Abstract
This study reports a new type of three-dimensional (3D) tissue model for studying interactions between cell types in collagen hydrogels. The aim was to create a 3D cell culture model containing separate cell populations in close proximity without the presence of a mechanical barrier, and demonstrate its relevance to modeling the axon growth-inhibitory cellular interfaces that develop in the central nervous system (CNS) in response to damage. This provides a powerful new tool to determine which aspects of the astroglial scar response and subsequent neuronal regeneration inhibition are determined by the presence of the other cell types. Astrocytes (CNS glia) and dissociated dorsal root ganglia (DRG; containing neurons and peripheral nervous system [PNS] glia) were seeded within collagen solution at 4°C in adjacent chambers of a stainless steel mould, using cells cultured from wild-type or green fluorescent protein expressing rats, to track specific populations. The divider between the chambers was removed using a protocol that allowed the gels to integrate without mixing of the cell populations. Following setting of the gels, they were maintained in culture for up to 15 days. Reciprocal astrocyte and neuronal responses were monitored using confocal microscopy and 3D image analysis. At DRG:astrocyte interfaces, by 5 days there was an increase in the number of astrocytes at the interface followed by hypertrophy and increased glial fibrillary acidic protein expression at 10 and 15 days, indicative of reactive gliosis. Neurons avoided crossing DRG:astrocyte interfaces, and neuronal growth was restricted to the DRG part of the gel. By contrast, neurons were able to grow freely across DRG:DRG interfaces, demonstrating the absence of a mechanical barrier. These results show that in a precisely controlled 3D environment, an interface between DRG and astrocyte cultures is sufficient to trigger reactive gliosis and inhibition of neuronal regeneration across the interface. Different aspects of the astrocyte response could be independently monitored, providing an insight into the formation of a glial scar. This technology has wide potential for researchers wishing to maintain and monitor interactions between adjacent cell populations in 3D culture.
Introduction
Tissue engineered cell culture models have the potential to provide powerful new tools for neuroscience research and the development of therapies. Recreating the three-dimensional (3D) spatial environment of the central nervous system (CNS) allows cells in vitro to behave more like their in vivo counterparts, providing robust and controllable model systems that mimic the cell biology present in the nervous system.1–3 Here we report a new type of 3D tissue model for studying interactions between cell types, and demonstrate its relevance to modeling the inhibitory interfaces that develop in the CNS in response to damage.
Neuronal growth following CNS damage is limited, resulting in permanent paralysis and loss of function in many patients. CNS neurons have the capacity to regenerate if a permissive environment is created, but a common finding of strategies aimed at bridging CNS lesions, including our work with biomaterial conduits,4,5 is that axons readily enter and traverse the bridging graft, but find difficulty in subsequently exiting the graft, or disassociating from engrafted cells, to re-enter host CNS parenchyma.6,7 An important feature of many current bridging devices aimed at spinal cord injury repair is that they contain or become populated by Schwann cells which accompany the neuronal regeneration. However, these peripheral nervous system (PNS) glia can trigger resident astrocytes to become reactive and growth inhibitory (forming a glial scar), preventing regenerating axons from crossing the Schwann cell - astrocyte transition zone.8,9 This graft/host cellular interface has similarities to the PNS/CNS interface of the dorsal root entry zone (DREZ).
Some success has been achieved in overcoming these cellular interfaces.10–12 However, research in this area will be facilitated by the development of appropriate experimental models in which to develop a better understanding of the formation and persistence of inhibitory interfaces in the CNS, and to develop new therapies to prevent interface formation or overcome the glial scar barrier.13,14 To understand the behavior of specific cell populations, in vitro models offer several advantages over whole animal studies when control over cellular components is required, and where real-time measurement of responses that cannot be monitored in vivo would be of benefit.1,3 While many two dimensional culture models of the glial scar have been developed,14–21 3D systems permit a similar level of control, manipulation and monitoring, yet they maintain cells in a more relevant spatial arrangement.22 Three dimensional coculture models that explore specific neuron-astrocyte interactions were initially developed more than 20 years ago,22 slice culture approaches have been used to investigate the role of extracellular matrix molecules,23 and 3D hydrogel systems have enabled the response of neural cells to exogenous forces24,25 and gradients of matrix stiffness26 to be assessed. The inhibitory interfaces that develop at the edges of a CNS lesion or repair site or the DREZ are 3D structures so recreating them in vitro should logically require a 3D culture approach. Further, it is clear that some of the limitations of previous culture models can be overcome using 3D matrices. Astrocytes on stiff matrices tend to adopt a reactive phenotype whereas in 3D hydrogels they are less reactive until stimulated,27 and neurons in culture also respond differently to two-dimensional (2D) and 3D environments.28,29
However, the compliant mechanical environment of hydrogel matrices can be problematic when trying to reduce variables in culture systems, since cells in such an environment will respond to relatively small mechanical cues,30–32 including the stiff surfaces of the surrounding mould.27 This means that approaches such as embedding cell-seeded conduits or even dorsal root ganglia (DRG) explants within astrocyte gels will provide mechanical cues that will introduce confounding factors. Making a 3D cell culture model that brings separate cell populations into close proximity without the presence of a mechanical interface is a challenge, but is necessary to isolate exactly which aspects of the glial response and the subsequent neuronal inhibition are determined by the presence of the other cell types.
The aim of this work was to develop novel methodology for engineering a 3D culture model that recreates the cellular interface which develops at the edge of a CNS lesion or the CNS/PNS boundary. Here we report that adjacent cell-seeded hydrogels integrated fully upon setting, providing no mechanical barrier to cells. This novel approach allowed astrocyte responses and neuronal behavior to be quantified, providing useful new insight into how each cell population responds to the presence of the other over time. Although we have developed this model for nervous system research, it is equally applicable to modeling interactions between a wide variety of cell types and is therefore suited to many different areas of research.
Methods
Astrocyte cultures
All experiments were performed according to the UK Animals (Scientific Procedures) Act (1986) and approved by the Open University animal ethics advisory group. Sprague–Dawley rats (a β-actin-green fluorescent protein reporter line or wild-type) were used from established in-house breeding colonies. Primary astrocyte cultures were prepared from postnatal 2-day-old rat cortices as previously reported.27 Cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with penicillin and streptomycin (100 U/mL and 100 mg/mL, respectively; Sigma) and 10% v/v fetal calf serum (Sigma) in 75 cm2 flasks (Greiner) coated with poly-D-lysine (Sigma). After 8 days in a humidified incubator at 37°C with 5% CO2: 95% air cells reached confluence, and then flasks were shaken at 150 rpm for 4 h to detach microglia and less adherent cells. Resulting cultures were 95% astrocytes and 5% microglia (as determined by immunoreactivity for glial fibrillary acidic protein [GFAP] and IB4 lectin, respectively). Cells were trypsinized, washed, and counted so that the correct densities could be calculated prior to seeding into collagen gels.
Dorsal root ganglia preparation and culture
DRG were dissected from adult rats (200–300 g). Nerve roots were stripped and DRGs incubated in collagenase (0.125%; Sigma) for 2 h at 37°C. Tissue was dissociated by trituration and washed twice by centrifugation with 25 mL of media to remove any remaining collagenase. Cell pellets were resuspended in DMEM and the resulting mixture of neurons and PNS glia was seeded within collagen gels.
3D interface culture construction
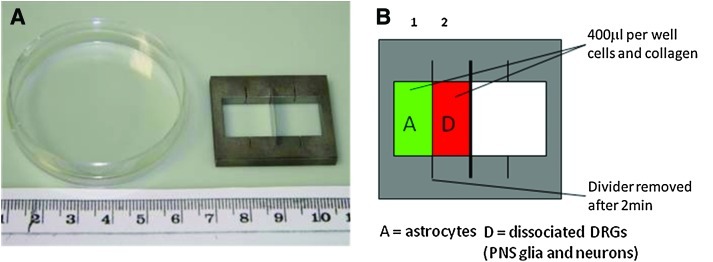
Interface cultures were prepared by simultaneously casting 400 μL collagen gels32 containing 3 million astrocytes per mL or 16 dissociated DRGs per mL in adjacent compartments of a stainless steel mould (Fig. 1). Gels were composed of 10% cell suspension in DMEM, 10% 10× minimum essential medium (MEM; Sigma), and 80% type I rat tail collagen (2 mg/mL in 0.6% acetic acid; First Link). The MEM and collagen were mixed together and neutralized using sodium hydroxide, assessed by color change of phenol red pH indicator; then the mixture was added to the cell suspension, mixed to ensure even distribution of cells throughout the suspension, and transferred to the mould. The two culture populations were separated by the presence of a 0.1 mm thick stainless steel divider which was removed after 2 min to allow gels to integrate. During the casting process the mould was maintained at 4°C until removal of the divider, whereupon it was incubated at room temperature for 10 min, then 37°C for 20 min. Controlling the temperature in this way ensured that the adjacent gels integrated prior to setting. In each integrated model, either the astrocyte or the DRG culture was generated using cells from green fluorescent protein (GFP) animals. After setting, interface cultures were carefully removed from the mould and maintained in a petri dish with 8 mL DMEM and incubated as before. Cultures were maintained for up to 15 days before fixing in 4% paraformaldehyde. In some experiments, control interface cultures were included which contained DRG cultures in both compartments.
FIG. 1.
Interface experimental set-up. (A) Photograph of stainless steel interface mould; one 0.1 mm thick stainless steel divider is shown; however, up to three dividers could be inserted into the frame. Two culture populations in collagen gels were seeded either side of a steel divider, which was then removed after 2 min to allow gels to integrate. (B) Diagrammatic representation of one astrocyte:DRG interface gel. DRG, dorsal root ganglia. Color images available online at www.liebertonline.com/tec
Immunofluorescence staining, microscopy, and image analysis
Antibody sources, dilutions, and immunofluorescence staining were carried out as previously described.27 Gels were stained for GFAP, CS56 and βIII tubulin, for astrocytic reactivity, chondroitin sulphate proteoglycans, and neurites, respectively. Hoechst 33258 was used to label cell nuclei. Confocal microscopy was performed using a Leica TCS SP5 confocal microscope (Leica Microsystems) to sample designated fields within the astrocyte and DRG compartments of the interface cultures.
Quantification of astrocyte cellular responses
Confocal fields for quantification of astrocytes were 400×400×40 μm (XYZ) with one image per 1 μm in the Z direction, three fields were sampled at the interface and three were sampled from a control region of the astrocyte compartment 5.5 mm away from the interface, according to a predefined protocol. The volumes of GFP labeling and GFAP and CS56 immunoreactivity were measured using Volocity image analysis software (Improvision, Perkin-Elmer). Automated analysis protocols were developed and cell number was determined by counting Hoechst stained nuclei, so that cell density could be assessed and GFP/GFAP volumes could be expressed per cell. For astrocyte:DRG interfaces, four independent interface gels were analyzed, from at least two separate cell preparations.
Quantification of neurite outgrowth
Confocal fields for quantification of neurons were 400×400×Z μm (XYZ) with one image per 1 μm in the Z direction. The Z displacement varied for neuronal quantification to include the full length of all neurites in each field. Five regions at the interface, and five regions 5.5 mm away from the interface were sampled according to a predefined protocol, ensuring that the orientation of the interface was aligned parallel to the edge of the field. The length of βIII-tubulin immunostained neurites was measured with Volocity Software (Improvision), the number of neurites per neuronal cell body was counted, and the angle of deviation of each neurite from growth perpendicular to the interface was calculated. The number of neurites which crossed the interface in each field was also assessed. For DRG:DRG interfaces three independent interface gels were analyzed from three separate cell preparations.
Statistical analysis
Data were analyzed using GraphPad Prism software (Version 4). Normality and quality of variance tests were performed on all data to determine which test was appropriate. A paired t-test was used with significance level 95% for comparison between “at the interface” and “away from the interface” data at comparable time points. For comparison between time points, an ANOVA with Tukey's post hoc test was performed with significance levels set at 95%. An unpaired t-test was used with significance level 95% for comparison of neuronal length, number of neurites per neuron, and crossing behavior of neurites at the interface. If variances of data sets were significantly different, then Welch's correction was applied. All values are indicated as mean±standard error of the mean. p-values were taken as an indicator of statistical significance as follows: *p<0.05, **p<0.01, and ***p<0.001 and where significant differences were present between two means in a figure these were indicated using a line.
Results
Methodology was optimized for the generation of 3D interface models. Specialized moulds were engineered from stainless steel which incorporated chambers separated by 0.1 mm thick dividers (Fig. 1). By optimizing the temperature and timing of gel setting and removal of the dividers, a protocol was developed that allowed the collagen in adjacent gels to integrate without the cell populations mixing. The result was that adjacent gels were physically contiguous, thus facilitating the investigation of subsequent cell-cell interactions without the presence of confounding mechanical cues.
Astrocytes undergo reactive gliosis at the interface
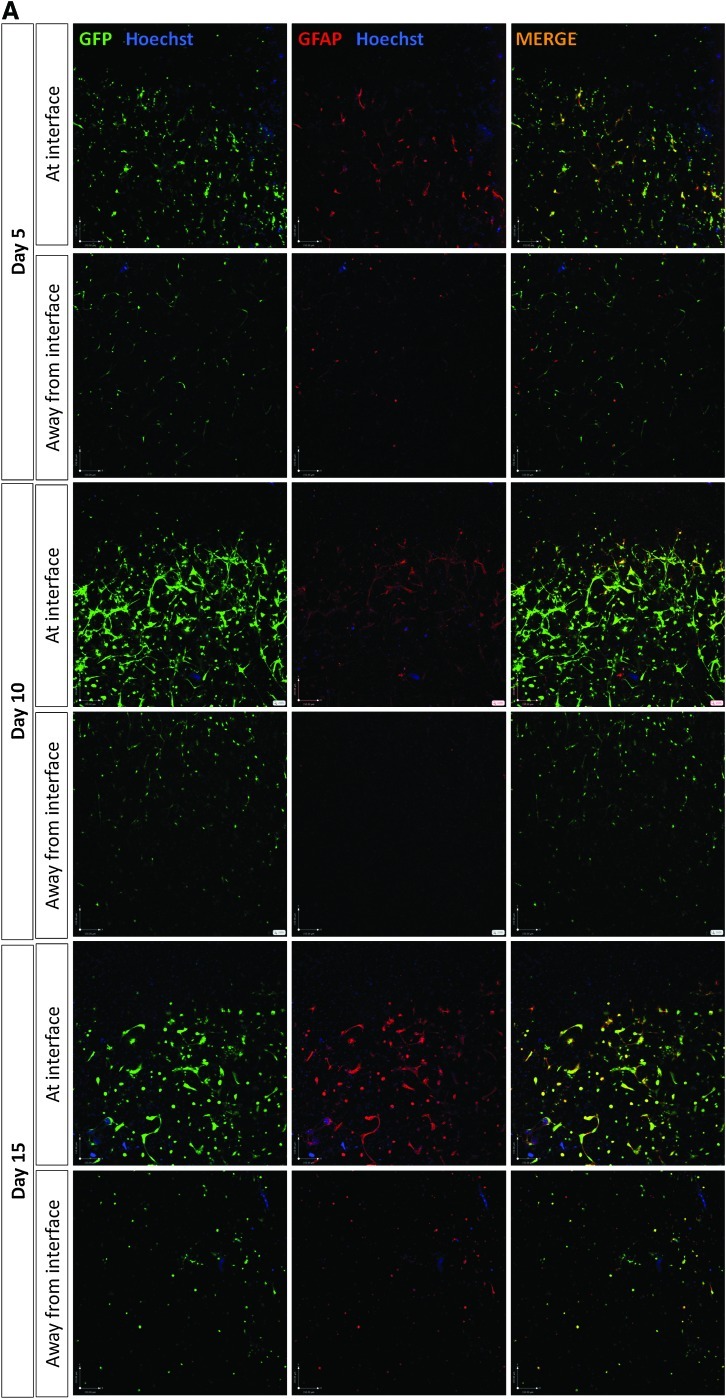
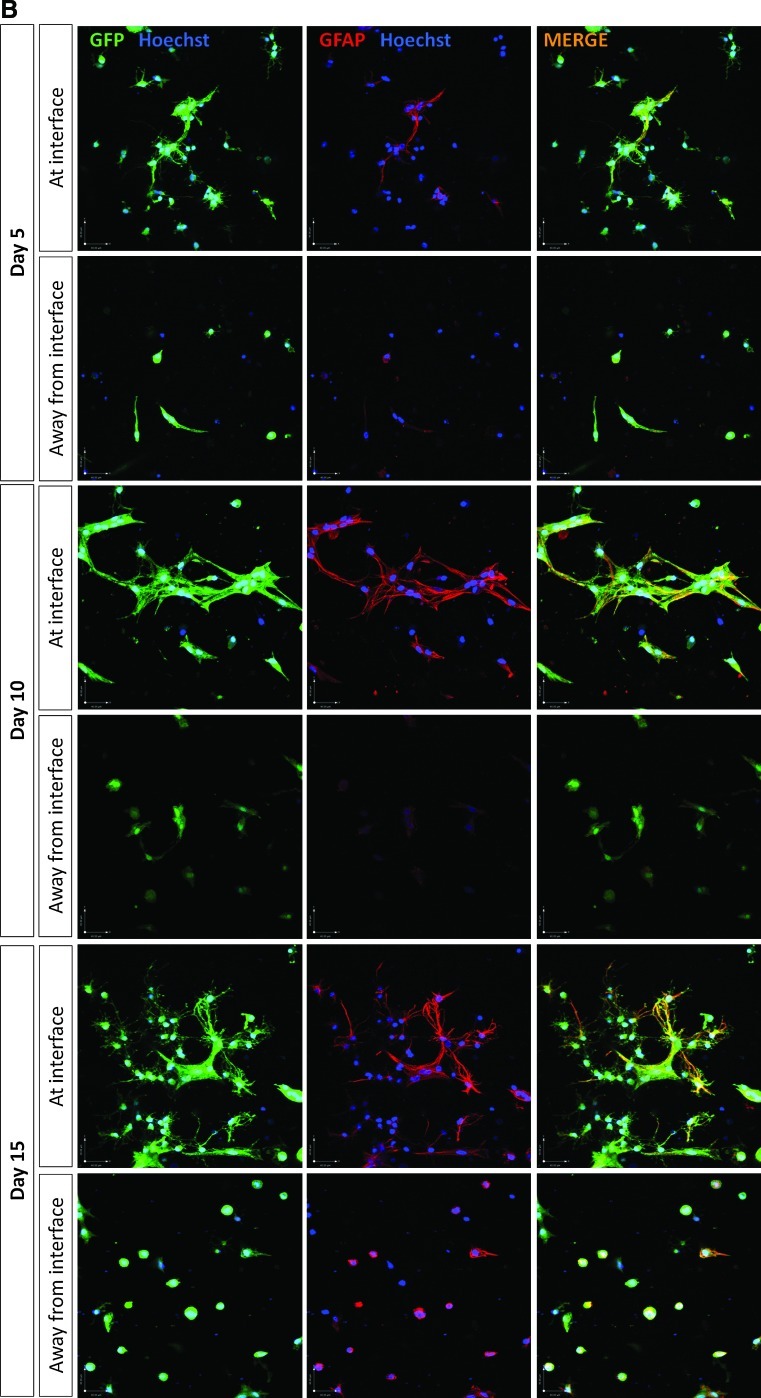
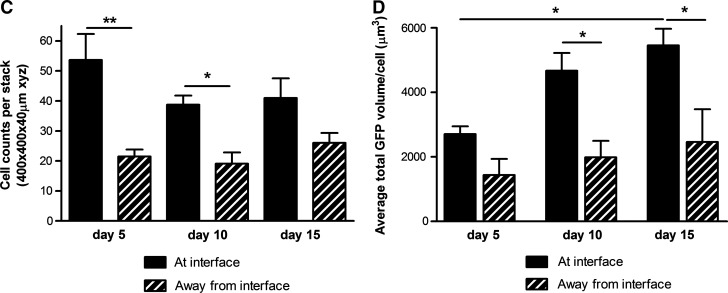
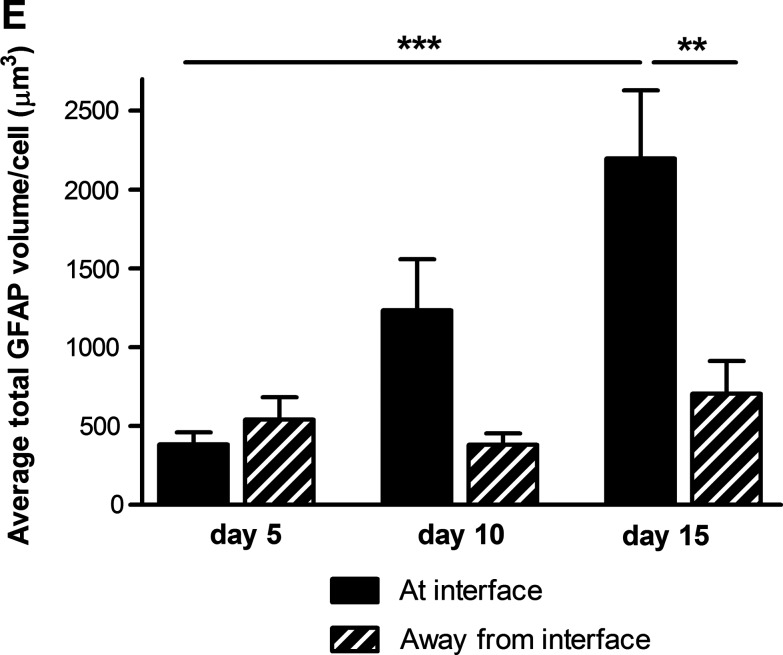
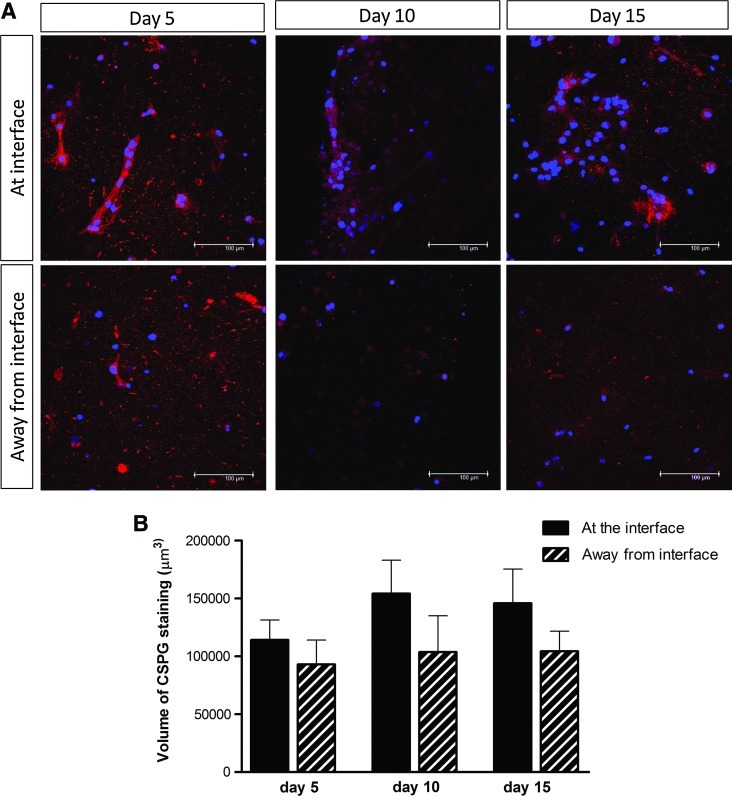
Reactive gliosis was monitored using confocal microscopy to detect astrocyte population density, volume, and GFAP expression after 5, 10, and 15 days in 3D culture (Fig. 2A, B). Astrocytes at the interface with the DRG culture were compared to those 5.5 mm away from the interface in the same gels. There was double the number of astrocytes at the interface compared with the control cells away from the interface at 5 days. At 10 and 15 days the numbers of astrocytes in the interface regions remained higher than those in the corresponding control regions (Fig. 2C). Over the course of the 15 days there was an increase in both astrocyte volume (based on cytoplasmic GFP measurement per cell) and GFAP expression (based on the amount of GFAP immunoreactivity per cell) indicative of both hypertrophy and activation. At 5 days there was a slight increase in astrocyte volume at the interface, then this increased to be significantly greater than control areas at 10 and 15 days (Fig. 2D). There was no difference in GFAP expression at 5 days but at 10 and 15 days there was considerably greater GFAP expression in the astrocytes at the interface compared with cells in the control regions 5.5 mm away from the interface (Fig. 2E). Chondroitin sulfate proteoglycan (CSPG) immunoreactivity was associated with cells and extracellular matrix (Fig. 3A) and there was a trend toward greater CSPG immunoreactivity at the interface than in control areas at all three time points (Fig. 3A, B), although this difference was not statistically significant.
FIG. 2.
Qualitative assessment of astrocyte reactivity at the interface with dissociated DRG cells. Astrocyte morphology and immunoreactivity was assessed using immunostaining and confocal microscopy for GFP (green), GFAP (red), and Hoechst (blue) at days 5, 10, and 15 in culture. Representative confocal projections are shown of astrocytes at the interface with dissociated DRG cells, and away from the interface, in an astrocyte only region at (A) low and (B) high magnification. Scale bar in (A)=150 μm and scale bar in (B)=40 μm. Over time, astrocytes in contact with/in close proximity to dissociated DRG cells become ramified and hypertrophic, which corresponds to an increase in staining for GFAP. GFAP, glial fibrillary acidic protein. GFP, green fluorescent protein. Color images available online at www.liebertonline.com/tec
Quantification of astrocyte reactivity at the interface with dissociated DRG cells: (C) GFP positive astrocytic cell nuclei (stained with Hoechst) were quantified at and away from the interface with dissociated DRG cells. Significantly more astrocytes were observed at the interface with dissociated DRG cells than away from the interface at day 5 and 10 in culture. (D) Quantification of GFP staining per cell revealed that astrocytes were becoming hypertrophic over time at the interface with dissociated DRG cells. No differences were observed in astrocyte size away from the interface. (E) Quantification of GFAP staining per cell revealed that astrocytes were becoming reactive at the interface with dissociated DRG cells, with significantly greater GFAP expression at day 15 in culture compared with away from the interface. *p<0.05, **p<0.01, ***p<0.001.
FIG. 3.
CSPG expression at the interface with dissociated DRG cells. (A) Astrocyte CSPG immunoreactivity was assessed using immunostaining and confocal microscopy, for CS56 (red) and Hoechst (blue) at days 5, 10, and 15 in culture. Representative confocal projections are shown of astrocytes at the interface with dissociated DRG cells, and away from the interface, in an astrocyte-only region at high magnification. Scale bar=100 μm. (B) Quantification of the volume of CSPG staining revealed a trend toward greater CSPG staining at the interface with DRG cells although these differences were not significantly different at any time point. CSPG, chondroitin sulfate proteoglycan. Color images available online at www.liebertonline.com/tec
Further experiments in models with astrocyte:astrocyte interfaces showed no changes in astrocyte reactivity between the interface and control regions. In addition, incubation of astrocyte gels with DRG-conditioned media did not increase reactivity (data not shown).
Neurons are inhibited from regenerating across an astrocyte interface
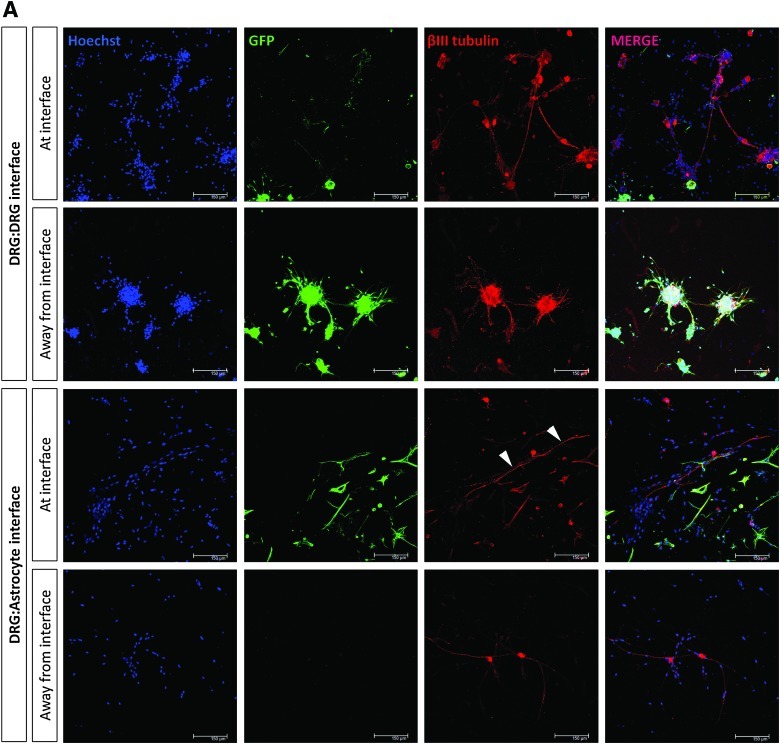
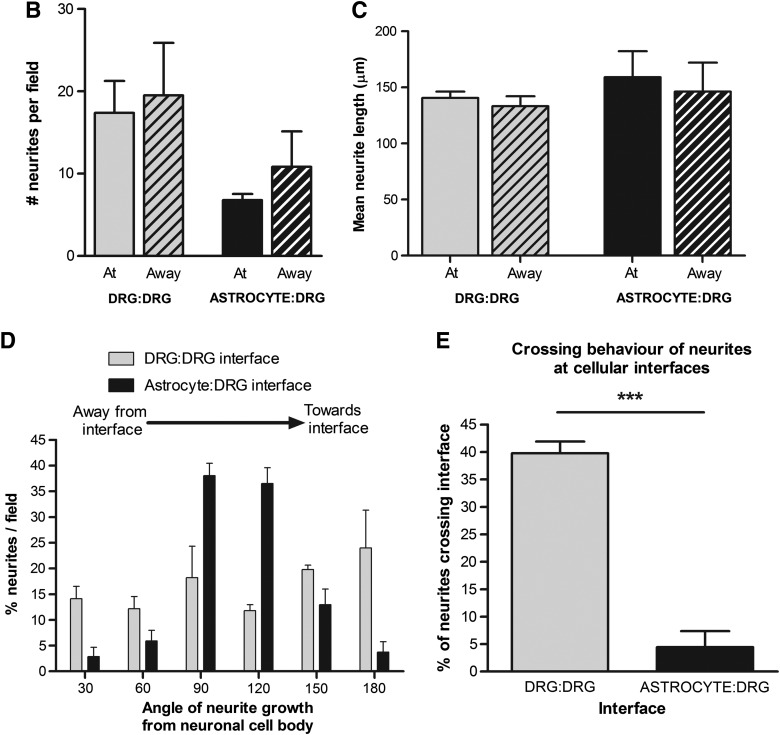
Neurite growth was assessed at day 5 at the interface between DRG:astrocyte gels, at the interface between DRG:DRG gels, and 5.5 mm away from these interfaces in control areas (Fig. 4A). There was a slight reduction in the number of neurites detected per 3D field in all regions within the DRG gels that were in contact with astrocyte gels compared with those in the DRG:DRG interface group although this was not statistically significant (Fig. 4B). Figure 4C shows that there was a similar amount of neurite outgrowth in all regions of all DRG gels, with mean neurite length in the range 133.3–159.1 μm. Interestingly, despite the overall amount of neurite growth being equivalent in the different groups, there was a marked difference in the orientation of neurites and their ability to penetrate the interfaces. In the DRG:DRG cultures the neurites projected equally in all directions at the interface, whereas in the DRG:astrocyte cultures neurites at the interface grew parallel to it (Fig. 4D). When the ability of neurites to cross the interface was analyzed there was a highly significant reduction (p<0.001) in the proportion of neurites that crossed DRG:astrocyte interfaces compared with those that crossed DRG:DRG interfaces (Fig. 4E).
FIG. 4.
Assessment of neuronal growth in interface gels. (A) Neuronal growth was investigated using immunostaining and confocal microscopy, for GFP (green), βIII tubulin (red), and Hoechst (blue) at days 5, 10, and 15 in culture. Representative confocal projections are shown of control gels (an interface gel of dissociated GFP DRG cells with dissociated WT DRG cells) and test gels (an interface gel of GFP astrocytes with dissociated WT DRG cells). Arrowheads indicate neurites growing parallel to the interface. Scale bar=150 μm. Neuronal growth was assessed in control gels and test gels at day 5 in culture. Color images available online at www.liebertonline.com/tec
(B) The number of neurites was quantified in confocal projections. While there was a trend for a reduced number of neurites in the DRG:astrocyte interface gels, this did not reach statistical significance (p=0.0547 at the interface vs. control DRG:DRG interfaces). (C) Mean neurite length did not significantly differ between the different interfaces, at or away from the interface. (D) Analysis of the angle of neurite growth revealed differences between the control and test interfaces. Neurites were present at all angles in control interfaces, whereas in test interfaces, neurites were predominantly orientated parallel to the interface with astrocytes. (E) Significantly fewer neurites in the test gels crossed the interface compared with control gels in which more neurites (GFP positive) crossed the interface. ***p<0.001.
Discussion
Here we report for the first time a method for making a physically integrated 3D interface between adjacent collagen hydrogels and its utilization to generate a model of the glial scar interface that demonstrates the development of reactive gliosis and neurite growth inhibition. Fluorescent tracking of different cell populations, combined with immunofluorescence, confocal microscopy, and 3D image analysis provided a method for monitoring neuronal and glial cell responses. Astrocytes at the interface responded to the presence of DRG cultures by increasing in number, hypertrophy, and increased reactive gliosis. The presence of the astrocyte interface caused no reduction in neurite growth within the DRG compartment, but significantly inhibited growth toward or penetration through the astrocyte interface by regenerating neurites. This was in contrast to similar DRG:DRG 3D cultures in which neurites grew in all directions and freely crossed the DRG:DRG interface, thus confirming the physical integration of the two gels.
It is interesting that in our system, adjacent compartments of astrocytes and DRG cells were necessary and sufficient to elicit the development of the neurite growth-inhibitory interface. This is the first time that this has been demonstrated using a 3D culture model and provides a reproducible and high-throughput method for detailed investigations of the dynamics of interface formation and regenerative failure. By maintaining the astrocytes in a 3D hydrogel environment it was possible to detect the events of reactive gliosis from a much earlier stage than would be achieved using a monolayer culture in which the astrocytes would exhibit a more reactive phenotype in the absence of a stimulus.27 When mature astrocytes and Schwann cells come into contact on 2D surfaces they react to each other's presence by producing migration-inhibitory molecules that concentrate at the cellular interface.33–36 Previous in vitro models have been used to explore this phenomenon further, for example studying the growth of axons across boundaries between monolayer cultures of astrocytes and Schwann cells,8 or across the DREZ in slice cultures from embryonic and mature spinal cord.37 Various studies have demonstrated the advantages of modeling astrocytes and neurons in 3D culture,3,22,27–29 but it is technically challenging to achieve a cellular interface in 3D culture without also generating a mechanical interface. Indeed, Yu and Bellamkonda showed that a mechanical barrier, formed by the elasticity mismatch between adjacent agarose gels, greatly influenced the ability of neurites to cross the resulting 3D interface.38 A key advantage of a culture model over an in vivo model is being able to limit the number of variables. It was therefore our aim to generate a culture system in which cell-cell interactions could be studied in isolation without the additional mechanical interface that can be present in simpler 3D culture systems or in vivo (particularly where an implanted material has mechanical properties that are mismatched in relation to the host tissue).
The behavior of astrocytes and neurons in our 3D system mimicked key aspects of the reactive gliosis and inhibition of neuronal regeneration that occurs in vivo at the site of a CNS lesion or at the mature PNS:CNS boundary.7,39 It is interesting to note that in our model we were able to explore the timing of three separate aspects of the astrocyte response: at day 5 there was an increase in cell numbers at the interface and a slight increase in cell volume with no apparent increase in GFAP volume/cell; while at subsequent time points there was little further change in the number of cells but a steady increase in both cell volume and GFAP volume/cell. It would be interesting to conduct further experiments using this system to unravel the relative importance of these three aspects of astrocyte behavior in the development of neurite growth-inhibitory interfaces. There was no change in astrocyte reactivity in the control regions away from the interface, in astrocyte:astrocyte interfaces, or in astrocyte gels treated with DRG conditioned media. This suggests that the astrocyte response in this case, which was consistent with previous reports of astrocyte responses to PNS glia,8,9 is likely to have been stimulated by direct cell-cell contact with DRG cells or a locally acting soluble factor rather than a general change in the concentration of some factor in the media.
In our previous study we showed that primary astrocytes in 3D culture exhibited relatively low reactivity and could then react to a stimulus (in that case TGFβ1) to become reactive.27 Here the astrocytes became reactive in response to contact with dissociated DRG cultures, which contained similar cellular components to the mature DREZ or the permissive environment within a CNS repair graft.4,34,40 In this case the astrocyte response was localized to the region of the gel immediately adjacent to the interface, and the increase in cell density, hypertrophy, and increased GFAP expression resulted in a 3D meshwork of reactive astrocyte processes resembling the inhibitory glial environment that forms at the boundary of a CNS lesion.6
The culture system reported here permits the simultaneous analysis of neuronal and glial responses in three dimensions, and would be amenable to supplementation of the culture media or incorporation of different cell types (e.g., transfected cells or primary cells from genetically altered animals) to allow investigation of neuron-glial biology or testing of CNS injury therapies. Further, it is straightforward to monitor the same culture over time (time-lapse) to allow detailed studies of cell:cell dynamics as the inhibitory interface develops and matures. In a wider context, this technology might be useful to researchers interested in modeling interfaces in non-neural tissues or tissue implants, where the ability to maintain and monitor interactions between adjacent cell populations in 3D would be of interest.41–44
In summary we have demonstrated a method for generating 3D cell-cell interfaces and used this to simulate the cellular interactions that occur during the formation of an inhibitory interface in the CNS. The results show that an interface between a DRG culture and an astrocyte culture is sufficient to trigger reactive gliosis in the astrocyte population and inhibition of neuronal regeneration across the interface after 5 days. Our method permitted different aspects of the astrocyte response to be independently monitored, providing an insight into the progression of events that leads to the formation of a glial scar.
Acknowledgments
This work was supported by the Wellcome Trust (080309). We are grateful for the technical support provided by the Open University Biomedical Resource Unit.
Disclosure Statement
The authors have no competing financial interests
References
- 1.East E. Phillips J.B. Tissue engineered cell culture models for nervous system research. In: Greco G.N., editor. Tissue Engineering Research Trends. New York: Nova Science Publishers; 2008. pp. 141–160. [Google Scholar]
- 2.Kaewkhaw R. Scutt A.M. Haycock J.W. Anatomical site influences the differentiation of adipose-derived stem cells for Schwann-cell phenotype and function. Glia. 2011;59:734. doi: 10.1002/glia.21145. [DOI] [PubMed] [Google Scholar]
- 3.LaPlaca M.C. Simon C.M. Prado G.R. Cullen D.K. CNS injury biomechanics and experimental models. Prog Brain Res. 2007;161:13. doi: 10.1016/S0079-6123(06)61002-9. [DOI] [PubMed] [Google Scholar]
- 4.King V.R. Phillips J.B. Hunt-Grubbe H. Brown R. Priestley J.V. Characterization of non-neuronal elements within fibronectin mats implanted into the damaged adult rat spinal cord. Biomaterials. 2006;27:485. doi: 10.1016/j.biomaterials.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Phillips J.B. King V.R. Ward Z. Porter R.A. Priestley J.V. Brown R.A. Fluid shear in viscous fibronectin gels allows aggregation of fibrous materials for CNS tissue engineering. Biomaterials. 2004;25:2769. doi: 10.1016/j.biomaterials.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 6.Geller H.M. Fawcett J.W. Building a bridge: engineering spinal cord repair. Exp Neurol. 2002;174:125. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 7.Silver J. Miller J.H. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 8.Adcock K.H. Brown D.J. Shearer M.C. Shewan D. Schachner M. Smith G.M., et al. Axon behaviour at Schwann cell—astrocyte boundaries: manipulation of axon signalling pathways and the neural adhesion molecule L1 can enable axons to cross. Eur J Neurosci. 2004;20:1425. doi: 10.1111/j.1460-9568.2004.03573.x. [DOI] [PubMed] [Google Scholar]
- 9.Lakatos A. Barnett S.C. Franklin R.J. Olfactory ensheathing cells induce less host astrocyte response and chondroitin sulphate proteoglycan expression than Schwann cells following transplantation into adult CNS white matter. Exp Neurol. 2003;184:237. doi: 10.1016/s0014-4886(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 10.Bradbury E.J. Carter L.M. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. 2011;84:306. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Davies J.E. Huang C. Proschel C. Noble M. Mayer-Proschel M. Davies S.J. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raisman G. Li Y. Repair of neural pathways by olfactory ensheathing cells. Nat Rev Neurosci. 2007;8:312. doi: 10.1038/nrn2099. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Rub M. McMahon S. Zeugolis D.I. Windebank A. Pandit A. Spinal cord injury in vitro: modelling axon growth inhibition. Drug Discov Today. 2010;15:436. doi: 10.1016/j.drudis.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Wanner I.B. Deik A. Torres M. Rosendahl A. Neary J.T. Lemmon V.P., et al. A new in vitro model of the glial scar inhibits axon growth. Glia. 2008;56:1691. doi: 10.1002/glia.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly E.M. Strappe P.M. McGinley L.M. Madigan N.N. Geurts E. Rooney G.E., et al. Lentiviral vector-mediated knockdown of the neuroglycan 2 proteoglycan or expression of neurotrophin-3 promotes neurite outgrowth in a cell culture model of the glial scar. J Gene Med. 2010;12:863. doi: 10.1002/jgm.1509. [DOI] [PubMed] [Google Scholar]
- 16.Ellis E.F. McKinney J.S. Willoughby K.A. Liang S. Povlishock J.T. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J Neurotrauma. 1995;12:325. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- 17.Kimura-Kuroda J. Teng X. Komuta Y. Yoshioka N. Sango K. Kawamura K., et al. An in vitro model of the inhibition of axon growth in the lesion scar formed after central nervous system injury. Mol Cell Neurosci. 2010;43:177. doi: 10.1016/j.mcn.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 18.McKeon R.J. Schreiber R.C. Rudge J.S. Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ness R. David S. Leptomeningeal cells modulate the neurite growth promoting properties of astrocytes in vitro. Glia. 1997;19:47. doi: 10.1002/(sici)1098-1136(199701)19:1<47::aid-glia5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Shearer M.C. Niclou S.P. Brown D. Asher R.A. Holtmaat A.J. Levine J.M., et al. The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signalling pathways. Mol Cell Neurosci. 2003;24:913. doi: 10.1016/j.mcn.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Yu A.C. Lee Y.L. Eng L.F. Astrogliosis in culture: I. The model and the effect of antisense oligonucleotides on glial fibrillary acidic protein synthesis. J Neurosci Res. 1993;34:295. doi: 10.1002/jnr.490340306. [DOI] [PubMed] [Google Scholar]
- 22.Fawcett J.W. Housden E. Smith-Thomas L. Meyer R.L. The growth of axons in three-dimensional astrocyte cultures. Dev Biol. 1989;135:449. doi: 10.1016/0012-1606(89)90193-0. [DOI] [PubMed] [Google Scholar]
- 23.Tom V.J. Doller C.M. Malouf A.T. Silver J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J Neurosci. 2004;24:9282. doi: 10.1523/JNEUROSCI.2120-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cullen D.K. Simon C.M. LaPlaca M.C. Strain rate-dependent induction of reactive astrogliosis and cell death in three-dimensional neuronal-astrocytic co-cultures. Brain Res. 2007;1158:103. doi: 10.1016/j.brainres.2007.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cullen D.K. Stabenfeldt S.E. Simon C.M. Tate C.C. LaPlaca M.C. In vitro neural injury model for optimization of tissue-engineered constructs. J Neurosci Res. 2007;85:3642. doi: 10.1002/jnr.21434. [DOI] [PubMed] [Google Scholar]
- 26.Sundararaghavan H.G. Monteiro G.A. Firestein B.L. Shreiber D.I. Neurite growth in 3D collagen gels with gradients of mechanical properties. Biotechnol Bioeng. 2009;102:632. doi: 10.1002/bit.22074. [DOI] [PubMed] [Google Scholar]
- 27.East E. Golding J.P. Phillips J.B. A versatile 3D culture model facilitates monitoring of astrocytes undergoing reactive gliosis. J Tissue Eng Regen Med. 2009;3:634. doi: 10.1002/term.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kofron C.M. Fong V.J. Hoffman-Kim D. Neurite outgrowth at the interface of 2D and 3D growth environments. J Neural Eng. 2009;6:016002. doi: 10.1088/1741-2560/6/1/016002. [DOI] [PubMed] [Google Scholar]
- 29.Li G.N. Livi L.L. Gourd C.M. Deweerd E.S. Hoffman-Kim D. Genomic and morphological changes of neuroblastoma cells in response to three-dimensional matrices. Tissue Eng. 2007;13:1035. doi: 10.1089/ten.2006.0251. [DOI] [PubMed] [Google Scholar]
- 30.Discher D.E. Janmey P. Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 31.East E. de Oliveira D.B. Golding J.P. Phillips J.B. Alignment of astrocytes increases neuronal growth in three-dimensional collagen gels and is maintained following plastic compression to form a spinal cord repair conduit. Tissue Eng Part A. 2010;16:3173. doi: 10.1089/ten.tea.2010.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips J.B. Brown R. Micro-structured materials and mechanical cues in 3D collagen gels. Methods Mol Biol. 2011;695:183. doi: 10.1007/978-1-60761-984-0_12. [DOI] [PubMed] [Google Scholar]
- 33.Ghirnikar R.S. Eng L.F. Chondroitin sulfate proteoglycan staining in astrocyte-Schwann cell co-cultures. Glia. 1995;14:145. doi: 10.1002/glia.440140209. [DOI] [PubMed] [Google Scholar]
- 34.Golding J.P. Bird C. McMahon S. Cohen J. Behaviour of DRG sensory neurites at the intact and injured adult rat dorsal root entry zone: postnatal neurites become paralysed, whilst injury improves the growth of embryonic neurites. Glia. 1999;26:309. [PubMed] [Google Scholar]
- 35.Golding J.P. Shewan D. Berry M. Cohen J. An in vitro model of the rat dorsal root entry zone reveals developmental changes in the extent of sensory axon growth into the spinal cord. Mol Cell Neurosci. 1996;7:191. doi: 10.1006/mcne.1996.0015. [DOI] [PubMed] [Google Scholar]
- 36.Wilby M.J. Muir E.M. Fok-Seang J. Gour B.J. Blaschuk O.W. Fawcett J.W. N-Cadherin inhibits Schwann cell migration on astrocytes. Mol Cell Neurosci. 1999;14:66. doi: 10.1006/mcne.1999.0766. [DOI] [PubMed] [Google Scholar]
- 37.Golding J.P. Cohen J. Border controls at the mammalian spinal cord: late-surviving neural crest boundary cap cells at dorsal root entry sites may regulate sensory afferent ingrowth and entry zone morphogenesis. Mol Cell Neurosci. 1997;9:381. doi: 10.1006/mcne.1997.0647. [DOI] [PubMed] [Google Scholar]
- 38.Yu X. Bellamkonda R.V. Dorsal root ganglia neurite extension is inhibited by mechanical and chondroitin sulfate-rich interfaces. J Neurosci Res. 2001;66:303. doi: 10.1002/jnr.1225. [DOI] [PubMed] [Google Scholar]
- 39.Faulkner J.R. Herrmann J.E. Woo M.J. Tansey K.E. Doan N.B. Sofroniew M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maro G.S. Vermeren M. Voiculescu O. Melton L. Cohen J. Charnay P., et al. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci. 2004;7:930. doi: 10.1038/nn1299. [DOI] [PubMed] [Google Scholar]
- 41.Brown R.A. Phillips J.B. Cell responses to biomimetic protein scaffolds used in tissue repair and engineering. Int Rev Cytol. 2007;262:75. doi: 10.1016/S0074-7696(07)62002-6. [DOI] [PubMed] [Google Scholar]
- 42.Grinnell F. Rocha L.B. Iucu C. Rhee S. Jiang H. Nested collagen matrices: a new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312:86. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Hadjipanayi E. Brown R.A. Mudera V. Interface integration of layered collagen scaffolds with defined matrix stiffness: implications for sheet-based tissue engineering. J Tissue Eng Regen Med. 2009;3:230. doi: 10.1002/term.157. [DOI] [PubMed] [Google Scholar]
- 44.Marenzana M. Kelly D.J. Prendergast P.J. Brown R.A. A collagen-based interface construct for the assessment of cell-dependent mechanical integration of tissue surfaces. Cell Tissue Res. 2007;327:293. doi: 10.1007/s00441-006-0316-z. [DOI] [PubMed] [Google Scholar]