Abstract
The hippocampal network comprises a large variety of locally connected GABAergic interneurons exerting a powerful control on network excitability and which are responsible for the oscillatory behaviour crucial for information processing. GABAergic interneurons receive an important cholinergic innervation from the medial septum-diagonal band complex of the basal forebrain and are endowed with a variety of muscarinic and nicotinic acetylcholine receptors (mAChRs and nAChRs) that regulate their activity. Deficits in the cholinergic system lead to the impairment of high cognitive functions, which are particularly relevant in neurodegenerative pathologies such as Alzheimer's and Parkinson's diseases as well as in schizophrenia. Here, we highlight some recent advances in the mechanisms by which cholinergic signalling via nAChRs regulates local inhibitory circuits in the hippocampus, early in postnatal life and in adulthood. We also discuss recent findings concerning the functional role of nAChRs in controlling short- and long-term modifications of synaptic efficacy. Insights into these processes may provide new targets for the therapeutic control of pathological conditions associated with cholinergic dysfunctions.
Enrico Cherubini (right) is Full Professor at SISSA, Trieste. He earned his MD and specialized in child neurology at the University of Rome. After being trained with H. Gastaut (Marseille), N. Buchwald (UCLA) and R. A. North (MIT) he moved to Paris where he was appointed ‘Directeur de Recherche’ at the INSERM unit directed by Y. Ben-Ari. His research interest focuses mainly on the molecular and cellular mechanisms regulating synaptic plasticity processes in the developing hippocampus including those mediated by nicotinic acetylcholine receptor (nAChR) signalling. Marilena Griguoli (left) obtained her PhD from SISSA, studying the effects of nAChRs on oriens-lacunosum moleculare interneurons in the hippocampus. As a post-doc she joined the group of C. Mulle in Bordeaux.
 |
Introduction
Since the pioneering studies of Ramón y Cajal (1899) and Lorente de Nó (1922) it has become clear that local circuit inhibitory interneurons constitute a very heterogeneous group of cells. By releasing γ-aminobutyric acid (GABA) into their postsynaptic targets they exert a powerful control on network excitability and are responsible for the oscillatory behaviour, crucial for information processing in the brain. Interneurons can be differentially classified according to their morphology, biophysical properties, molecular expression profile and connectivity (Freund & Buzsaki, 1996; McBain & Fisahn, 2001). In the CA1 hippocampal region for instance, relatively uniform excitatory pyramidal cells are supported by more than 20 different types of interneurons (Klausberger & Somogyi, 2008).
In contrast to principal cells that exhibit long axons projecting information to distant brain areas, GABAergic interneurons present short axons that selectively innervate different domains of pyramidal cells, thus providing the main source of feedback and feed-forward inhibition (Miles et al. 1996; Maccaferri & Lacaille, 2003; Kullmann, 2011). The spatio-temporal dynamics between the activity of interneurons and pyramidal cells leads to coherent oscillations (Klausberger et al. 2003, 2004; Somogyi & Klausberger, 2005), which support different behavioural states and high cognitive tasks (Klausberger & Somogyi, 2008). Oscillatory rhythms are facilitated by the intrinsic properties of GABA-releasing cells (Maccaferri & McBain, 1996) and by their electrical coupling via gap junctions (Hestrin & Galarreta, 2005).
Interestingly hippocampal interneurons receive an important cholinergic innervation from the medial septum–diagonal band complex of the basal forebrain (Frotscher & Léránth, 1985) and are endowed with nicotinic acetylcholine receptors (nAChRs), the activation of which contributes to the setting of the cooperative temporal framework that provides the basis for high cognitive functions (Rezvani & Levin, 2001).
Neuronal nAChRs belong to the large cysteine loop of the ligand-gated ion channel superfamily and are composed of five subunits organized in a variety of allosteric oligomers (Changeux & Edelstein, 2005). Several different nAChR subunits, α2–α10 and β2−β4, have been cloned. They may assemble in various combinations to generate a large variety of nAChR subtypes with different biophysical and pharmacological properties (Le Novère & Changeux, 1995). While α7−α10 subunits form channels sensitive to the snake venom α-bungarotoxin (α-BGTx), α2 and α6 combine with β2−β4 subunits to produce channels insensitive to α-BGTx. The two major nAChR subtypes present in the hippocampus are homomeric α7 and heteromeric α4β2 nAChRs (Alkondon & Albuquerque, 2004). These receptors are permeable to cations including calcium. Calcium permeability varies among different receptor types, being highest in the homomeric α7 nAChRs (Fucile, 2004). This characteristic allows nAChRs to play a key role in calcium-mediated events including neurotransmitter release, regulation of a variety of signal transduction cascades, cell survival and apoptosis.
Deficits in the cholinergic system produce impairment of cognitive functions, which are particularly relevant during senescence and in age-related neurodegenerative pathologies (Selkoe, 2002), and nicotine is known to enhance cognitive functions, via nAChRs, in some Alzheimer's disease patients (Nordberg, 1994).
This review examines how cholinergic signalling controls via nAChRs the correlated network activity present in the hippocampus early in postnatal life and orchestrates the functional properties of GABAergic interneurons in a cell-specific manner. In particular, recent advances in nAChR-mediated modulation of short- and long-term synaptic plasticity processes in local inhibitory circuits are highlighted.
nAChRs control correlated network activity in the immature hippocampus
It has been well established that nAChRs contribute to the functional maturation of the brain (Chang & Berg, 1999; Aramakis et al. 2000; Rossi et al. 2001; Kawa, 2002) and that their excessive activation by perinatal exposure to nicotine impairs cognitive functions by interfering with the development of areas involved in these processes (Johns et al. 1982; Levin et al. 1993; Ernst et al. 2001; Linnet et al. 2003).
In the rat hippocampus, mRNAs for the α7 and β2 subunits are present early during embryogenesis but their expression patterns differ. The density of {3H}-epibatidine binding sites, an indicator of heteromeric nAChRs, remains stable during postnatal development (Tribollet et al. 2004). Conversely, the expression of α7 mRNA and the density of {125I}-α-BGTx binding sites, an indicator of α7 nAChRs, are particularly high during the first postnatal week and decrease subsequently (Shacka & Robinson, 1998; Tribollet et al. 2004). This suggests that, at least in the CA1 region of the hippocampus, the balance between α7- and β2-containing nAChRs changes during postnatal development. This may lead to differences in nicotine-induced modulation of synaptic and network activity.
At the network level, the immature hippocampus is characterized by a correlated activity (giant depolarizing potentials or GDPs; Ben-Ari et al. 1989), generated by the synergistic action of glutamate and GABA which, at this developmental stage, is depolarizing and excitatory (Cherubini et al. 1991; Ben-Ari et al. 2007). This activity represents a primordial form of synchrony between neurons preceding more organized forms such as the theta and the gamma rhythms and it is instrumental in enhancing synaptic efficacy at poorly developed GABAergic and glutamatergic synapses (Kasyanov et al. 2004; Mohajerani et al. 2007). A previous report on CA3 pyramidal neurons in the hippocampus has demonstrated that nAChRs are present and functional from the first postnatal day and that nicotine cholinergic signalling via α7 and non-α7 nAChRs exerts a powerful regulatory action on network-driven GDPs (Maggi et al. 2001). Since glutamatergic terminals projecting to pyramidal neurons are controlled only by α7 nAChRs, the nicotine-induced increase in GDP frequency observed in α7-/- mice can be attributed to the enhancement of GABA release from GABAergic interneurons via β2-containing nAChRs (Le Magueresse et al. 2006; Fig. 1). It is worth noting that, in α7-/- mice, nicotine failed to increase the frequency of interictal discharges obtained towards the end of the first postnatal week by blocking the GABAA receptor with bicuculline (Le Magueresse et al. 2006). Activation of α7 nAChRs, probably localized on associative-commissural fibres involved in the generation of bursting activity (Miles & Wong, 1987), may account for this effect. Therefore, while modulation of glutamatergic signalling needs the activation of α7 nAChRs (see also Maggi et al. 2003), regulation of GABAergic transmission needs the activation of both α7- and β2-containing nAChRs (Le Magueresse et al. 2006).
Figure 1. Different regulation of GDPs and interictal discharges by nAChRs.
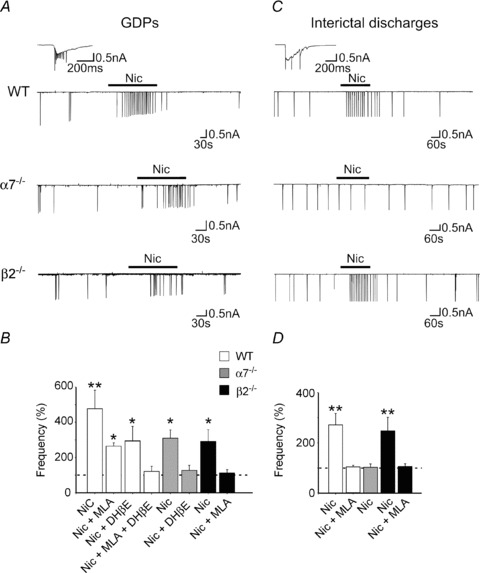
A, representative traces recorded at P5–P6 from CA1 pyramidal neurons in hippocampal slices obtained from WT, α7-/- and β2-/- mice, respectively, in control conditions and in the presence of nicotine (1 μm). The inset above the traces represents a GDP at an expanded time scale. B, each column represents nicotine-induced changes of GDP frequency as a percentage of control (dashed line); n = 6–12. C and D, as in A and B but for interictal discharges induced by bicuculline at P9–P10 (see the inset above the traces); n = 3–13; *P < 0.05; **P < 0.01. While nicotine enhanced GDPs frequency via activation of α7- and β2-containing nAChRs, it increased the frequency of interictal discharges only via α7 nAChRs, indicating a different distribution of nAChRs between GABAergic interneurons and principal cells. Modified from Le Magueresse et al. 2006.
In addition, at least in the CA1 region of the hippocampus, the potency of the observed effects and the involved nAChR subtypes vary among different lamina in a neuron-type-specific way (Le Magueresse et al. 2006). This may differently affect the fine regional tuning of GABAergic and glutamatergic transmission and hippocampal wiring. It is important to mention that during the first week of postnatal life nicotinic cholinergic activity drives, mainly via α7 nAChRs, maturation of GABAergic signalling, contributing in this way to the shift of GABA from the depolarizing to the hyperpolarizing direction (Liu et al. 2006).
nAChRs regulate the functional properties of GABAergic interneurons in a cell-specific manner
In the rat and mouse hippocampus, nAChRs are expressed at both pre- and post-synaptic sites (Zoli et al. 1998; Sudweeks & Yakel, 2000; Fabian-Fine et al. 2001). The activation of presynaptic nAChRs induces a calcium-dependent increase in the probability of transmitter release (Gray et al. 1996; Vizi & Lendvai, 1999; Alkondon & Albuquerque, 2001). In particular, α7 nAChRs that are expressed on both glutamatergic and GABAergic terminals modulate the release of both glutamate and GABA. Calcium increase in presynaptic nerve terminals occurs through nAChR channels, high voltage-dependent calcium channels (of the N, P/Q and R types) activated by the depolarizing action of nicotine or endogenously released ACh and calcium-induced calcium release from internal stores (Le Magueresse & Cherubini, 2007). Interestingly, activation of α3β4 nAChRs localized on axon terminals of parvalbumin-positive cells can boost tetrodotoxin-insensitive GABA release via low voltage-gated calcium channels (of the T-type) and calcium-induced calcium release (Tang et al. 2011). The cholinergic enhancement of GABA release from perisomatic-targeting parvalbumin-expressing cells may affect gamma oscillations which, together with theta waves, occur during spatial navigation, memory tasks and rapid-eye-movement sleep (Klausberger & Somogyi, 2008). Furthermore, α3β4β2 nAChRs, present on glutamatergic axons synapsing on stratum radiatum interneurons, exert a powerful control on their resting excitability (Alkondon et al. 2011). Thus, mecamylamine (a non selective nAChRs antagonist) is able to reduce the frequency of action currents recorded in cell-attached mode from basket cells in stratum radiatum. The lack of methyllycaconitine (MLA; a selective α7 nAChR antagonist) in the modification of the firing frequency of stratum radiatum interneurons may be attributed to the low level of ‘ambient’ acetylcholine (ACh) in hippocampal slices, insufficient to trigger interneuronal firing via low-affinity α7 nAChRs, apparently localized together with α3β4β2 on glutamatergic axons.
The activation of nAChRs localized postsynaptically on the somato-dendritic compartments produces specific responses in pyramidal cells and interneurons. However, while in pyramidal cells nAChR agonists produce no responses or barely detectable responses (Frazier et al. 1998b; McQuiston & Madison, 1999; Khiroug et al. 2003; but see Ji et al. 2001), in interneurons they induce responses whose kinetics and pharmacology vary among different cell types (Frazier et al. 1998a). Previous studies, using conventional pharmacological tools, have indicated that a local application of ACh to interneurons present in stratum radiatum and stratum lacunosum moleculare induces fast and slow decaying responses selectively blocked by α-BGTx/MLA or DHβE, indicating that they are mediated by α7 and β2 containing nAChRs, respectively (Frazier et al. 1998a, Alkondon et al. 1999). It is worth noting that α7 nAChRs undergo rapid desensitization (Hogg et al. 2003), a condition that would limit, in case of excessive agonist stimulation, membrane excitability and action potential firing (Alkondon et al. 2000). Fast and slow responses to ACh can be also recorded in stratum oriens interneurons (McQuiston & Madison, 1999). These cells are innervated by axon collaterals of pyramidal cells (Blasco-Ibanez & Freund, 1995) and project back to principal cells in stratum lacunosum moleculare (Lacaille et al. 1987; Ali & Thompson, 1998; Maccaferri et al. 2000; Maccaferri, 2005), contributing in this way to local feedback circuits. The kinetics correlation of currents evoked by ACh in stratum radiatum and stratum oriens interneurons with single-cell RT-PCR analysis revealed responses with fast kinetics, mediated by α3 and α7 subunits and responses with slow kinetics mediated by α2 and α4 subunits (Sudweeks & Yakel, 2000). The α4 and α2 subunits, certainly in combination with one or more β subunits, may be the major contributors to slow activating non-α7 responses detected in stratum radiatum and stratum oriens interneurons. In particular, stratum oriens interneurons express high levels of α2 subunits (Wada et al. 1989; Yakel & Shao, 2004), which support sustained non-desensitizing responses (Jia et al. 2009). According to McQuiston & Madison (1999), interneurons localized near the stratum pyramidale with axons providing perisomatic inhibition are insensitive to nAChR agonists. A schematic simplified view of different subtypes of nAChRs expressed on pyramidal cells and GABAergic interneurons of the CA1 hippocampal region is represented in Fig. 2.
Figure 2. Simplified view of the expression of different nAChR subtypes on pyramidal cells and GABAergic interneurons present in the CA1 hippocampal region.
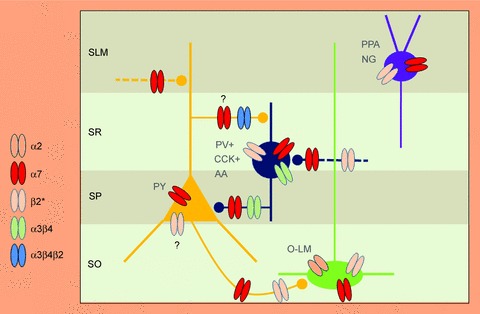
SLM, stratum lacunosum moleculare; SR, stratum radiatum; SP, stratum pyramidale; SO, stratum oriens; PY, pyramidal cells; PV+, parvalbumin-positive; CCK+, cholecystokinin-positive; AA, axo-axonic interneurons; O-LM, oriens-lacunosum moleculare; PPA, perforant path-associated lacunosum moleculare or lacunosum moleculare-radiatum interneurons; NG, neurogliaform cell. Dashed lines represent glutamatergic terminals from pyramidal cells (yellow) or from GABAergic interneurons (blue).
nAChRs can be endogenously activated by acetylcholine released from the septal cholinergic projection (Frotscher & Léránth, 1985) or from intrinsic cholinergic interneurons (Frotscher et al. 1986). The latter comprise a small number of cells localized in the dentate gyrus and in the hippocampus proper, immunopositive for the acetylcholine-synthesizing enzyme choline acetyltransferase (Frotscher et al. 2000) and projecting specifically to GABAergic interneurons (Freund & Buzsaki, 1996). ACh released from cholinergic interneurons would regulate, via nAChRs present on GABAergic cells, network activity generated in the rat and mouse CA3 hippocampal region (Cobb et al. 1999). Thus, after degeneration of septal cholinergic terminals, the hippocampal network is still able to support nAChR-dependent theta-mode activity, suggesting that intrinsic cholinergic circuits may provide the neurotransmitter necessary for nAChR activation (Cobb et al. 1999).
It is important to mention that cholinergic fibres arising from the medial septum–diagonal band complex have a number of transmitter-containing varicosities which only in a few cases face postsynaptic specializations (Descarries et al. 2004). This has led to the idea that cholinergic signalling may occur mainly via non-synaptic volume transmission (Umbriaco et al. 1995). In the volume transmission mode, ACh released from the synaptic cleft and/or from non-synaptic varicosities diffuses away to activate extrasynaptic nAChRs. This may explain the higher probability of producing slow nAChRs-mediated responses upon sustained stimulation of cholinergic fibres (Ren et al. 2011).
Fast cholinergic synaptic signalling involving vesicle exocytosis has been clearly detected in interneurons, while its presence in principal cells has been questioned (but see Hefft et al. 1999 and Grybko et al. 2011, for fast nAChR-mediated EPSCs in rats and mice, respectively). Hence, electrical stimulation of cholinergic fibres, in the presence of blockers of ionotropic glutamatergic and GABAergic transmission, elicits in stratum radiatum interneurons fast α7-mediated synaptic responses (Alkondon et al. 1998; Frazier et al. 1998b, 2003).
It is clear from this overview that nAChRs enhance GABAergic transmission from hippocampal interneurons. The magnitude and the final output of the response would depend on the subtypes of receptors involved and on neuronal connectivity. This may inhibit or disinhibit principal cells (Ji & Dani, 2000). Disinhibition of pyramidal neurons has been shown to facilitate gamma oscillations (Wang & Buzsaki, 1996).
nAChRs regulate the activity of O-LM interneurons
O-LM interneurons constitute a well-defined cellular population with soma and horizontal dendrites running parallel to the alveus and long axons that target the apical dendritic tufts of CA1 pyramidal cells aligned with entorhinal cortical inputs in stratum lacunosum moleculare (Lacaille et al. 1987; Maccaferri & McBain, 1996; Ali & Thomson, 1998; Maccaferri et al. 2000). O-LM interneurons, which contain somatostatin and express mGluR1α and neuropeptide Y receptors (Baude et al. 1993; Freund & Buzsaki, 1996; Katona et al. 1999; Maccaferri et al. 2000; Losonczy et al. 2002), exhibit fast spiking firing patterns caused by high densities of expression of voltage-gated sodium and potassium channels (Atzori et al. 2000; Martina et al. 2000; Lien et al. 2002; Lien & Jonas, 2003; Lawrence et al. 2006a). Moreover, they are endowed with hyperpolarization-activated channels (HCNs) carrying Ih, which underlies their pacemaker properties (Maccaferri & McBain, 1996; Ali & Thomson, 1998; Minneci et al. 2007) and with calcium-dependent potassium channels (BK and SK), which control action potential repolarization and the afterhyperpolarization (AHP), respectively (Zhang & McBain, 1995a,b). In vivo studies have demonstrated that during theta oscillations, O-LM cells become very active and, in cooperation with bistratified cells, modulate the dendrites of pyramidal cells one-quarter of a theta cycle after parvalbumin-expressing basket cells discharge (Klausberger & Somogyi, 2008). The O-LM firing is suppressed during ripple episodes (Klausberger et al. 2003). Furthermore, in vitro studies have shown that O-LM interneurons exhibit intrinsic resonance and spike transfer frequency preference within the theta range (Gillies et al. 2002; Pike et al. 2000; Hájos et al. 2004; Gloveli et al. 2005).
We used transgenic mice expressing enhanced green fluorescent protein in a subpopulation of stratum oriens interneurons containing somatostatin (Oliva et al. 2000) to assess the functional role of nAChRs on the firing properties of O-LM cells. Hence, we found that the postsynaptic calcium increase through calcium-permeable nAChRs and voltage-dependent calcium channels, activated by the depolarizing action of nicotine, facilitates the mobilization of calcium from intracellular stores.
This, in turn, activates apamin-sensitive calcium-dependent potassium conductances responsible for cell firing adaptation (Griguoli et al. 2009; Fig. 3). This effect follows the initial one consisting in an enhanced cell firing caused by the opening of cation-permeable channels (see also McQuiston & Madison, 1999) and probably mediated by non-desensitizing α2-containing nAChRs (Jia et al. 2009). Calcium increase via calcium-induced calcium release mechanisms will contribute to the prolongation of the effects of nicotine on firing adaptation. Like O-LM interneurons, auditory outer hair cells in the cochlea present a unique inhibitory synapse that uses a calcium-permeable excitatory acetylcholine receptor to activate hyperpolarizing currents mediated by SK channels (Art et al. 1984; Fuchs & Murrow, 1992; Blanchet et al. 1996). Previous studies from stratum oriens interneurons have demonstrated that cholinergic signalling via muscarinic receptors is crucial for tuning active conductances and for enhancing cell firing reliability (Lawrence et al. 2006a,b). Therefore, the dynamic integration of muscarinic and nicotinic signals will differentially control the firing properties of O-LM interneurons and rhythmogenesis.
Figure 3. Activation of nAChRs by nicotine or endogenously released ACh reduces the firing rate of O-LM interneurons.
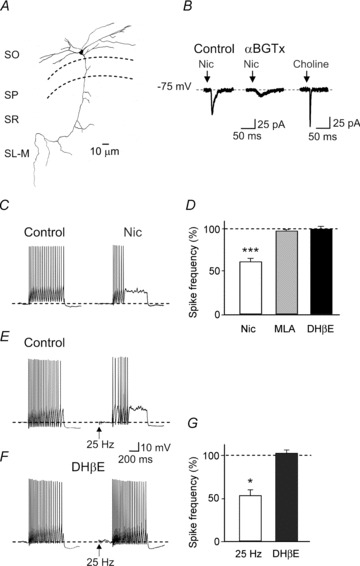
A, camera lucida reconstruction of a O-LM cell. B, puff application of nicotine induces a fast inward current followed by a slow one. In the presence of αBGTx (100 nm, middle) a slow component is unveiled. Pressure application of choline to another cell induces a fast response. C, regular spiking interneuron in control and after bath application of nicotine. D, each column represents the mean spike frequency values (expressed as percentage of control, dashed line), obtained in the presence of nicotine (n = 21) and nicotine plus MLA (n = 16) or DHβE (n = 16). E, a regular spiking neuron recorded before (Control) and immediately after delivering one train of high-frequency stimuli (2 s duration at 25 Hz) to cholinergic fibres in the alveus (in the presence of atropine). F, as in E but in the presence of a high (50 μm) concentration of DHβE. G, each column represents the mean spike frequency values obtained after stimulation of cholinergic fibres in the absence (open, n = 8) or in the presence (filled, n = 8) of DHβE and expressed as percentage of control (dashed line). *P < 0.05; ***P < 0.001. Modified from Griguoli et al. 2009.
nAChRs control activity-dependent synaptic plasticity processes
It is well known that nicotine, the neuroactive component of tobacco, enhances certain forms of memory (Rezvani & Levin, 2001). This occurs through nAChRs, highly expressed at pre- and post-synaptic sites in brain areas controlling learning and memory processes. In the hippocampus, a brain structure essential for encoding new declarative memories, nicotine has been shown to facilitate long-term potentiation (LTP) and convert short-term potentiation (STP) to LTP (Fujii et al. 1999; McGehee, 2002; Mann & Greenfield, 2003; Nashmi et al. 2007). The direction of synaptic changes (LTP or long-term depression, LTD) is strictly dependent on the localization of nAChRs and on the timing of their activation (Ji et al. 2001; Ge & Dani, 2005). For example, activation of nAChRs on CA1 pyramidal neurons can boost STP to LTP while stimulation of nAChRs on nearby interneurons can block LTP (Ji et al. 2001). In addition, exogenously applied ACh may convert STP to LTP or LTD, depending on the timing relative to afferent stimulation (Ge & Dani, 2005). In a series of elegant experiments, Gu & Yakel (2011), using electrophysiological and optogenetic tools, have demonstrated that different types of synaptic plasticity could be elicited in CA1 principal cells depending on the timing of the septal cholinergic input related to the Schaffer collateral input. Thus, stimulation of cholinergic afferents 100 ms or 10 ms prior to the Schaffer collateral resulted in α7 nAChR-dependent LTP or short-term depression (STD), respectively. It would be of interest to know how the precise timing of cholinergic modulation of synaptic plasticity at glutamatergic synapses affects local inhibitory circuits and rhythmogenesis.
In stratum radiatum interneurons, activation of α7 nAChRs by local photolysis of caged carbachol has been shown to significantly enhance cytoplasmic calcium levels in the perisomatic area (Khiroug et al. 2003). However, in these experiments, the extension of nAChR-mediated responses was probably underestimated due to dendritic filtering. More recently, calcium transients induced by activation of extrasynaptic nAChRs could be revealed also on dendrites of stratum radiatum interneurons (Rózsa et al. 2008). Dendritic calcium signalling, which increases as a function of the distance from the soma, interacts with back-propagating action potentials and, depending on the timing of α7 nAChR activation, may either potentiate or depress excitatory postsynaptic potentials. This cholinergic switch may be relevant for memory encoding and retrieval (Chang & Gold, 2003).
It is worth mentioning that a novel form of short-term plasticity involving extrasynaptic nAChRs, which closely depends on the time of agonist exposure and on the interval between exposures, has been described in stratum radiatum interneurons. By combining a dual-pulse uncaging protocol with patch clamp recordings, Klein & Yakel (2005) have found that at short intervals (less than 200 ms) the second α7 nAChR-mediated response evoked by photolysis of caged carbachol is potentiated whereas at longer intervals it is depressed, probably because of nAChR desensitization. The potentiating effect is mediated by calcium-dependent processes and requires receptor phosphorylation. Calcium-permeable nAChRs may also modulate the activity of other nearby localized receptors. Two recent studies (Wanaverbecq et al. 2007; Zhang & Berg, 2007) have demonstrated that calcium increase via α7 nAChRs, activated by direct application of nicotine or by endogenously released ACh, down-regulates GABAA-mediated synaptic currents. This effect can be favoured by the co-clustering of α7 nAChRs with GABAA receptors (Zago et al. 2006) but may occur also at distant sites via volume transmission (Umbriaco et al. 1995). The depressant effect which involved the activation of PKC, calcium–calmodulin-dependent protein kinase II and mitogen-activated protein kinase (MAPK), was clearly postsynaptic since it was blocked by chelating calcium in the postsynaptic cell and was not associated with modification in the paired-pulse ratio, a clear index of presynaptic release probability (Zucker, 1989). Whether the observed effect can be attributed to PKC-driven GABAA receptor phosphorylation or receptor internalization remains to be clarified. Interestingly, in the presence of α7 nAChR antagonists no run-down of whole cell GABAergic currents occurred, suggesting that in physiological conditions, GABAA receptors are controlled by ACh endogenously released from cholinergic fibres (Zhang & Berg, 2007). Down-regulation of GABAergic signalling may potentiate NMDA-mediated synaptic currents in principal cells and facilitate LTP induction, as demonstrated at CA3–CA1 synapses (Yamazaki et al. 2005, 2006),
At excitatory synapses between CA1 pyramidal cells and O-LM interneurons, a novel NMDA-independent form of LTP has been recently described (Lamsa et al. 2007). This form of LTP requires the activation of calcium-permeable AMPA receptors and type I mGluRs, and it has been named anti-Hebbian because presynaptic activation coincides with postsynaptic quiescence. This occurs when high-frequency stimulation (HFS) of presynaptic fibres is delivered to postsynaptic neurons maintained hyperpolarized (Lamsa et al. 2007; Le Duigou & Kullmann, 2011). Calcium-permeable AMPA receptors exhibit, in fact, a strong inward rectification that favours calcium entry at hyperpolarizing membrane potentials. Calcium rise via calcium-permeable AMPA receptors and mGluRs would activate a transduction pathway necessary for LTP induction. It would be of interest to test whether cholinergic signalling via nAChRs and mAChRs (activated by ACh released from cholinergic fibres during HFS) may contribute to anti-Hebbian LTP. It is known that, as calcium-permeable AMPA receptors, nAChRs exhibit a pronounced inward rectification (Bertarnd et al. 1993) due to polyamine block at depolarizing potentials (Haghighi & Cooper, 1998), a condition that favours calcium entry at relatively negative membrane potentials. In addition, since the concentration of polyamines in the cytoplasm could be dynamically regulated and nAChRs are several times more sensitive to spermine block than AMPA receptors (Haghighi & Cooper, 1998), it may be possible that their attenuation following repetitive synaptic activation (Rozov & Burnashev, 1999) will preferentially block nAChRs, promoting in this manner calcium flux via these receptor types.
Conclusions
The data reviewed here clearly indicate that cholinergic signalling via nAChRs plays a crucial role in regulating local GABAergic circuits in the hippocampus. Much remains to be discovered about the underlying cellular and molecular processes. In particular, most of the reported studies failed to characterize how nAChR signalling regulates the activity of well-identified interneuronal subtypes. In addition, it is unclear how nAChR-mediated changes in synaptic efficacy may affect the dynamic properties of the hippocampal circuit, rhythmogenesis, and how these functional changes relate to behaviour. These have important implications not only for the understanding of how information is stored and processed in the brain but also for pathological conditions including Alzheimer's and Parkinson's diseases and schizophrenia in which a cholinergic dysfunction parallels the loss of high cognitive functions (Kenney & Gould, 2008).
Selectively activating or silencing cholinergic input in specific neuronal ensembles using optogenetic tools will allow the correlation of hippocampal microcircuit functional structure with animal behaviour in both physiological and pathological conditions.
Acknowledgments
We are grateful to Manuela Schipizza Lough for carefully reading the manuscript. This work was supported by a Grant from Ministero Istruzione Universita’ e Ricerca (PRIN 2008) to E.C. We wish to thank our colleagues who contributed to some of the original work reported in this review and all members of the laboratory for useful discussions.
Glossary
Abbreviations
- ACh
acetylcholine
- α-BGTx
alpha-bungarotoxin
- DHβE
dihydro-beta-eritroidine
- GDP
giant depolarizing potential
- HFS
high-frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- mAChR
muscarinic acetylcholine receptor
- MAPK
mitogen-activated protein kinase
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- O-LM
oriens-lacunosum moleculare
- STP
short-term potentiation
Author's present address
M. Griguoli: University of Bordeaux and CNRS, Interdisciplinary Institute for Neuroscience, UMR 5297, F-33000 Bordeaux, France.
References
- Ali AB, Thomson AM. Facilitating pyramid to horizontal oriens-alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. J Physiol. 1998;507:185–199. doi: 10.1111/j.1469-7793.1998.185bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptorα7 and α4β2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004;145:109–120. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Braga MF, Pereira EF, Maelicke A, Albuquerque EX. α7 nicotinic acetylcholine receptors and modulation of GABAergic synaptic transmission in the hippocampus. Eur J Pharmacol. 2000;393:59–67. doi: 10.1016/s0014-2999(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. α-Bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. Endogenous activation of nAChRs and NMDA receptors contributes to the excitability of CA1 stratum radiatum interneurons in rat hippocampal slices: effects of kynurenic acid. Biochem Pharmacol. 2011;82:842–851. doi: 10.1016/j.bcp.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramakis VB, Hsieh CY, Leslie FM, Metherate R. A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20:6106–6116. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Fettiplace R, Fuchs PA. Synaptic hyperpolarization and inhibition of turtle cochlear hair cells. J Physiol. 1984;356:525–550. doi: 10.1113/jphysiol.1984.sp015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori M, Lau D, Tansey EP, Chow A, Ozaita A, Rudy B, McBain CJ. H2 histamine receptor-phosphorylation of Kv3.2 modulates interneuron fast spiking. Nat Neurosci. 2000;3:791–798. doi: 10.1038/77693. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiéry A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal α7 nicotinic receptor. Proc Natl Acad Sci U S A. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet C, Eróstegui C, Sugasawa M, Dulon D. Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J Neurosci. 1996;16:2574–2584. doi: 10.1523/JNEUROSCI.16-08-02574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco-Ibáñez JM, Freund TF. Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feed-back activation. Eur J Neurosci. 1995;7:2170–2180. doi: 10.1111/j.1460-9568.1995.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Chang KT, Berg DK. Nicotinic acetylcholine receptors containing α7 subunits are required for reliable synaptic transmission in situ. J Neurosci. 1999;19:3701–3710. doi: 10.1523/JNEUROSCI.19-10-03701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. 2005;308:1424–1428. doi: 10.1126/science.1108595. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Bulters DO, Suchak S, Riedel G, Morris RG, Davies CH. Activation of nicotinic acetylcholine receptors patterns network activity in the rodent hippocampus. J Physiol. 1999;518:131–140. doi: 10.1111/j.1469-7793.1999.0131r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Mechawar N, Aznavour N, Watkins KC. Structural determinants of the roles of acetylcholine in cerebral cortex. Prog Brain Res. 2004;145:45–58. doi: 10.1016/S0079-6123(03)45002-4. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the α7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via α-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998a;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an α-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998b;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Strowbridge BW, Papke RL. Nicotinic receptors on local circuit neurons in dentate gyrus: a potential role in regulation of granule cell excitability. J Neurophysiol. 2003;89:3018–3028. doi: 10.1152/jn.01036.2002. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Léránth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Schlander M, Léránth C. Cholinergic neurons in the hippocampus. A combined light- and electron-microscopic immunocytochemical study in the rat. Cell Tissue Res. 1986;246:293–301. doi: 10.1007/BF00215891. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Vida I, Bender R. Evidence for the existence of non-GABAergic, cholinergic interneurons in the rodent hippocampus. Neuroscience. 2000;96:27–31. doi: 10.1016/s0306-4522(99)00525-4. [DOI] [PubMed] [Google Scholar]
- Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. Cholinergic inhibition of short (outer) hair cells of the chick's cochlea. J Neurosci. 1992;12:800–809. doi: 10.1523/JNEUROSCI.12-03-00800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846:137–143. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- Ge S, Dani JA. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci. 2005;25:6084–6091. doi: 10.1523/JNEUROSCI.0542-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MJ, Traub RD, LeBeau FE, Davies CH, Gloveli T, Buhl EH, Whittington MA. A model of atropine-resistant theta oscillations in rat hippocampal area CA1. J Physiol. 2002;543:779–793. doi: 10.1113/jphysiol.2002.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562:131–147. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Griguoli M, Scuri R, Ragozzino D, Cherubini E. Activation of nicotinic acetylcholine receptors enhances a slow calcium-dependent potassium conductance and reduces the firing of stratum oriens interneurons. Eur J Neurosci. 2009;30:1011–1022. doi: 10.1111/j.1460-9568.2009.06914.x. [DOI] [PubMed] [Google Scholar]
- Grybko MJ, Hahm ET, Perrine W, Parnes JA, Chick WS, Sharma G, Finger TE, Vijayaraghavan S. A transgenic mouse model reveals fast nicotinic transmission in hippocampal pyramidal neurons. Eur J Neurosci. 2011;33:1786–1798. doi: 10.1111/j.1460-9568.2011.07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi AP, Cooper E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J Neurosci. 1998;18:4050–4062. doi: 10.1523/JNEUROSCI.18-11-04050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Pálhalmi J, Mann EO, Németh B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Hulo S, Bertrand D, Muller D. Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. J Physiol. 1999;515:769–776. doi: 10.1111/j.1469-7793.1999.769ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 2005;28:304–309. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Jia Y, Yamazaki Y, Nakauchi S, Sumikawa K. α2 nicotine receptors function as a molecular switch to continuously excite a subset of interneurons in rat hippocampal circuits. Eur J Neurosci. 2009;29:1588–1603. doi: 10.1111/j.1460-9568.2009.06706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Louis TM, Becker RF, Means LW. Behavioral effects of prenatal exposure to nicotine in guinea pigs. Neurobehav Toxicol Teratol. 1982;4:365–369. [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:3967–3972. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Acsady L, Freund TF. Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience. 1999;88:37–55. doi: 10.1016/s0306-4522(98)00302-9. [DOI] [PubMed] [Google Scholar]
- Kawa K. Acute synaptic modulation by nicotinic agonists in developing cerebellar Purkinje cells of the rat. J Physiol. 2002;538:87–102. doi: 10.1113/jphysiol.2001.012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Klein RC, Fayuk D, Yakel JL. Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci. 2003;23:9024–9031. doi: 10.1523/JNEUROSCI.23-27-09024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RC, Yakel JL. Paired-pulse potentiation of α7-containing nAChRs in rat hippocampal CA1 stratum radiatum interneurones. J Physiol. 2005;568:881–889. doi: 10.1113/jphysiol.2005.096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM. Interneuron networks in the hippocampus. Curr Opin Neurobiol. 2011;21:1–8. doi: 10.1016/j.conb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, Grinspan ZM, Statland JM, McBain CJ. Muscarinic receptor activation tunes mouse stratum oriens interneurones to amplify spike reliability. J Physiol. 2006a;571:555–562. doi: 10.1113/jphysiol.2005.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, Statland JM, Grinspan ZM, McBain CJ. Cell type-specific dependence of muscarinic signalling in mouse hippocampal stratum oriens interneurones. J Physiol. 2006b;570:595–610. doi: 10.1113/jphysiol.2005.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Duigou C, Kullmann DM. Group I mGluR agonist-evoked long-term potentiation in hippocampal oriens interneurons. J Neurosci. 2011;31:5777–5781. doi: 10.1523/JNEUROSCI.6265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Cherubini E. Presynaptic calcium stores contribute to nicotine-elicited potentiation of evoked synaptic transmission at CA3-CA1 connections in the neonatal rat hippocampus. Hippocampus. 2007;17:316–325. doi: 10.1002/hipo.20271. [DOI] [PubMed] [Google Scholar]
- Le Magueresse C, Safiulina V, Changeux JP, Cherubini E. Nicotinic modulation of network and synaptic transmission in the immature hippocampus investigated with genetically modified mice. J Physiol. 2006;576:533–546. doi: 10.1113/jphysiol.2006.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novère N, Changeux JP. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J Mol Evol. 1995;40:155–172. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol. 1993;15:251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- Lien CC, Jonas P. Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J Neurosci. 2003;23:2058–2068. doi: 10.1523/JNEUROSCI.23-06-02058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien CC, Martina M, Schultz JH, Ehmke H, Jonas P. Gating, modulation and subunit composition of voltage-gated K+ channels in dendritic inhibitory interneurones of rat hippocampus. J Physiol. 2002;538:405–419. doi: 10.1113/jphysiol.2001.013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, Kotimaa A, Moilanen I, Thomsen PH, Olsen J, Jarvelin MR. 2003). Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. La corteza cerebral de ratón (Primera contribución – La corteza acústica) Trabajos del Laboratorio de Investigaciones Biológicas de la Universidad de Madrid. 1922;20:41–78. [Google Scholar]
- Losonczy A, Zhang L, Shigemoto R, Somogyi P, Nusser Z. Cell type dependence and variability in the short-term plasticity of EPSCs in identified mouse hippocampal interneurons. J Physiol. 2002;542:193–210. doi: 10.1113/jphysiol.2002.020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- Maccaferri G. Stratum oriens horizontal interneurone diversity and hippocampal network dynamics. J Physiol. 2005;562:73–80. doi: 10.1113/jphysiol.2004.077081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Lacaille JC. Interneuron diversity series: hippocampal interneuron classifications – making things as simple as possible, not simpler. Trends Neurosci. 2003;26:564–571. doi: 10.1016/j.tins.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol. 2000;524:91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS. Nicotinic receptors and hippocampal synaptic plasticity … it's all in the timing. Trends Neurosci. 2002;25:171–172. doi: 10.1016/s0166-2236(00)02127-5. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature ‘silent’ connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Greenfield SA. Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol. 2003;551:539–550. doi: 10.1113/jphysiol.2003.045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Vida I, Jonas P. Distal initiation and active propagation of action potentials in interneuron dendrites. Science. 2000;287:295–300. doi: 10.1126/science.287.5451.295. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Latent synaptic pathways revealed after tetanic stimulation in the hippocampus. Nature. 1987;329:724–726. doi: 10.1038/329724a0. [DOI] [PubMed] [Google Scholar]
- Minneci F, Janahmadi M, Migliore M, Dragicevic N, Avossa D, Cherubini E. Signaling properties of stratum oriens interneurons in the hippocampus of transgenic mice expressing EGFP in a subset of somatostatin-containing cells. Hippocampus. 2007;17:538–553. doi: 10.1002/hipo.20291. [DOI] [PubMed] [Google Scholar]
- Mohajerani M, Sivakumaran S, Zacchi P, Aguilera P, Cherubini E. Correlated network activity enhances synaptic efficacy via BDNF and the ERK pathway at immature CA3–CA1 connections in the hippocampus. Proc Natl Acad Sci U S A. 2007;104:13176–13181. doi: 10.1073/pnas.0704533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA. Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg A. Human nicotinic receptors – their role in aging and dementia. Neurochem Int. 1994;25:93–97. doi: 10.1016/0197-0186(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike FG, Goddard RS, Suckling JM, Ganter P, Kasthuri N, Paulsen O. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J Physiol. 2000;529:205–213. doi: 10.1111/j.1469-7793.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Textura del sistema nervioso del hombre y de los vertebrados. Madrid: Moya; 1899. [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69:445–452. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptorβ2 subunit for the anatomical and functional development of the visual system. Proc Natl Acad Sci U S A. 2001;98:6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401:594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- Rózsa B, Katona G, Kaszás A, Szipöcs R, Vizi ES. Dendritic nicotinic receptors modulate backpropagating action potentials and long-term plasticity of interneurons. Eur J Neurosci. 2008;27:364–377. doi: 10.1111/j.1460-9568.2007.05999.x. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Robinson SE. Postnatal developmental regulation of neuronal nicotinic receptor subunitα7 and multiple α4 and β2 mRNA species in the rat. Brain Res Dev Brain Res. 1998;109:67–75. doi: 10.1016/s0165-3806(98)00058-3. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527:515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Karson MA, Nagode DA, McIntosh JM, Uebele VN, Renger JJ, Klugmann M, Milner TA, Alger BE. Nerve terminal nicotinic acetylcholine receptors initiate quantal GABA release from perisomatic interneurons by activating axonal T-type (Cav3) Ca2+ channels and Ca2+ release from stores. J Neurosci. 2011;31:13546–13561. doi: 10.1523/JNEUROSCI.2781-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Garcia S, Beaulieu C, Descarries L. Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1) Hippocampus. 1995;5:605–620. doi: 10.1002/hipo.450050611. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Lendvai B. Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res Brain Res Rev. 1999;30:219–235. doi: 10.1016/s0165-0173(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution ofα2, α3, α4, and β2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wanaverbecq N, Semyanov A, Pavlov I, Walker MC, Kullmann DM. Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynapticα7 nicotinic receptors. J Neurosci. 2007;27:5683–5693. doi: 10.1523/JNEUROSCI.1732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel JL, Shao Z. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat hippocampal interneurons. Prog Brain Res. 2004;145:95–107. doi: 10.1016/S0079-6123(03)45006-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Fujii S, Jia Y, Sumikawa K. Nicotine withdrawal suppresses nicotinic modulation of long-term potentiation induction in the hippocampal CA1 region. Eur J Neurosci. 2006;24:2903–2916. doi: 10.1111/j.1460-9568.2006.05160.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Jia Y, Hamaue N, Sumikawa K. Nicotine-induced switch in the nicotinic cholinergic mechanisms of facilitation of long-term potentiation induction. Eur J Neurosci. 2005;22:845–860. doi: 10.1111/j.1460-9568.2005.04259.x. [DOI] [PubMed] [Google Scholar]
- Zago WM, Massey KA, Berg DK. Nicotinic activity stabilizes convergence of nicotinic and GABAergic synapses on filopodia of hippocampal interneurons. Mol Cell Neurosci. 2006;31:549–559. doi: 10.1016/j.mcn.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berg DK. Reversible inhibition of GABAA receptors byα7-containing nicotinic receptors on the vertebrate postsynaptic neurons. J Physiol. 2007;579:753–763. doi: 10.1113/jphysiol.2006.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McBain CJ. Voltage-gated potassium currents in stratum oriens-alveus inhibitory neurones of the rat CA1 hippocampus. J Physiol. 1995a;488:647–660. doi: 10.1113/jphysiol.1995.sp020997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McBain CJ. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. J Physiol. 1995b;488:661–672. doi: 10.1113/jphysiol.1995.sp020998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Léna C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors usingb2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]


