Abstract
Adequate iron availability is essential to human development and overall health. Iron is a key component of oxygen-carrying proteins, has a pivotal role in cellular metabolism, and is essential to cell growth and differentiation. Inadequate dietary iron intake, chronic and acute inflammatory conditions, and obesity are each associated with alterations in iron homeostasis. Tight regulation of iron is necessary because iron is highly toxic and human beings can only excrete small amounts through sweat, skin and enterocyte sloughing, and fecal and menstrual blood loss. Hepcidin, a small peptide hormone produced mainly by the liver, acts as the key regulator of systemic iron homeostasis. Hepcidin controls movement of iron into plasma by regulating the activity of the sole known iron exporter ferroportin-1. Downregulation of the ferroportin-1 exporter results in sequestration of iron within intestinal enterocytes, hepatocytes, and iron-storing macrophages reducing iron bioavailability. Hepcidin expression is increased by higher body iron levels and inflammation and decreased by anemia and hypoxia. Importantly, existing data illustrate that hepcidin may play a significant role in the development of several iron-related disorders, including the anemia of chronic disease and the iron dysregulation observed in obesity. Therefore, the purpose of this article is to discuss iron regulation, with specific emphasis on systemic regulation by hepcidin, and examine the role of hepcidin within several disease states, including iron deficiency, anemia of chronic disease, and obesity. The relationship between obesity and iron depletion and the clinical assessment of iron status will also be reviewed.
Introduction
Maintenance of iron homeostasis throughout human life is essential for proper growth, development, and overall health. The recent discovery of the peptide hepcidin requires the nutrition and medical professional to reexamine the fundamentals of iron regulation. Hepcidin acts as the main regulator of systemic iron homeostasis by controlling the flux of iron into plasma through regulation of the sole known iron exporter, ferroportin-1 (Fpn) (1). Hepcidin is produced mainly by the liver and concentrations are regulated by body iron status, anemia, hypoxia, and inflammation (1–5). Increased serum levels of hepcidin are associated with reduced dietary iron absorption and decreased systemic iron bioavailability. Importantly, hepcidin appears to play a significant role in the development of several iron-related disorders including the anemia of chronic disease (ACD) (1, 2, 6). Also, hepcidin has been shown to be increased in obese compared to lean individuals suggesting that hepcidin may play an important role in the iron dysregulation observed in obesity (7–11). Therefore, keeping abreast of the recent discoveries in the field of iron metabolism, particularly hepcidin’s role in iron homeostasis, and its function in the manifestation of several iron-related disorders, is critical for the precise evaluation of patients and selection of the most suitable treatment options. Thus, the purpose of this article is to discuss iron regulation, with specific emphasis on systemic regulation by hepcidin, and the role of hepcidin within several disease states including iron deficiency (ID), the ACD, and obesity. Additionally, the relationship between obesity and iron depletion and the clinical assessment of iron status will be reviewed.
Methodology for Selection of Obesity-related Articles
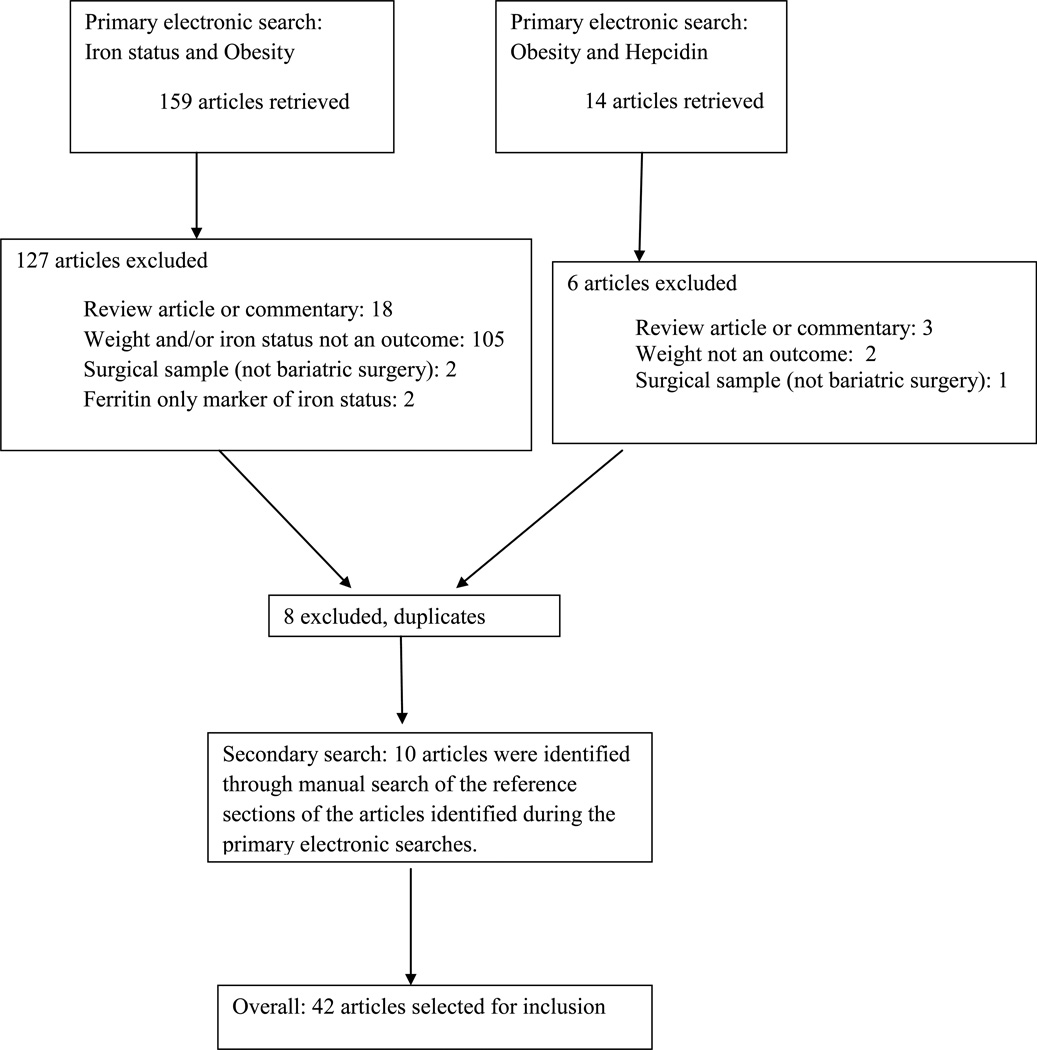
Obesity-related articles were identified through an electronic database search using PubMed and a manual search of the reference sections of articles identified following the electronic search. Key search terms included a combination of iron status and obesity as well as hepcidin and obesity. Search limiters included publication between the years 1960–2010, human studies, and English language. Exclusion criteria included review articles or commentaries, studies focused on pregnant or lactating women, studies conducted in surgical patients not undergoing a bariatric procedure, and studies in which ferritin was the only marker of iron status. Figure 1 details the article attrition and the number of publications included at each step of the vetting process. Briefly, the electronic search of iron status and obesity returned a total of 159 articles and the search of obesity and hepcidin returned 14 articles. The titles and abstracts were reviewed by the primary author for possible inclusion. In the event that abstracts did not allow for a clear decision regarding inclusion or exclusion, the full manuscript was reviewed. Following the primary search of iron status and obesity, a total of 32 articles were included and 127 articles excluded. Following the primary search of obesity and hepcidin a total of 8 articles were included and 6 excluded; all of the articles meeting the inclusion criteria were duplicates from the iron status and obesity search and were therefore dropped. Manual assessment of the reference sections from the papers retrieved from the electronic searches identified an additional 10 articles. In total, 42 articles met the inclusion criteria.
Figure 1.
Flow chart describing the selection process for obesity with iron status and obesity with hepcidin-related articles
Iron Regulation
Iron is an essential element that acts as a key component of oxygen-carrying proteins, is a vital player in cellular metabolism, and is essential to cell growth and differentiation (1). Tight regulation of iron is necessary because iron is highly toxic to cells and humans lack a regulated pathway to excrete large amounts (12). A small amount of iron, approximately 1–2 milligrams, is lost by the body on a daily basis (13). These small, daily, ordinary losses occur through the shedding of intestinal enterocytes, sweat, fecal blood loss, and skin sloughing (3). Women of reproductive age can also excrete significant amounts of iron through monthly menstrual blood losses (14). Effective communication between key sites of iron utilization, absorption, and storage at both the cellular and systemic level is necessary to maintain appropriate iron balance (15).
Within the cell, iron levels are sensed by two iron regulatory proteins (IRPs): IRP-1 and 2 (16). When cytoplasmic iron is low, IRPs bind to the iron regulatory element (IRE) sequences in the mRNAs of iron-regulated proteins including transferrin receptor and ferritin (1). Binding of IRPs to the IRE in the 3’ region of mRNA results in increased mRNA stability and increased protein synthesis. Conversely, when cytoplasmic iron is adequate, binding of IRPs to the IRE in the 5’ region of the mRNA occurs, which results in translational inhibition and decreased protein synthesis. Therefore, if extracellular iron concentrations are within the normal range, iron homeostasis within each cell is maintained by the IRE/IRP system and its effects on protein production (1).
Systemic iron regulation ensures a stable concentration of the iron-transferrin complex in plasma and extracellular fluid, and is accomplished by maintaining the major iron flows into plasma (1). Iron can be introduced into the plasma via three major routes: (1) absorption of dietary iron via the enterocytes of the proximal duodenum; (2) release of stored iron from the hepatocytes; or (3) the release of stored iron from the reticuloendothelial macrophages. These three different cell types use the same membrane iron exporter, Fpn, for the release of iron into plasma. Therefore, obliteration of Fpn at any one of these key sites results in iron sequestration inside these cells and diminished iron bioavailability (1, 3).
Regulation of Systemic Iron Metabolism by Hepcidin
Hepcidin is a small peptide hormone that functions as both the homeostatic regulator of systemic iron metabolism and mediator of host defense and inflammation, and is measurable in human urine, plasma, and serum (1–3, 17). Sensing of circulating iron and iron stores is thought to occur in the liver, which is the primary site of hepcidin production and secretion (1, 4). Hepcidin is also produced to a lesser degree in the adipose tissue, heart, placenta, and kidneys although in vivo secretion and contribution to circulating levels from these sites is currently unknown (3). Also, production in the adipose tissue, heart, placenta, and kidneys is likely not regulated by body iron status but most likely by inflammation (IL-6 mediated) (3).
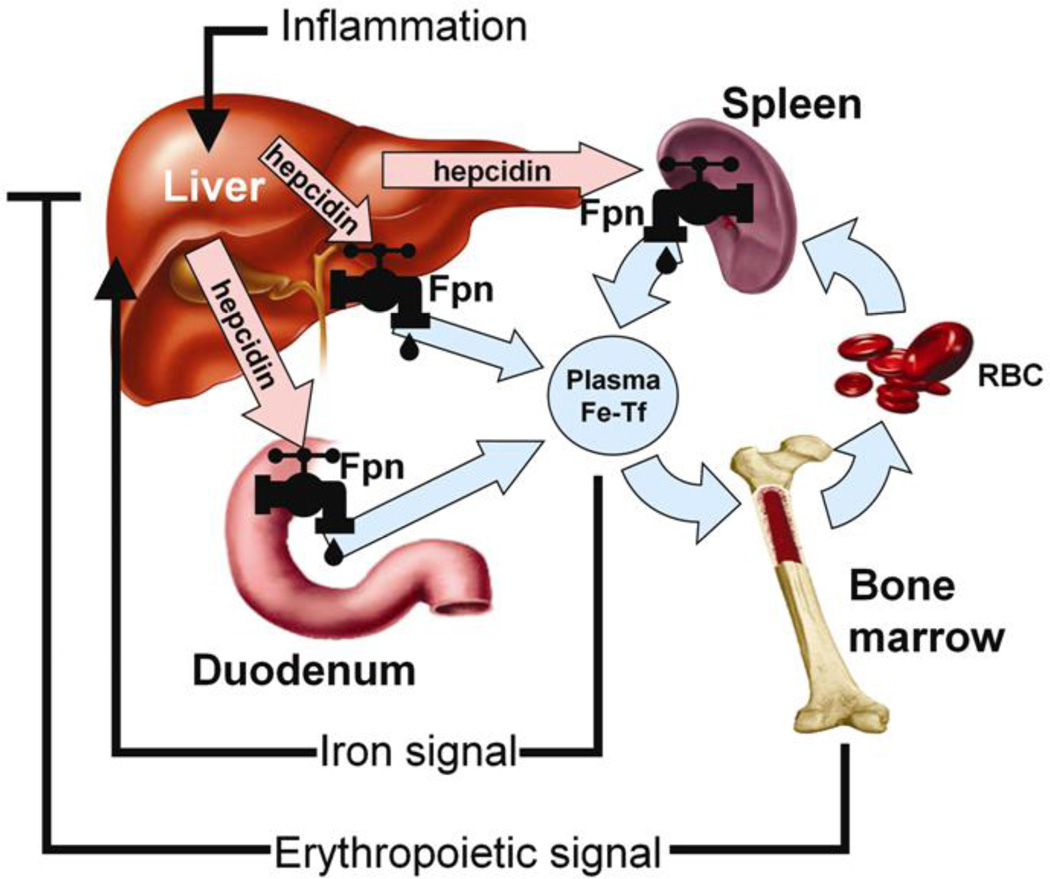
Hepcidin controls the flux of iron into plasma by regulating the Fpn exporter (Figure 2). When hepcidin binds to Fpn the two proteins are internalized and degraded within lysosomes (1). Hepcidin production by the liver is simultaneously regulated by iron status, anemia, hypoxia, and inflammation (1–5). Therefore, at any time, hepcidin expression can be determined by the interplay of pathways controlled by iron status, erythropoietic activity, and inflammation, and the relative strength of each of the individual signals (1–5). When body iron levels are elevated or inflammation or infection is present, liver hepcidin production is increased resulting in diminished Fpn expression. Down-regulation of Fpn results in reduced iron export from, and increased iron sequestration within intestinal enterocytes, hepatocytes and iron-recycling macrophages (1, 3, 4). When body iron levels are elevated hepcidin blocks absorption of iron from the diet. In infection, elevated hepcidin and consequent hypoferremia reduce dietary and cellular iron bioavailability; slowing microbial growth. Conversely, when body iron levels are depleted or anemia or hypoxia exists, hepcidin expression is minimal, allowing for increased dietary iron absorption and mobilization from body stores via active Fpn transporters. Researchers reported that hepcidin concentrations are inversely associated with iron absorption from supplemental and food-based non-heme iron sources in iron-replete women (18). Therefore, hepcidin concentrations markedly influence iron absorption and can impact the efficacy of iron repletion via supplemental or dietary sources. Unfortunately, at this time, assessment of hepcidin is limited in the clinical setting. However, the first immunoassay for human hepcidin was recently developed and validated but, at this time, is primarily available for research purposes (17). Based on this assay, the reference ranges for serum hepcidin are 29–254 ng/ml in healthy men and 17–286 ng/ml in healthy women while concentrations have been shown to vary significantly in those with ID, the ACD, and obesity (9–11, 17, 19).
Figure 2.
Hepcidin in iron homeostasis. Hepcidin is released into circulation by the liver, and its production is increased by higher circulating iron levels (Fe-Tf), or in an inflammatory state. Hepcidin acts to block iron efflux from various cell types by binding to the iron transporter ferroportin (Fpn), and downregulating its expression. In contrast, increases in erythropoietic activity or low circulating iron act to decrease hepcidin expression.
Abbreviations: Fpn, ferroportin-1; Fe-TF, transferrin-bound circulating iron; RBC, red blood cells
Original Source: Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122(2–3):78–86. Published by S. Karger AG, Basel, Switzerland.
Iron Deficiency: Hepcidin Expression and Clinical Assessment
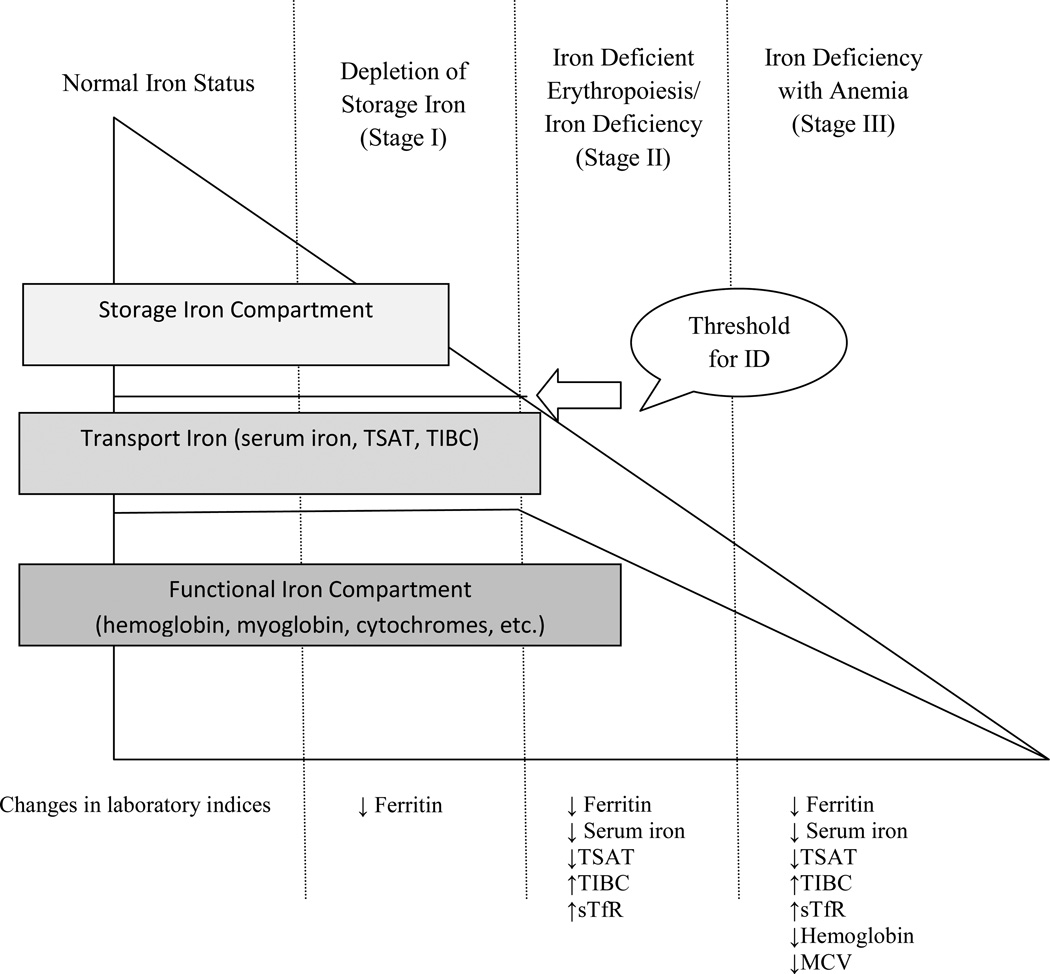
Iron deficiency remains the most common nutritional deficiency and cause of anemia worldwide. Populations in developing countries, premenopausal females, pregnant women, children, vegetarians, and frequent blood donors are largely affected by ID due to low dietary intake, inadequate bioavailable iron, increased iron demand required for growth and development, iron losses, and changes in blood volume (20–22). Iron deficiency is a condition in which there is inadequate iron to maintain normal function of bodily tissues (23). Iron deficiency develops in three stages beginning with depletion of iron stores (I), followed by diminished iron transport and iron deficient erythropoiesis referred to as ID (II), and lastly depletion of functional iron-containing proteins and enzymes referred to as ID with anemia (IDA) (III) (24, 25) (Figure 3). Iron deficiency with anemia is characterized by a defect in Hb synthesis which results in red blood cells that are small (microcytic) and contain a reduced amount of Hb (hypochromic) (26). Consequences of ID and IDA can include fatigue, weakness, decreased work capacity, palpitations, pallor, and alterations in immune function (27, 28).
Figure 3.
Stages of Advancing Iron Deficiency. The gradual reduction of different iron compartments and the concomitant changes seen in the laboratory parameters are presented schematically in relation to the three separate stages of advancing iron deficiency*
Abbreviations: ID, iron deficiency; TSAT, transferrin saturation; TIBC, total iron binding capacity; sTfR, soluble transferrin receptor; MCV, mean corpuscular volume
*Adapted from: Suominen P, Punnonen K, Rajamaki A, Irjala K. Serum transferrin receptor and transferrin receptor-ferritin index identify healthy subjects with subclinical iron deficits. Blood. 1998;92(8):2934–2939.
In persons with ID and IDA, hepcidin is suppressed to very low or undetectable levels (8, 17) (Table 1). Pak et al. (29) demonstrated that suppression of hepcidin is not directly mediated by ID or anemia but appears to be mediated by an uncharacterized substance released during erythropoiesis. The low concentration of serum hepcidin observed in those with ID and IDA would allow for increased efflux of dietary iron from the basolateral enterocytes and iron from body stores via abundantly expressed Fpn exporters (15). In persons with ID and IDA, iron repletion via dietary food-stuffs or pharmacological sources is an efficacious choice for therapy as low hepcidin facilitates increased dietary iron absorption (12).
Table 1.
Distinguishing Features of Select Clinical Parameters Related to Iron Status in Persons with Iron Deficiency, Iron Deficiency with Anemia, the Anemia of Chronic Disease, the Anemia of Chronic Disease with coexistent Iron Deficiency/Iron Deficiency Anemia, and Obesity*
| ID | IDA | ACD | ACD + ID/IDA |
Obesity | |
|---|---|---|---|---|---|
| Select Hematologic Parameters | |||||
| Hemoglobin Concentration (g/l) Threshold for IDA: 11.0 g/dl, 12–23 month children 11.1 g/dl, 2–5 year old children 11.5 g/dl, 5–8 year old children 11.9 g/dl, 8–12 year old children 11.8 g/dl, 12–14 year old females 12.0g/dl, 15 + year old females 12.5 g/dl, 12–14 year old males 13.3 g/dl, 15–17 year old males 13.5 g/dl, 18+ year old males |
↔ | ↓ | ↓ | ↔ ↓ | ↔ |
| Mean Cell Volume (fl) Threshold for IDA (all genders): < 77 fl, 1–2 years old < 79 fl, 3–5 years old < 80 fl, 6–11 years old < 82 fl, 12–15 years old > 85 fl, >15 years old |
↔ | ↓ | ↔ ↓ | ↔ ↓ | ↔ |
| Select Biochemical Parameters of Iron Status | |||||
| Serum Ferritin Concentration Threshold for IDA (all genders): ≤ 15µg/l, > 6 months old |
↓ | ↓ | ↔ ↑ | ↔ ↑ | ↔ ↑ |
| Serum Iron Concentration (µg/dl) Reference Ranges (adults only): 40–150 µg/dl, adult female 50–160 µg/dl, adult male |
↓ | ↓ | ↓ | ↓ | ↓ |
| Transferrin Saturation (%) Threshold for IDA (all ages/genders): < 16% |
↓ | ↓ | ↔ ↓ | ↓ | ↓ |
| Total Iron Binding Capacity Reference Ranges (all genders): 250–400 µg/dl, 4 months-10 years old 240–450 µg/dl, 11+ years old |
↑ | ↑ | ↔ ↓ | ↔ ↑ | ↔ ↑ |
| Soluble Transferrin Receptor Concentration† Reference Ranges (adults only) Immunoturbidometric method 2.2 – 5.0 mg/L, adult male 1.9 – 4.4 mg/L, adult female ELISA method 8.7–28.1 nmol/L, adults |
↑ | ↑ | ↔ ↓ | ↑ | ↑ |
| Other Select Biochemical Parameters | |||||
| Inflammatory Cytokine Concentrations (including IL-6, TNF-α, and CRP) |
↔ | ↔ | ↑ | ↑ | ↑ |
| Serum Hepcidin Concentration (ng/ml)† Reference range (adults only): ELISA method 29–254 ng/ml males 17–286 ng/ml females |
↓ | ↓ | ↑ | ↔ ↑ | ↔ ↑ |
Abbreviations: ↓ decreased; ↑ increased; ↔ within normal limits
ID, iron deficiency; IDA, iron deficiency with anemia, ACD, anemia of chronic disease; decreased; ELISA, enzyme-linked immunosorbent assay; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; CRP, C-reactive protein
Adapted from Wians FH,Jr, Urban JE, Keffer JH, Kroft SH. Discriminating between iron deficiency anemia and anemia of chronic disease using traditional indices of iron status vs transferrin receptor concentration. Am J Clin Pathol. 2001;115(1):112–118.
References ranges are not standardized and vary depending on the methodology and assay used.
Sources: Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency anemia in the United States. Morbidity and Mortality Weekly Report. 1998: 47:1–29; Yee DL, Bollard CM, Geaghan SM. Appendix: Normal Blood Values: Selected Reference Values for Neonatal, Pediatric, And Adult Populations. In: Hoffman R, Benz EJ, Shattil SS, eds. Hematology: Basic Principles and Practice. 5th ed. Philadelphia, Pa: Elsevier Churchill Livingstone; 2008: chapter 164; National Health and Nutrition Examination Survey Laboratory Procedures Manual for Serum Iron. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/lab_methods_07_08.htm. Accessed June 6, 2011; National Health and Nutrition Examination Survey Laboratory Procedures Manual for Soluble Transferrin Receptor. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/lab_methods_07_08.htm. Accessed June 6, 2011; Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–4297.
In healthy populations free of inflammatory disorders, chronic disease, parasitic infection, or obesity, the assessment of iron status includes evaluation of iron stores, circulating iron, and hematological parameters. The biochemical markers commonly used to assess iron stores and circulating iron include: ferritin, serum iron, total iron binding capacity (TIBC), and percent saturation of transferrin (TSAT). Typical hematological indices include, but are not limited to, Hb and mean corpuscular volume (MCV) (30). In conjunction with the more traditional biochemical and hematological indicators, soluble transferrin receptor (sTfR) can be used to detect early changes in iron status. Soluble transferrin receptor is generated by the cleavage of the cell-bound transferrin receptor, which is essential for the uptake of diferric transferrin, and is sensitive to the delivery of iron to bone marrow and tissue and is also an indicator of erythrpoetic activity (31). Soluble transferrin receptor is an indicator of iron status only when iron stores are depleted and no underlying cause of abnormal erythropoiesis exists (32). Soluble transferrin receptor is upregulated in ID and IDA due an increased abundance of transferrin receptors on the cell surface competing for available iron (33). It is important to note that no single test should be used to diagnose ID or IDA (23, 25). As depicted in Figure 3, the biochemical indices can detect early changes in iron status while the hematological markers typically demonstrate abnormality after anemia has developed (25) Therefore, several laboratory markers should be used in conjunction to properly assess iron status because each test assesses a different aspect of iron metabolism and the result of one test may not agree with the result of another test given the stage of ID (Figure 3) (23, 25)
Anemia of Chronic Disease: Hepcidin Expression and Clinical Assessment
Although not related to inadequate dietary iron intake or increased iron demand for specific biological processes, persons suffering from acute and chronic infection, parasitic infestation, inflammatory disorders, neoplastic diseases, trauma, and critical illness commonly suffer from alterations in iron metabolism referred to as the ACD or anemia of inflammation (34). The ACD is a normochromic, microcytic anemia that is thought to be a consequence of the host defense response mediated by inflammatory cytokines [interleukin-6 (IL-6), interleukin-1 (IL-1), and tumor necrosis factor-α (TNF-α)] that evolved to deprive bacteria of iron (1, 6, 35–37). The major pathophysiological processes underlying the ACD include iron-restriction, a blunted response to erythropoietin, and decreased red blood cell half-life (36). The ACD remains one of the most common syndromes encountered clinically (6).
The ACD is characterized by hypoferremia and anemia despite adequate iron stores and the presence of iron in bone marrow (38). Thus, anemia develops due to impaired mobilization of iron from diet and stores rather than inadequate dietary intake (6). Several animal and human studies suggest that inflammation leads to hypoferremia and decreased iron bioavailability and ultimately the ACD through an IL-6-mediated increase in hepcidin production which would diminish Fpn expression at the intestinal enterocytes and body iron stores (1, 2, 6). Elevated serum and urinary hepcidin levels have been observed in several conditions associated with the ACD including multiple myeloma and chronic kidney disease (17). As a result of elevated hepcidin concentrations, oral iron supplementation, in persons with the ACD, may have minimal impact on repletion efforts (39). In most cases, treatment of the underlying inflammatory condition can resolve the ACD. However, often times, the underlying inflammatory condition cannot be resolved. In such cases, a hepcidin antagonist could be an efficacious therapy to treat the ACD (1), however, such therapy is not currently available. Interestingly, individuals with concomitant ACD and ID/IDA have shown positive response to oral iron supplementation and subsequent improvement in Hb (40). These findings suggest that persons with a mixed anemia (ACD and ID/IDA), may have lower hepcidin levels (due to the simultaneous regulation by iron status, anemia, hypoxia, and inflammation), compared to that observed in individuals suffering exclusively with the ACD. The negative feedback on liver hepcidin expression due to the coexistent ID/IDA may allow for some dietary iron mobilization resulting in the iron repletion observed in these individuals. Further studies would be needed to confirm this hypothesis.
Without the selection of the most appropriate laboratory indices, the ACD may be misdiagnosed as ID/IDA, since both conditions share several common characteristics, including low serum iron and Hb (Table 1). Additionally, both conditions may coexist making diagnosis that much more challenging. In persons with the ACD (unlike ID/IDA) ferritin levels may be normal or even elevated and may reflect both the storage of iron within cells and an increase in ferritin as an acute phase reactant (41). When the ACD and ID/IDA co-exist, ferritin levels may remain elevated secondary to the inflammatory process and can be misleading of actual cellular iron levels (Table 1) (42). Therefore, the sole use of serum ferritin to accurately assess iron status in chronically ill or inflamed individuals is inadequate (43). However, coupling serum ferritin with the measurement of another acute phase reactant, such as CRP, can help to indicate whether underlying inflammation exists and will help to explain elevated serum ferritin despite underlying ID (23). The World Health Organization Consultation Panel on Assessing Iron Status of Populations (23) proposed the use of both serum ferritin and sTfR as the best approach to measuring the iron status of populations. Soluble transferrin receptor is less affected by the acute phase response (23) and is therefore considered to be a more useful clinical marker to assess iron status in chronically ill and inflamed individuals. Soluble transferrin receptor can be used to differentiate between ID, the ACD, and conditions in which ID/IDA and the ACD coexist (Table 1) (44, 45). In ID where transferrin receptor expression is increased due to greater cellular iron demand, sTfR concentrations are elevated, whereas in ACD, sTfR is often not elevated due to adequate iron stores (46). In the case of a “mixed anemia”, in which both ACD and ID/IDA coexist, sTfR has been shown to be elevated (Table 1) (47–49). However, it is important to note that some studies have reported that sTfR may not be useful in identifying ID in chronic conditions in which erythropoiesis is impacted by cytokines (50). In addition to sTfR, the ACD and ID can also be distinguished from one another by assessing TIBC, inflammatory markers such as IL-6, C-reactive protein (CRP) and TNF-α, as well as serum hepcidin (Table 1) (17, 30, 36, 51). Another, more conclusive, but relatively uncommon clinical approach to differentiate between ID and the ACD would be to examine bone marrow aspirates for the presence (ACD) or absence of iron (ID) using Perl’s Prussian Blue stain (52).
Obesity: Iron Status, Absorption, Hepcidin Ex pression, and Clinical Features
Obesity and Iron Depletion
In addition to the traditional populations at risk for ID there is an expanding body of literature demonstrating that obesity has an important role in modifying iron homeostasis. As early as the 1960’s, researchers observed a strong correlation between decreased serum iron and increased adiposity in adolescents (53, 54). Decades later, results from National Health and Nutritional Examination Survey III showed that overweight children and adolescents were two times more likely to be ID, based on two of three abnormal clinical indicators of iron status (TSAT, ferritin, and erythrocyte protoporphyrin), than those of a normal weight (55). Similar results were reported in a cross-sectional study of 321 Israeli children and adolescents. Those with a body mass index (BMI) above the 85th percentile were 1.75 times more likely to have decreased serum iron levels than those below this threshold (56). In obese Iranian children between 11–17 years of age, ID, defined as serum ferritin <12 ng/ml and TSAT <16%, was three times more prevalent compared to those of normal weight (57). Shi et al. (58) reported that IDA, defined as Hb < 12.0 g/dl and <13.0 g/dl for boys aged 14 years and older, was higher in obese (26.3%) compared to normal weight children (19.0%). In another study, ID was significantly more prevalent in overweight and obese U.S. adolescent females and was positively associated with BMI and inflammation (as measured by CRP) but not associated with race, age, dietary iron intake, years since beginning menstruation, or physical activity (59).
The relationship between obesity and iron status has also been explored in adults. Micozzi and colleagues (60) reported that serum iron was significantly lower in the highest (15.9 µmol/l) compared to lowest (18.6 µmol/l) quartile of BMI in a large cohort of women; no differences were observed in m en. Also, in a study of obese adult males, serum iron concentrations were comparable to that of normal weight controls (61). Ausk and Ioannou (62) found that overweight and obesity was associated with lower serum iron and TSAT; similarly overweight was found to be associated with low transferrin saturation in a group of South African women (63). Chambers and colleagues (64) found a significant inverse association between central (r=−0.19; p<0.05) and total fat mass (r=−0.19; p<0.05) with serum iron concentrations in Hispanic women; although no significant associations were observed among men or women from other ethnic groups. In another study, anemia, defined as Hb < 12.0 g/dl, was significantly more common in women with a BMI > 25 kg/m2 compared to women with a lower BMI (65). When the nutritional status of obese men and women was assessed prior to bariatric surgery, decreased levels of both serum iron and Hb were observed (66, 67). A case-control study of obese and lean postmenopausal women found obese women had significantly higher sTfR levels (Obese: 1.38 mg/dl vs. Lean: 1.16 mg/dl; p <0.001), indicative of ID, and BMI was positively correlated with log sTfR (r=0.48; p<0.001) (68). Yanoff and colleagues (44) reported mean serum iron (obese: 75.8 µg/dl vs. non-obese: 86.5 µg/dl), MCV (obese: 85.9 fl vs. non-obese: 88.0 fl), and TSAT (obese: 20.3% vs. non-obese: 23.0) were significantly lower, while mean sTFR (obese:22.6 nmol/l vs. non-obese 21.0 nmol/l), CRP (obese: 0.75 mg/dl vs. non-obese: 0.34 mg/dl), and ferritin (obese: 81.1 µg/l vs. non-obese: 57.6 µg/l) were significantly higher in obese men and women. They hypothesized that the increased prevalence of ID in obese individuals may be associated with increased hepcidin expression due to chronic low-grade inflammation. Also, Menzie and colleagues (69) determined that obesity-related hypoferremia in adults was not associated with differences in reported intake of heme and non-heme iron or intake or other dietary factors that can affect iron absorption including vitamin C and calcium.
Obesity and Dietary Iron Absorption
The association between excess weight, iron status, and iron absorption has been elegantly assessed in women and children from transition countries (70). Confirming previous studies, poorer iron status was notably more common in heavier participants. Independent of iron status, both BMI and inflammation (as measured by CRP) were negatively correlated with absorption of isotopically-labeled iron from fortified foods. Additionally, those with greater adiposity were unable to improve their iron status by dietary means as compared to lean controls. Although hepcidin was not measured, it was suggested by the authors that inflammation-induced hepcidin was likely causing a decrease in dietary iron absorption by diminishing the expression of Fpn located on the basolateral membrane of intestinal enterocytes thus impairing dietary repletion efforts.
Obesity and Hepcidin
A seminal paper by Bekri et al. (7) reported that hepcidin mRNA expression was significantly higher in visceral and subcutaneous adipose tissue from obese compared to lean pre-menopausal women and visceral and subcutaneous adipose hepcidin mRNA expression was positively correlated with BMI and IL-6. Also, ex vivo protein expression of hepcidin was detected in the subcutaneous and visceral adipose tissue explants from these women. The authors hypothesized that hepcidin secreted by the sizeable subcutaneous and visceral adipose tissue mass in obesity may be associated with the alterations in iron status observed in these individuals. However, the ability of subcutaneous or visceral adipose tissue to secrete bioactive hepcidin, in vivo, was not assessed and remains unknown. Furthermore, serum or plasma hepcidin levels were not measured or compared between the two groups of women. Recently, several researchers have demonstrated that serum hepcidin is significantly elevated in obese compared to lean women and children (8–11). One study reported that overweight children have higher circulating serum hepcidin and poorer iron status, despite similar dietary iron intake, when compared to normal weight children (10). Serum hepcidin was positively correlated with BMI and body iron but surprisingly no relationship was observed with the inflammatory markers including CRP, IL-6 and leptin. In another study, overweight children were found to have higher serum hepcidin and lower serum iron and TSAT compared to normal weight children and serum hepcidin was inversely correlated with iron absorption and positively with serum leptin (11). Both groups concluded that ID is likely due to hepcidin-mediated reduction in iron absorption and/or sequestration, and that increased serum hepcidin levels may be due in part to subcutaneous and visceral adipose tissue secretion of the protein as well as increased liver hepcidin production mediated by inflammation. Extending the literature, a recent study reported that serum hepcidin was positively correlated with liver (r= 0.61; p=0.04) and not significantly correlated with subcutaneous (r=0.01; p=0.95) or visceral (r=−0.19; p=0.43) adipose hepcidin mRNA expression in severely obese women, and liver hepcidin mRNA expression was 700 times greater than the mRNA expression in subcutaneous or visceral adipose tissue (8). Also, ferric iron accumulation, assessed using Perl’s Prussian blue stain, a classic method for assessing iron in tissue, was minimal in liver and subcutaneous and visceral adipose tissue suggesting negligible iron sequestration. However, a quantitative approach assessing tissue iron content is necessary to completely rule out iron sequestration. Based on their findings, the researchers suggest that adipose tissue may actually contribute very little to circulating serum hepcidin levels based on the low level of adipose compared to liver hepcidin mRNA expression although this has yet to be confirmed and also, reduced dietary absorption and not iron sequestration may be contributing to the alterations in iron metabolism observed in obesity. Although associations have been established linking obesity with ID, reduced dietary iron absorption, and increased serum hepcidin, it remains unclear, mechanistically, how weight gain impacts iron status. Therefore, it is important that additional studies investigating the mechanistic link between weight gain and depleted iron status are conducted.
Iron Status and Hepcidin Concentrations Following Weight Loss
Several studies have investigated the impact of diet-induced weight loss on iron status in both adults and children however, results are conflicting. Di Toro et al. (71) reported that hematological indices including serum iron, serum ferritin, and TSAT were within normal limits at baseline and no significant change was observed after a 13-week hypocaloric diet producing significant reduction of % ideal body weight in a group of obese children. Similarly in adults, diet-induced weight loss was associated with maintenance or improvement in serum iron, TSAT, and Hb (72, 73). However, Beard et al. (74) reported a significant decrease in TSAT after just one week of adherence to a very low calorie diet. Similarly, Kretsch et al. (75) reported a significant decrease in Hb, hematocrit, and red blood cell count after a 15 week calorie-restricted diet in a group of obese women.
The results detailing the impact of weight loss on iron status following bariatric surgery is also inconsistent. Several studies have reported increased incidence of ID, decreased Hb, as well as decreased iron absorption following gastric bypass surgery (76–80). Bariatric surgery is thought to negatively impact iron status through decreased gastric acid secretion (needed to convert dietary ferric iron to the ferrous state which is necessary for absorption); reduced tolerance to red meat; and decreased intestinal surface area for the absorption of iron (81). However, Anty and colleagues (82) reported that six months after bariatric surgery (94% gastric bypass), in a cohort of pre-menopausal women, TSAT was significantly higher (18% vs. 25%; p<0.0001). Also, several other studies have reported no significant change in iron status following gastric bypass or gastric banding surgery (83–85) While another study reported a significant decrease in sTfR concentrations at 18 months post-surgery (baseline: 1.40 vs. follow-up 1.27 mg/l; p <0.05) suggesting improvement in iron status (86).
Few studies have assessed change in both iron status and serum hepcidin following weight loss. One study (9), reported that weight loss six months post-restrictive bariatric surgery (gastric band and gastric sleeve procedures only), in pre-menopausal women, was associated with significantly lower serum hepcidin (baseline: 111.25 ng/ml vs. follow-up: 31.35 ng/ml; p<0.0001), CRP (baseline: 10.83 mg/l vs. follow-up: 5.71 mg/l; p<0.0001), IL-6 (baseline: 2.90 pg/ml vs. follow-up: 1.78 pg/ml; p=0.01) and sTfR (baseline: 29.97 nmol/l vs. follow-up: 23.08 nmol/l; p=0.001) and increased Hb (baseline: 12.10 g/dl vs. follow-up: 13.30 g/dl; p<0.0001). However, no significant changes in serum iron (baseline: 51.0 µg/ml vs. follow-up:57.0 µg/ml), TSAT (baseline: 13.0% vs. follow-up:16.5%) or ferritin (28.0 ng/ml vs. 25.0 ng/ml) were observed. Considering that hepcidin is the main determinant of the rate of iron absorption (18, 35, 87), the postoperative decrease in serum hepcidin likely accounted for the improvement in Hb and sTfR detected in these women, while notably total dietary iron intake (food and supplemental sources) remained constant (baseline: 19.75 mg vs. follow-up: 21.20 mg; p=0.10). Unfortunately, independent associations between change in weight, CRP or IL-6 with change in serum hepcidin, were not identified. The minimal change in serum iron, TSAT, and ferritin may reflect the excess weight and inflammation that persisted at six months post-surgery which could have impeded the replenishment of iron stores. Recently, Amato et al. (88) reported that following a six month weight loss program overweight children had significantly lower serum hepcidin (2.1 vs. 1.1 nmol/l; p=0.003), based on a cation-exchange chromatography method, and improved intestinal iron absorption. Iron absorption was assessed using an iron loading test in which a ferrous sulfate solution was provided after an overnight fast and change in serum iron concentration was measured at 120 minutes. Collectively, these findings suggest weight loss can lower serum hepcidin levels and improve iron absorption and iron status in obese persons. However, due to inconsistencies, it is important that prospective studies examining the impact of weight reduction (by dietary and surgical means) on iron status are conducted in obese adults and children so that the relationship between excess weight and iron regulation can be better understood.
Clinical Features
Although obesity is a chronically inflamed condition, the ID phenotype in obese individuals is vastly different from the ACD (Table 1). Unlike the ACD, obesity does not appear to be associated with iron sequestration or impaired mobilization of iron from stores but instead iron depletion (8). Also, obesity is associated with ID, and not IDA as indicated by Hb concentrations (adult range: 12.25– 13.7 g/dl; children and adolescents 9–17 years old 12.8–13.7 g/dl) (8, 44, 68, 89). Obesity-associated ID is more like a “mixed anemia” in which the clinical hallmarks of both ID and the ACD co-exist (Table 1) (44). Interestingly, serum hepcidin concentrations observed in obese women [median: 103.55 ng/ml (IQR:107.1)] are well below values observed in women with the ACD (range: 396–989 ng/ml), significantly higher than lean individuals with a similar degree of ID [median: 16.25 ng/ml (IQR: 30.45)], but well within the reference range for healthy, lean, iron-replete women (17–286 ng/ml) based on results from the same immunoassay (9, 10, 17, 18). The level of serum hepcidin observed in obesity could impede repletion efforts, but, may not completely obliterate Fpn expression allowing for some dietary iron mobilization as indicated by the iron absorption studies conducted in obese women and children (10, 70, 88). Hence, the amount of dietary iron absorbed may be adequate to allow tissues that need iron to maintain normal physiologic function, as indicated by the Hb concentrations (9, 44, 68, 89), but may not be adequate to maintain iron stores resulting in the ID observed in obese individuals. However, the clinical significance of obesity coupled with ID remains unknown and deserves further exploration.
Conclusions and Future Direction
After decades of evaluating and treating patients for iron disorders, the recent discovery of hepcidin requires the reexamination of iron metabolism. Hepcidin is the main regulator of systemic iron homeostasis and has an important role in the etiology of several iron-related disorders including the ACD. Importantly, hepcidin is markedly increased in obese compared to lean individuals suggesting that hepcidin may play an important role in the iron depletion observed in obesity. However, the exact mechanistic link between obesity, hepcidin and iron depletion and the clinical significance of obesity coupled with ID remains unknown suggesting the urgent need for research in this area (90, 91). Future studies should include: a longitudinal assessment of the relationship between weight gain/loss on iron status; an experiment assessing the secretion of hepcidin from subcutaneous and visceral adipose tissue in lean and obese individuals; and an examination of the health impact of suffering from both nutritional comorbidities concurrently. Furthermore, variations in dietary iron requirements based on BMI have not been explored and are needed given the burgeoning obesity epidemic. For now, understanding hepcidin’s role in iron metabolism coupled with the proper selection and interpretation of the biochemical, hematological, and inflammatory assays clinically available to aid in the assessment of iron status can provide clinicians with insight into the etiology of the iron disturbance, type of anemia, degree of iron depletion, and potential treatment options. Finally, it is important that medical professionals recognize obesity as a non-traditional risk factor for ID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lisa Tussing-Humphreys, US Department of Agriculture/Agricultural Research Service (USDA/ARS), Baton Rouge, LA.
Cenk Pustacioglu, Department of Kinesiology and Nutrition, University of Illinois at Chicago.
Elizabeta Nemeth, Division of Pulmonary and Critical Care Medicine, University of California at Los Angeles.
Carol Braunschweig, Department of Kinesiology and Nutrition, University of Illinois at Chicago.
References
- 1.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 2.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 3.Collins JF, Wessling-Resnick M, Knutson MD. Hepcidin regulation of iron transport. J Nutr. 2008;138(11):2284–2288. doi: 10.3945/jn.108.096347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122(2–3):78–86. doi: 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darshan D, Anderson GJ. Interacting signals in the control of hepcidin expression. Biometals. 2009;22(1):77–87. doi: 10.1007/s10534-008-9187-y. [DOI] [PubMed] [Google Scholar]
- 6.Means RT., Jr Hepcidin and anaemia. Blood Rev. 2004;18(4):219–225. doi: 10.1016/S0268-960X(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 7.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I, Saint-Paul MC, Huet PM, Sadoul JL, Gugenheim J, Srai SK, Tran A, Le Marchand-Brustel Y. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131(3):788–796. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Guzman G, Holterman AX, Braunschweig C. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity (Silver Spring) 2009;18(7):1449–1456. doi: 10.1038/oby.2009.319. [DOI] [PubMed] [Google Scholar]
- 9.Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Holterman AX, Galvani C, Ayloo S, Vitello J, Braunschweig C. Decreased serum hepcidin and improved functional iron status 6 months after restrictive bariatric surgery. Obesity (Silver Spring) 2010;18(10):2010–2016. doi: 10.1038/oby.2009.490. [DOI] [PubMed] [Google Scholar]
- 10.Aeberli I, Hurrell RF, Zimmermann MB. Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. Int J Obes (Lond) 2009;33(10):1111–1117. doi: 10.1038/ijo.2009.146. [DOI] [PubMed] [Google Scholar]
- 11.del Giudice EM, Santoro N, Amato A, Brienza C, Calabro P, Wiegerinck ET, Cirillo G, Tartaglione N, Grandone A, Swinkels DW, Perrone L. Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J Clin Endocrinol Metab. 2009;94(12):5102–5107. doi: 10.1210/jc.2009-1361. [DOI] [PubMed] [Google Scholar]
- 12.Leong WI, Lonnerdal B. Hepcidin, the recently identified peptide that appears to regulate iron absorption. J Nutr. 2004;134(1):1–4. doi: 10.1093/jn/134.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Hunt JR, Zito CA, Johnson LK. Body iron excretion by healthy men and women. Am J Clin Nutr. 2009;89(6):1792–1798. doi: 10.3945/ajcn.2009.27439. [DOI] [PubMed] [Google Scholar]
- 14.Hallberg L, Hulthen L, Garby L. Iron stores and haemoglobin iron deficits in menstruating women. Calculations based on variations in iron requirements and bioavailability of dietary iron. Eur J Clin Nutr. 2000;54(8):650–657. doi: 10.1038/sj.ejcn.1601069. [DOI] [PubMed] [Google Scholar]
- 15.Steele TM, Frazer DM, Anderson GJ. Systemic regulation of intestinal iron absorption. IUBMB Life. 2005;57(7):499–503. doi: 10.1080/15216540500149904. [DOI] [PubMed] [Google Scholar]
- 16.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: Molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 17.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 18.Young MF, Glahn RP, Ariza-Nieto M, Inglis J, Olbina G, Westerman M, O'Brien KO. Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women. Am J Clin Nutr. 2009;89(2):533–538. doi: 10.3945/ajcn.2008.26589. [DOI] [PubMed] [Google Scholar]
- 19.Dallalio G, Fleury T, Means RT. Serum hepcidin in clinical specimens. Br J Haematol. 2003;122(6):996–1000. doi: 10.1046/j.1365-2141.2003.04516.x. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370(9586):511–520. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 21.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1 Suppl):257S–264S. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann MB, Chaouki N, Hurrell RF. Iron deficiency due to consumption of a habitual diet low in bioavailable iron: A longitudinal cohort study in Moroccan children. Am J Clin Nutr. 2005;81(1):115–121. doi: 10.1093/ajcn/81.1.115. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization, Centers for Disease Control. Assessing the iron status of populations: Report of a joint WHO/CDC Technical Consultation on the assessment of iron status at the population level. [Accessed May 4, 2011]; http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/en/index.html.
- 24.Johnson MA. I ron: Nutrition monitoring and nutrition status assessment. J Nutr. 1990;120(Suppl 11):1486–1491. doi: 10.1093/jn/120.suppl_11.1486. [DOI] [PubMed] [Google Scholar]
- 25.Suominen P, Punnonen K, Rajamaki A, Irjala K. Serum transferrin receptor and transferrin receptor-ferritin index identify healthy subjects with subclinical iron deficits. Blood. 1998;92(8):2934–2939. [PubMed] [Google Scholar]
- 26.Bainton DF, Finch CA. The diagnosis of iron deficiency anemia. Am J Med. 1964;37:62–70. doi: 10.1016/0002-9343(64)90212-8. [DOI] [PubMed] [Google Scholar]
- 27.Basta SS, Soekirman, Karyadi D, Scrimshaw NS. Iron deficiency anemia and the productivity of adult males in Indonesia. Am J Clin Nutr. 1979;32(4):916–925. doi: 10.1093/ajcn/32.4.916. [DOI] [PubMed] [Google Scholar]
- 28.Annibale B, Marignani M, Monarca B, Antonelli G, Marcheggiano A, Martino G, Mandelli F, Caprilli R, Delle Fave G. Reversal of iron deficiency anemia after helicobacter pylori eradication in patients with asymptomatic gastritis. Ann Intern Med. 1999;131(9):668–672. doi: 10.7326/0003-4819-131-9-199911020-00006. [DOI] [PubMed] [Google Scholar]
- 29.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wians FH, Jr, Urban JE, Keffer JH, Kroft SH. Discriminating between iron deficiency anemia and anemia of chronic disease using traditional indices of iron status vs transferrin receptor concentration. Am J Clin Pathol. 2001;115(1):112–118. doi: 10.1309/6L34-V3AR-DW39-DH30. [DOI] [PubMed] [Google Scholar]
- 31.Cook JD, Dassenko S, Skikne BS. Serum transferrin receptor as an index of iron absorption. Br J Haematol. 1990;75(4):603–609. doi: 10.1111/j.1365-2141.1990.tb07806.x. [DOI] [PubMed] [Google Scholar]
- 32.Cook JD, Flowers CH, Skikne BS. An assessment of dried blood-spot technology for identifying iron deficiency. Blood. 1998;92(5):1807–1813. [PubMed] [Google Scholar]
- 33.Clark SF. Iron deficiency anemia: Diagnosis and management. Curr Opin Gastroenterol. 2009;25(2):122–128. doi: 10.1097/MOG.0b013e32831ef1cd. [DOI] [PubMed] [Google Scholar]
- 34.Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev. 2002;16(2):87–96. doi: 10.1054/blre.2002.0193. [DOI] [PubMed] [Google Scholar]
- 35.Ganz T. Hepcidin--a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18(2):171–182. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 37.Jurado RL. Iron, infections, and anemia of inflammation. Clin Infect Dis. 1997;25(4):888–895. doi: 10.1086/515549. [DOI] [PubMed] [Google Scholar]
- 38.Cartwright GE. The anemia of chronic disorders. Semin Hematol. 1966;3(4):351–375. [PubMed] [Google Scholar]
- 39.Jensen NM, Brandsborg M, Boesen AM, Yde H, Dahlerup JF. Low-dose oral iron absorption test in anaemic patients with and without iron deficiency determined by bone marrow iron content. Eur J Haematol. 1999;63(2):103–111. doi: 10.1111/j.1600-0609.1999.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 40.Ahluwalia N, Lammi-Keefe CJ, Bendel RB, Morse EE, Beard JL, Haley NR. Iron deficiency and anemia of chronic disease in elderly women: A discriminant-analysis approach for differentiation. Am J Clin Nutr. 1995;61(3):590–596. doi: 10.1093/ajcn/61.3.590. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson BJ, Skikne BS, Simpson KM, Baynes RD, Cook JD. Serum transferrin receptor distinguishes the anemia of chronic disease from iron deficiency anemia. J Lab Clin Med. 1992;119(4):385–390. [PubMed] [Google Scholar]
- 42.Fitzsimons EJ, Brock JH. The anaemia of chronic disease. BMJ. 2001;322(7290):811–812. doi: 10.1136/bmj.322.7290.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavill I. Iron status as measured by serum ferritin: The marker and its limitations. Am J Kidney Dis. 1999;34(4 Suppl 2):S12–S17. doi: 10.1053/AJKD034s00012. [DOI] [PubMed] [Google Scholar]
- 44.Yanoff LB, Menzie CM, Denkinger B, Sebring NG, McHugh T, Remaley AT, Yanovski JA. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes (Lond) 2007;31(9):1412–1419. doi: 10.1038/sj.ijo.0803625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freixenet N, Remacha A, Berlanga E, Caixas A, Gimenez-Palop O, Blanco-Vaca F, Bach V, Baiget M, Sanchez Y, Felez J, Gonzalez-Clemente JM. Serum soluble transferrin receptor concentrations are increased in central obesity. Results from a screening programme for hereditary hemochromatosis in men with hyperferritinemia. Clin Chim Acta. 2009;400(1–2):111–116. doi: 10.1016/j.cca.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Looker AC, Loyevsky M, Gordeuk VR. Increased serum transferrin saturation is associated with lower serum transferrin receptor concentration. Clin Chem. 1999;45(12):2191–2199. [PubMed] [Google Scholar]
- 47.Martini A, Ravelli A, Di Fuccia G, Rosti V, Cazzola M, Barosi G. Intravenous iron therapy for severe anaemia in systemic-onset juvenile chronic arthritis. Lancet. 1994;344(8929):1052–1054. doi: 10.1016/s0140-6736(94)91710-8. [DOI] [PubMed] [Google Scholar]
- 48.Suominen P, Mottonen T, Rajamaki A, Irjala K. Single values of serum transferrin receptor and transferrin receptor ferritin index can be used to detect true and functional iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum. 2000;43(5):1016–1020. doi: 10.1002/1529-0131(200005)43:5<1016::AID-ANR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Das Gupta A, Abbi A. High serum transferrin receptor level in anemia of chronic disorders indicates coexistent iron deficiency. Am J Hematol. 2003;72(3):158–161. doi: 10.1002/ajh.10260. [DOI] [PubMed] [Google Scholar]
- 50.Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta. 2003;329(1–2):9–22. doi: 10.1016/s0009-8981(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 51.Cook JD, Skikne BS. Iron deficiency: Definition and diagnosis. J Intern Med. 1989;226(5):349–355. doi: 10.1111/j.1365-2796.1989.tb01408.x. [DOI] [PubMed] [Google Scholar]
- 52.Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: An overview. J Gen Intern Med. 1992;7(2):145–153. doi: 10.1007/BF02598003. [DOI] [PubMed] [Google Scholar]
- 53.Wenzel BJ, Stults HB, Mayer J. Hypoferraemia in obese adolescents. Lancet. 1962;2(7251):327–328. doi: 10.1016/s0140-6736(62)90110-1. [DOI] [PubMed] [Google Scholar]
- 54.Seltzer CC, Mayer J. Serum iron and iron-binding capacity in adolescents. II. Comparison of obese and nonobese subjects. Am J Clin Nutr. 1963;13:354–361. doi: 10.1093/ajcn/13.6.354. [DOI] [PubMed] [Google Scholar]
- 55.Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M. Overweight children and adolescents: A risk group for iron deficiency. Pediatrics. 2004;114(1):104–108. doi: 10.1542/peds.114.1.104. [DOI] [PubMed] [Google Scholar]
- 56.Pinhas-Hamiel O, Newfield RS, Koren I, Agmon A, Lilos P, Phillip M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord. 2003;27(3):416–418. doi: 10.1038/sj.ijo.0802224. [DOI] [PubMed] [Google Scholar]
- 57.Moayeri H, Bidad K, Zadhoush S, Gholami N, Anari S. Increasing prevalence of iron deficiency in overweight and obese children and adolescents (Tehran Adolescent Obesity Study) Eur J Pediatr. 2006;165(11):813–814. doi: 10.1007/s00431-006-0178-0. [DOI] [PubMed] [Google Scholar]
- 58.Shi Z, Lien N, Kumar BN, Dalen I, Holmboe-Ottesen G. The sociodemographic correlates of nutritional status of school adolescents in Jiangsu Province, China. J Adolesc Health. 2005;37(4):313–322. doi: 10.1016/j.jadohealth.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 59.Tussing-Humphreys LM, Liang H, Nemeth E, Freels S, Braunschweig CA. Excess adiposity, inflammation, and iron-deficiency in female adolescents. J Am Diet Assoc. 2009;109(2):297–302. doi: 10.1016/j.jada.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 60.Micozzi MS, Albanes D, Stevens RG. Relation of body size and composition to clinical biochemical and hematologic indices in US men and women. Am J Clin Nutr. 1989;50(6):1276–1281. doi: 10.1093/ajcn/50.6.1276. [DOI] [PubMed] [Google Scholar]
- 61.Ozata M, Mergen M, Oktenli C, Aydin A, Sanisoglu SY, Bolu E, Yilmaz MI, Sayal A, Isimer A, Ozdemir IC. Increased oxidative stress and hypozincemia in male obesity. Clin Biochem. 2002;35(8):627–631. doi: 10.1016/s0009-9120(02)00363-6. [DOI] [PubMed] [Google Scholar]
- 62.Ausk KJ, Ioannou GN. Is obesity associated with anemia of chronic disease? A population-based study. Obesity (Silver Spring) 2008;16(10):2356–2361. doi: 10.1038/oby.2008.353. [DOI] [PubMed] [Google Scholar]
- 63.Wolmarans P, Dhansay MA, Mansvelt EP, Laubscher JA, Benade AJ. Iron status of South African women working in a fruit-packing factory. Public Health Nutr. 2003;6(5):439–445. doi: 10.1079/PHN2003460. [DOI] [PubMed] [Google Scholar]
- 64.Chambers EC, Heshka S, Gallagher D, Wang J, Pi-Sunyer FX, Pierson RN., Jr Serum iron and body fat distribution in a multiethnic cohort of adults living in New York City. J Am Diet Assoc. 2006;106(5):680–684. doi: 10.1016/j.jada.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Hassan EO, el-Hussinie M, el-Nahal N. The prevalence of anemia among clients of family planning clinics in Egypt. Contraception. 1999;60(2):93–99. doi: 10.1016/s0010-7824(99)00066-9. [DOI] [PubMed] [Google Scholar]
- 66.Schweiger C, Weiss R, Berry E, Keidar A. Nutritional deficiencies in bariatric surgery candidates. Obes Surg. 2010;20(2):193–197. doi: 10.1007/s11695-009-0008-3. [DOI] [PubMed] [Google Scholar]
- 67.Flancbaum L, Belsley S, Drake V, Colarusso T, Tayler E. Preoperative nutritional status of patients undergoing Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg. 2006;10(7):1033–1037. doi: 10.1016/j.gassur.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Lecube A, Carrera A, Losada E, Hernandez C, Simo R, Mesa J. Iron deficiency in obese postmenopausal women. Obesity (Silver Spring) 2006;14(10):1724–1730. doi: 10.1038/oby.2006.198. [DOI] [PubMed] [Google Scholar]
- 69.Menzie CM, Yanoff LB, Denkinger BI, McHugh T, Sebring NG, Calis KA, Yanovski JA. Obesity-related hypoferremia is not explained by differences in reported intake of heme and nonheme iron or intake of dietary factors that can affect iron absorption. J Am Diet Assoc. 2008;108(1):145–148. doi: 10.1016/j.jada.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmermann MB, Zeder C, Muthayya S, Winichagoon P, Chaouki N, Aeberli I, Hurrell RF. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes (Lond) 2008;32(7):1098–1104. doi: 10.1038/ijo.2008.43. [DOI] [PubMed] [Google Scholar]
- 71.Di Toro A, Marotta A, Todisco N, Ponticiello E, Collini R, Di Lascio R, Perrone L. Unchanged iron and copper and increased zinc in the blood of obese children after two hypocaloric diets. Biol Trace Elem Res. 1997;57(2):97–104. doi: 10.1007/BF02778192. [DOI] [PubMed] [Google Scholar]
- 72.Weinsier RL, Bacon JA, Birch R. Time-calorie displacement diet for weight control: A prospective evaluation of its adequacy for maintaining normal nutritional status. Int J Obes. 1983;7(6):539–548. [PubMed] [Google Scholar]
- 73.Rodríguez-Rodríguez E, López-Sobaler AM, Andrés P, Aparicio A, Navia B, Ortega RM. Modification of iron status in young overweight/mildly obese women by two dietary interventions designed to achieve weight loss. Ann Nutr Metab. 2007;51(4):367–373. doi: 10.1159/000107680. [DOI] [PubMed] [Google Scholar]
- 74.Beard J, Borel M, Peterson FJ. Changes in iron status during weight loss with very-low-energy diets. Am J Clin Nutr. 1997;66(1):104–110. doi: 10.1093/ajcn/66.1.104. [DOI] [PubMed] [Google Scholar]
- 75.Kretsch MJ, Fong AK, Green MW, Johnson HL. Cognitive function, iron status, and hemoglobin concentration in obese dieting women. Eur J Clin Nutr. 1998;52(7):512–518. doi: 10.1038/sj.ejcn.1600598. [DOI] [PubMed] [Google Scholar]
- 76.Avinoah E, Ovnat A, Charuzi I. Nutritional status seven years after Roux-en-Y gastric bypass surgery. Surgery. 1992;111(2):137–142. [PubMed] [Google Scholar]
- 77.Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, Kalfarentzos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12(4):551–558. doi: 10.1381/096089202762252334. [DOI] [PubMed] [Google Scholar]
- 78.Ruz M, Carrasco F, Rojas P, Codoceo J , Inostroza J, Rebolledo A, Basfi-fer K, Csendes A, Papapietro K, Pizarro F, Olivares M, Sian L, Westcott JL, Hambidge KM, Krebs NF. Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90(3):527–532. doi: 10.3945/ajcn.2009.27699. [DOI] [PubMed] [Google Scholar]
- 79.Carr ND, Harrison RA, Tomkins A, Baughen R, Demmer S, Godfrey J, Clark CG. Vertical banded gastroplasty in the treatment of morbid obesity: Results of three year follow up. Gut. 1989;30(8):1048–1053. doi: 10.1136/gut.30.8.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25(11–12):1150–1156. doi: 10.1016/j.nut.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 81.Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11–12):1031–1037. doi: 10.1016/j.nut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 82.Anty R, Dahman M, Iannelli A, Gual P, Staccini-Myx A, Amor IB, Luciani N, Saint-Paul MC, Huet PM, Sadoul JL, Srai SK, Unwin R, Gugenheim J, Le Marchand-Brustel Y, Tran A, Bekri S. Bariatric surgery can correct iron depletion in morbidly obese women: A link with chronic inflammation. Obes Surg. 2008;18(6):709–714. doi: 10.1007/s11695-007-9276-y. [DOI] [PubMed] [Google Scholar]
- 83.Coupaye M, Puchaux K, Bogard C, Msika S, Jouet P, Clerici C, Larger E, Ledoux S. Nutritional consequences of adjustable gastric banding and gastric bypass: A 1-year prospective study. Obes Surg. 2009;19(1):56–65. doi: 10.1007/s11695-008-9571-2. [DOI] [PubMed] [Google Scholar]
- 84.Updegraff TA, Neufeld NJ. Protein, iron, and folate status of patients prior to and following surgery for morbid obesity. J Am Diet Assoc. 1981;78(2):135–140. [PubMed] [Google Scholar]
- 85.Gasteyger C, Suter M, Calmes JM, Gaillard RC, Giusti V. Changes in body composition, metabolic profile and nutritional status 24 months after gastric banding. Obes Surg. 2006;16(3):243–250. doi: 10.1381/096089206776116381. [DOI] [PubMed] [Google Scholar]
- 86.Ramalho R, Guimarães C, Gil C, Neves C, Guimarães JT, Delgado L. Morbid obesity and inflammation: A prospective study after adjustable gastric banding surgery. Obes Surg. 2009;19(7):915–920. doi: 10.1007/s11695-009-9848-0. [DOI] [PubMed] [Google Scholar]
- 87.Roe MA, Collings R, Dainty JR, Swinkels DW, Fairweather-Tait SJ. Plasma hepcidin concentrations significantly predict interindividual variation in iron absorption in healthy men. Am J Clin Nutr. 2009;89(4):1088–1091. doi: 10.3945/ajcn.2008.27297. [DOI] [PubMed] [Google Scholar]
- 88.Amato A, Santoro N, Calabro P, Grandone A, Swinkels DW, Perrone L, Miraglia Del Giudice E. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int J Obes (Lond) 2010;34(12):1772–1774. doi: 10.1038/ijo.2010.204. [DOI] [PubMed] [Google Scholar]
- 89.Fricker J, Le Moel G, Apfelbaum M. Obesity and iron status in menstruating women. Am J Clin Nutr. 1990;52(5):863–866. doi: 10.1093/ajcn/52.5.863. [DOI] [PubMed] [Google Scholar]
- 90.McClung JP, Karl JP. Iron deficiency and obesity: The contribution of inflammation and diminished iron absorption. Nutr Rev. 2009;67(2):100–104. doi: 10.1111/j.1753-4887.2008.00145.x. [DOI] [PubMed] [Google Scholar]
- 91.Zafon C, Lecube A, Simo R. Iron in obesity. An ancient micronutrient for a modern disease. Obes Rev. 2010;11(4):322–328. doi: 10.1111/j.1467-789X.2009.00638.x. [DOI] [PubMed] [Google Scholar]