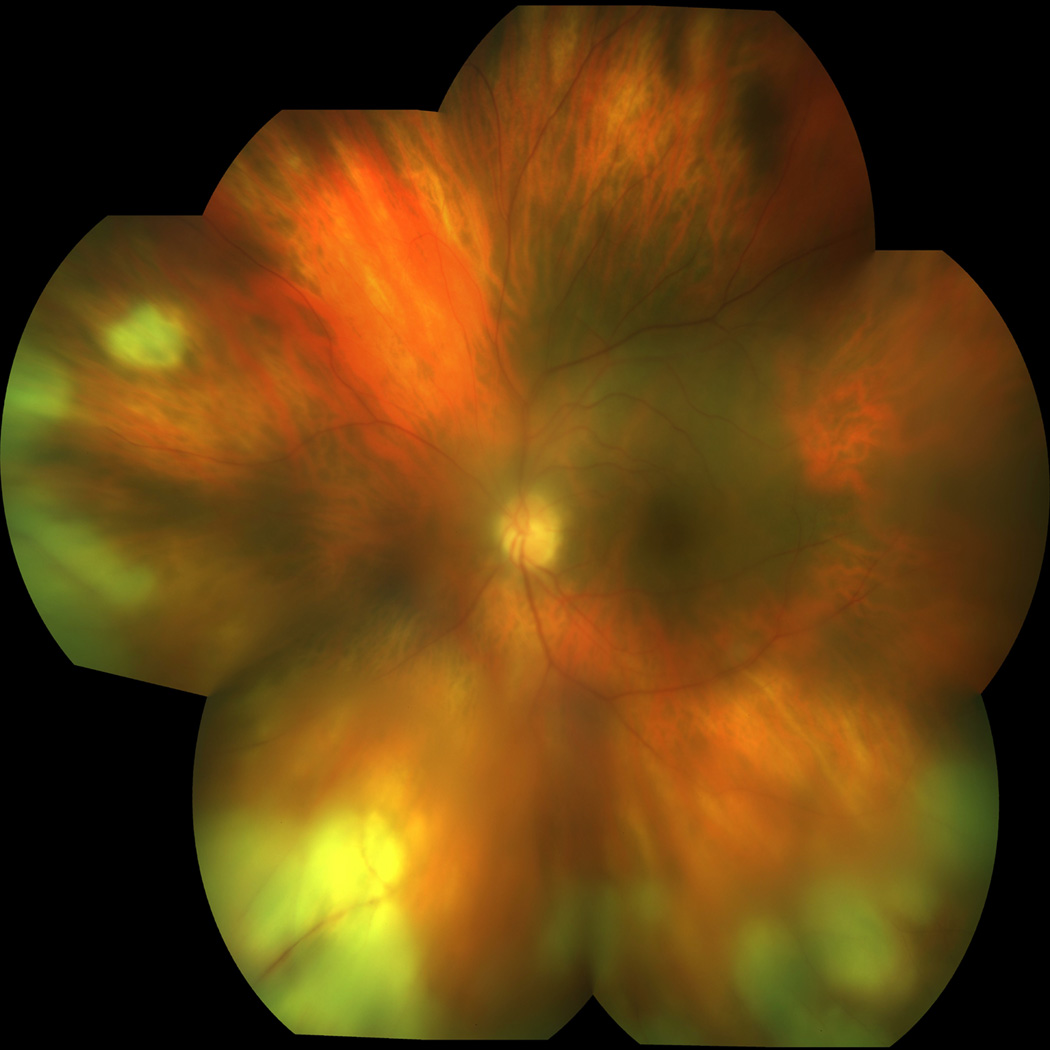
A 75-year-old woman developed post-operative iritis in the right eye attributed to poor compliance with topical steroids after uncomplicated cataract surgery. The iritis was quickly and uneventfully treated topically with steroid eye drops. Given the history of non-compliance with post-operative drops, 1.5 mg preservative-free TA was injected into the anterior chamber at the end of cataract surgery of the left eye. Two weeks later, she presented with floaters and decreased vision in the left eye. Visual acuity was 20/40 in the left eye, pinholing to 20/25. Intraocular pressure was 9 mmHg in the left eye. Slit-lamp examination of the left eye revealed 3+ anterior chamber cells. Fundus examination revealed vitreous haze and debris, vessel attenuation, and peripheral confluent areas of retinal whitening (Figure 1). Fluorescein angiogram demonstrated staining of vessels and peripheral lesions in the left eye. Examination of the right eye revealed no sign of active or prior intraocular inflammation.
Figure 1.
Color photograph OS: Acute retinal necrosis with vitreous haze, vessel attenuation, and peripheral confluent areas of retinal whitening, most notable inferiorly.
The patient was placed on a 10-day course of intravenous acyclovir (10 mg/kg every 8 hours) for management of ARN, in addition to topical prednisolone acetate 1% drops every 2 hours and atropine 1% drops three times a day. An extensive infectious and autoimmune work-up revealed positive serologies for both herpes simplex virus type 1 and varicella zoster virus IgG. She responded well with no further progression of retinitis and developed early pigment changes indicative of scarring of the lesions. Four days after initiation of anti-viral therapy, oral prednisone 40 mg daily was started to decrease vitritis. She was discharged on oral valacyclovir 1g 3 times a day and a prednisone taper.
Five months after treatment, the patient underwent repair for an inferior rhegmatogenous retinal detachment of the left eye with vitrectomy, laser retinopexy, and gas tamponade. Her visual acuity 7 months after surgery was 20/25 in the right eye and 20/50 in the left eye. The right eye remained unaffected.
Discussion
To the best of our knowledge, this is the first report of ARN following intracameral injection of TA. In the absence of treatment, ARN can progress rapidly and be visually devastating.4 Poor vision generally results from rhegmatogenous retinal detachment or optic neuritis. Causative agents include varicella zoster virus, herpes simplex viruses types 1 and 2, and rarely, cytomegalovirus and Epstein-Barr virus.4
Intraocular TA has been promising in the treatment of many ocular disorders, as corticosteroids have anti-inflammatory, anti-angiogenic, and anti-permeability properties.1 Infectious endophthalmitis is a rare, but severe, complication with an incidence of 0–0.87%.1,3,5 ARN has also been described following intravitreal TA (4 mg) in the treatment of macular edema and central retinal vein occlusion, as well as in combination with photodynamic therapy for treatment of choroidal neovascularization.6,7
In contrast with these previous reports, ARN developed in our patient following cataract extraction and concomitant anterior chamber TA injection. To the best of our knowledge, no report of ARN after uneventful cataract surgery alone has been documented. The temporal association between triamcinolone injection and the development of ARN is suggestive of a causative link. Intraocular corticosteroids may decrease the ocular immune response allowing for primary infection or latent viral reactivation.
Cytotoxic effects on rabbit retina have been demonstrated after a single intravitreal injection of TA at doses greater than 4 mg but not at lower doses of 0.5 mg or 1 mg.8 Considering differences between human and rabbit eyes, such as ocular volume and presence of intraocular inflammation, a 4 mg TA injection in human eyes may correlate to the lower dose injection in rabbit eyes, and thus, would not be cytotoxic in human eyes. Electroretinogram studies in patients have also shown no effect on retinal function 3 months after intravitreal injection of 4 mg Kenalog (commercial TA).9 Moreover, benzyl alcohol, a preservative in Kenalog, and not TA itself, has been shown to be toxic to the retina.10 Thus, TA-induced retinal toxicity is unlikely in our patient, in whom retinal necrosis developed despite using a low dose (1.5 mg), preservative-free form of TA. However, a direct toxic effect of triamcinolone layering in contact with the inferior retina, inducing localized retinal necrosis, cannot be excluded.
Although widespread application of intravitreal TA has resulted in favorable outcomes in the treatment of many ocular diseases its use must be judicious, as it is not without the possibility for significant complications. Likewise, we recommend judicious use of intracameral TA and close monitoring of these injected patients given the possible association with ARN. The authors encourage others to report cases of post-intracameral ARN if they have encountered them.
Footnotes
The authors have no proprietary interest in this report.
References
- 1.Jonas JB, Kreissig I, Degenring R. Intravitreal triamcinolone acetonide for treatment of intraocular proliferative, exudative, and neovascular diseases. Prog Retin Eye Res. 2005;24:587–611. doi: 10.1016/j.preteyeres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Gills JP, Gills P. Effect of intracameral triamcinolone to control inflammation following cataract surgery. J Cataract Refract Surg. 2005;31:1670–1671. doi: 10.1016/j.jcrs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Gillies MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004;122:336–340. doi: 10.1001/archopht.122.3.336. [DOI] [PubMed] [Google Scholar]
- 4.Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis: features, management and outcomes. Ophthalmology. 2007;143:536–538. doi: 10.1016/j.ophtha.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003;136:791–796. doi: 10.1016/s0002-9394(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 6.Toh TY, Borthwick JH. Acute retinal necrosis post intravitreal injection of triamcinolone acetonide. Clin Experiment Ophthalmol. 2006;34:380–382. doi: 10.1111/j.1442-9071.2006.01229.x. [DOI] [PubMed] [Google Scholar]
- 7.Aggermann T, Stolba U, Brunner S, Binder S. Endophthalmitis with retinal necrosis following intravitreal triamcinolone acetonide injection. Ophthalmologica. 2006;220:131–133. doi: 10.1159/000090579. [DOI] [PubMed] [Google Scholar]
- 8.Yu SY, Damico FM, Viola F, et al. Retinal toxicity of intravitreal triamcinolone acetonide: a morphological study. Retina. 2006;26:531–536. doi: 10.1097/00006982-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lang Y, Leibu R, Shoham N, et al. Evaluation of intravitreal kenalog toxicity in humans. Ophthalmology. 2007;114:724–731. doi: 10.1016/j.ophtha.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 10.Lang Y, Zemel E, Miller B, Perlman I. Retinal toxicity of intravitreal kenalog in albino rabbits. Retina. 2007;27:778–788. doi: 10.1097/IAE.0b013e318030c517. [DOI] [PubMed] [Google Scholar]