Abstract
Human functional neuroimaging techniques provide a powerful means of linking neural level descriptions of brain function and cognition. The exploration of the functional anatomy underlying human memory comprises a prime example. Three highly reliable findings linking memory-related cognitive processes to brain activity are discussed. First, priming is accompanied by reductions in the amount of neural activation relative to naive or unprimed task performance. These reductions can be shown to be both anatomically and functionally specific and are found for both perceptual and conceptual task components. Second, verbal encoding, allowing subsequent conscious retrieval, is associated with activation of higher order brain regions including areas within the left inferior and dorsal prefrontal cortex. These areas also are activated by working memory and effortful word generation tasks, suggesting that these tasks, often discussed as separable, might rely on interdependent processes. Finally, explicit (intentional) retrieval shares much of the same functional anatomy as the encoding and word generation tasks but is associated with the recruitment of additional brain areas, including the anterior prefrontal cortex (right > left). These findings illustrate how neuroimaging techniques can be used to study memory processes and can both complement and extend data derived through other means. More recently developed methods, such as event-related functional MRI, will continue this progress and may provide additional new directions for research.
Keywords: implicit memory, prefrontal, visual, functional MRI, positron-emission tomography
Human memory is a remarkably complex cognitive function. It is inherently intertwined with all other aspects of cognition, shaping (and being shaped by) our thoughts and behaviors as we interact with the world. The study of the neurobiological basis of memory is therefore, not surprisingly, an enormous undertaking, requiring understanding at many levels ranging from the molecular to neural systems to cognitive approaches. Unfortunately, our experimental methods to bridge the gaps between these levels are limited. Linking neural systems and cognitive approaches requires observations or inferences about neuronal function in relation to human memory processes. Key methods that long have been available include the use of primate and other animal models (allowing both single-unit physiological recordings and ablation studies) and the study of human clinical populations (examination of patients with lesions and intraoperative recordings). Human functional neuroimaging techniques recently have emerged as promising alternative methods, providing a new window through which to view the neurobiological basis of human cognition (1). These techniques are extremely powerful in that they allow researchers to identify specific brain areas and pathways that are recruited and differentially activated during the performance of various cognitive tasks—including memory tasks—and to do so noninvasively, in normal, awake humans. These techniques are limited, however, in that the spatial scale within which different activations can be resolved is presently a few millimeters and the temporal resolution is ≈1 s. The topic we address in this paper is how these techniques can be applied to the study of human memory and findings that have emerged so far.
Memory refers to a function that unfolds over time. Within the context of human long term memory, the phenomenon to be examined concerns how information processing at one point in time influences processing at another—later—point in time. Such a notion has inherent in it the idea that memory can be differentiated into two kinds of processes: (i) active information processing that is isolated in time and (ii) processes or mechanisms that maintain and consolidate information over extended periods of time. Classic models of memory capture this idea and conceptualize memory processes as involving three separate stages: encoding, storage, and retrieval (or more elaborate expansions of these three stages; see, e.g., ref. 2). Encoding and retrieval are active processes that occur at relatively specific points in time; encoding refers to the initial processing of information that potentially instantiates a memory trace, and retrieval refers to newly evolved processing that results from, and often requires access to, prior encoding episodes. Somewhere between these two sets of active processes occur the more temporally distributed processes involved in storage and consolidation, the mechanisms that convert the otherwise transient encoding event into a more enduring form. (For review and discussion see, for example, refs. 3 and 4.)
The question relevant here is: Which, if any, of these processes can we observe with neuroimaging techniques? At present, it seems likely that neuroimaging methods allow us to observe the subset of memory processes described above as active memory processes that can be isolated in time. The two most commonly used neuroimaging techniques [PET blood flow and blood oxygenation level-dependent (BOLD) functional MRI (fMRI)] appear to be sensitive to physiologic events that correlate with acute changes in neuronal activity on the order of seconds (5–8). This means that we are capable of asking a range of questions: Which brain areas are active during tasks known to promote long term memory storage (e.g., deep encoding tasks)? Which brain areas are active when information is intentionally retrieved from memory (e.g., explicit recall and recognition)? How do the brain areas that are initially activated by a task change after repeated performance of the task or prior exposure to information involved in the task (e.g., skill learning and priming)? However, it seems unlikely that we can observe the temporally distributed processes related to storage and consolidation directly.
A consequence of this notion is that, when we are observing active memory processes with neuroimaging alone, we are, to a greater or lesser degree, simply observing what might be thought of as “specialized” instances of typical information processing—tasks that are known to create, or benefit from, storage- and consolidation-related processes that bridge gaps over time. Often these memory tasks are the same tasks that are elsewhere described as language tasks or attention tasks (9, 10). The fundamental basis for this interdependency might be that many processes subsumed under the concept of memory are a by-product of normal information processing (11–13). Where information processing ends and “memory encoding” begins, for example, is a blurry distinction at best. After all, in typical everyday life how often do we actively try to memorize something? Yet, at the end of the day, we can recall a wide range and number of details regarding what happened to us. The exception to this comes in relation to explicit (or episodic) memory retrieval. During explicit memory retrieval, the active task demand is to intentionally retrieve information acquired at another time. In our discussion of neuroimaging studies of memory, we will examine both findings related to memory encoding and memory retrieval.
Functional neuroimaging techniques are also unlikely to resolve directly the flow of information processing that occurs over very brief time scales, such as on the order of 10s of milliseconds (14). However, these processes can be observed with electrophysiological recording techniques, and perhaps the integration of functional neuroimaging and electrical recording techniques will provide a comprehensive description of the spatio-temporal orchestration of human neural processing (8, 15–20).
Tasks that Promote Long Term Memory Encoding.
Functional neuroimaging studies of memory encoding were first conducted serendipitously (see discussions in refs. 9 and 10). Early studies of language function required subjects to generate and/or elaborate on the meanings of words (21, 22). Although not specifically intended as such, these tasks, as well as similar tasks that followed (23–25), were excellent long term memory encoding tasks. Tulving and colleagues (26, 77) directly demonstrated this by testing subjects for recognition following performance of a task used by Petersen et al. (21) to explore language function. The task was word (verb) generation, in which individuals are presented with nouns (e.g., “dog”) and are asked to generate associated verbs (e.g., “bark”). Tulving found that subjects showed high levels of recognition performance for words encountered during this word generation task. The PET imaging studies showed that word generation activated a pathway of brain regions including left prefrontal areas, the anterior cingulate, and the right-lateral cerebellum. Thus, the first insight from functional neuroimaging related to memory was arrived at: Active encoding of verbal information is tied to activation of a brain pathway including the left prefrontal cortex and functionally related structures. A series of more directed studies followed.
Kapur et al. (26), by using PET, sought to identify brain areas activated by deep encoding. Subjects were instructed to decide whether visually presented words represented entities that were either living or nonliving. This meaning-based word processing task led to 75% correct recognition of the words. Imaging data contrasting this deep encoding task and a shallow encoding task (decide whether the word contains the letter “a,” 57% correct recognition) demonstrated robust left prefrontal activation overlapping with the regions previously activated by the word generation tasks. This basic pattern of findings also has been demonstrated with fMRI. For example, in a series of fMRI studies, Gabrieli and colleagues have explored the functional–anatomic correlates of another deep encoding task, in which participants view words and then decide whether they fall into the category of abstract (e.g., hope) or concrete (e.g., tree) words. They found significantly greater left prefrontal activation during this deep encoding task than during a shallow encoding task in which subjects simply decided whether words were presented in uppercase or lowercase letters (23, 27). Our laboratory, by using fMRI, has followed up on some of these findings, and representative data are shown in Fig. 1.
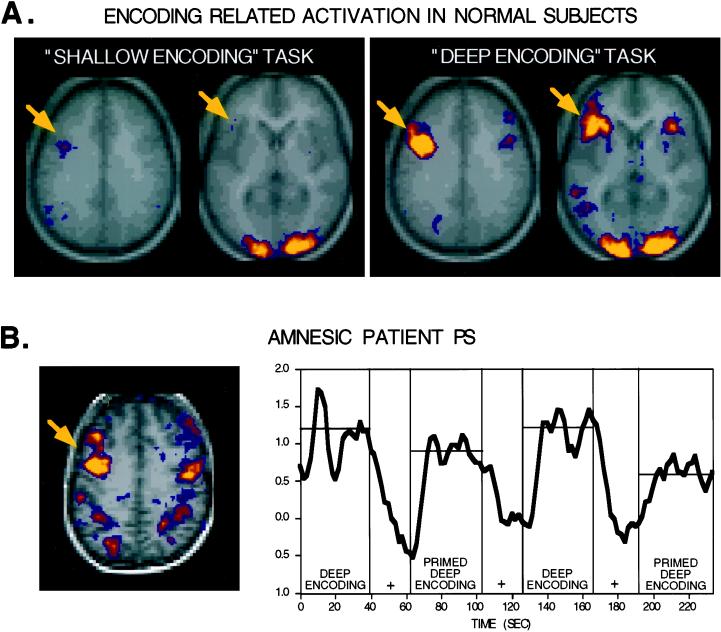
Figure 1.
(A) Horizontal sections from two levels show fMRI activation maps for a “shallow encoding” task contrasted with fixation and for a “deep encoding” task contrasted with fixation (averaged data from 12 normal subjects; K-S statistical activation map threshold = P < 0.001; brighter colors indicate greater significance; functional data overlie averaged anatomic image; right shown on the right). Both tasks share certain brain areas in common such as posterior visual areas whereas only the deep encoding task shows increased activation of left inferior and dorsal prefrontal areas (indicated with yellow arrows). These robust activations (P < 10−8) are at peak coordinates [Talairach 1998 atlas (91) (x, y, z)] -40, 9, 34 and -46, 6, 28 for the more dorsal activations and -40, 19, 3 and -43, 19, 12 for the more ventral prefrontal activations. The direct contrast between the deep and shallow encoding tasks also indicated that these regions differed significantly. (B) A horizontal section showing left dorsal prefrontal cortex activation in an amnesic patient during a “deep encoding” task, collected in collaboration with Verfaellie, Schacter, and Gabrieli. Robust activation was detected at -46, 3, 31 similar to normal subjects. The time course of activation within this region is shown to the right. Repeating items across the deep encoding task revealed significantly reduced activation (priming) as indicated by the time course (+, fixation control condition). This latter finding suggests that priming-related changes are present at a functional–anatomic level in amnesia, consistent with preserved behavioral priming often observed in amnesia.
Fletcher et al. (28) approached the issue in a different manner and compared later recall performance when participants first engaged in word generation concurrently with an easy distractor task (yielding high levels of recall, 83%) to a dual task situation in which word generation was paired with a difficult distractor task (yielding moderate levels of recall, 69%). Word generation paired with easy distraction, which presumably allows for more elaborate encoding, showed significantly greater left prefrontal activation than was observed during word generation in conjunction with the difficult distractor task. The conclusion across all of these studies is that the left prefrontal cortex, at or near Brodmann areas 44 and/or 45 and sometimes extending anteriorally and dorsally, is activated when subjects are engaging in tasks that lead to long term storage as assessed by later explicit retrieval tasks.
As can be seen across a wide range of manipulations, the data suggest that brain areas actively used to elaborate on word meaning or to access new words (word generation) are the same areas that lead to encoding of these events. Verbal working memory tasks also activate these areas (ref. 29; for reviews, see refs. 30 and 31). In a particularly elegant demonstration, prefrontal activation was shown to increase parametrically in relation to working memory load (32). The prefrontal activations tracking memory load were located in several areas of dorsolateral prefrontal cortex including portions of anterior frontal-operculum and the inferior frontal gyrus that directly overlap with areas activated by the “deep encoding” task shown in Fig. 1. What might this mean?
Buckner and Tulving (10) proposed that these functional neuroimaging studies demonstrate how multiple kinds of information processing might interact to promote encoding of long term memory. Effortful word generation tasks, verbal working memory tasks, and long term memory encoding tasks all activate similar brain pathways including left prefrontal regions and related structures. One possible account of this regularity is that the imaging studies conducted to date have not yet teased apart the various memory processes or separated distinct subregions within prefrontal cortex. This possibility currently is being explored further by several different laboratories. Alternatively, brain regions in left prefrontal cortex might be part of the neural substrate that maintains representations on-line (in working memory) while the representations are manipulated and used to guide further functions such as word access and generation. The basis of these representations may involve information coding in terms of phonology, lexical representations, more abstract semantic representations, or even coding of response alternatives. Further work is needed to explore the nature of the information that is being operated on in these left prefrontal regions.
The key point here is that these representations may themselves be the “encoding” that leads to storage and consolidation. This simple framework can be used to explain the depth of processing effect (12) at a functional anatomic level. Processing that requires verbal elaboration (deep processing) appears to activate left prefrontal cortex selectively whereas well automated tasks involving verbal information (shallow tasks) do not (see, e.g., ref. 23; Fig. 1). Shallow encoding tasks thus may lead less often to the formation of explicit long term memories because they do not initially require representation of the information in prefrontal cortex, the anatomic substrate that supports higher level representations necessary for conscious retrieval.
Two pieces of additional data come from functional neuroimaging studies of elderly and patient populations. Grady, Haxby, and colleagues (33, 34) examined brain areas activated during encoding of faces in young subjects and in elderly subjects. Consistent with the data discussed earlier, young subjects showed left prefrontal activation during this task. Elderly subjects, however, did not show significant left prefrontal activation, and the direct comparison between young and old subjects indicated that younger adults showed significantly greater activation in the left inferior prefrontal cortex. The relevance of this finding is tied to the behavioral result: Elderly subjects also recognized significantly fewer of the faces than young subjects. The intriguing interpretation, although somewhat speculative, is that the neuroimaging study captured the breakdown in engaging normal encoding processes that may accompany aging (35–37). Elderly subjects may have failed to spontaneously adopt appropriate encoding strategies that would bring the information into active working memory and activate left prefrontal regions. Their recognition performance suffers as a consequence.
Such a finding can be contrasted directly with a result we obtained in an amnesic patient. In collaboration with Verfaellie, Schacter, and Gabrieli, we imaged an amnesic subject (patient P.S., a 47 year-old with a prior anoxic insult) performing a deep encoding task on two separate occasions. During both sessions, behavioral performance during the “encoding” task (abstract/concrete word judgments) was close to normal. Consistent with this, robust and reproducible activations were observed in left prefrontal cortex [Fig. 1; similar to findings with several other amnesic patients (38)]. However, immediately after the fMRI session, the subject could not freely recall the words and was even uncertain as to whether words or pictures had been presented. Such a finding suggests that normal memory storage and/or retrieval did not occur despite the normal active and deep processing that was engaged during encoding. Thus, memory impairment was due to the failure or malfunctioning of processes after encoding, unlike the elderly subjects of Grady et al. (33) who presumably failed to encode the information properly at the time of presentation.
In contrast to the consistent findings regarding left prefrontal involvement in memory encoding, there have been sporadic findings of medial temporal lobe involvement in encoding processes. Deep vs. shallow and other verbal encoding manipulations often have failed to detect differential activation in these regions (26, 28, 39). One possible account of this failure is that the techniques are not capable of observing activation within these regions at all. However, this seems unlikely. A number of studies have reported medial temporal lobe involvement in encoding when novel, complex visual scenes or faces are presented (33, 34, 40–42). These activations often have extended posteriorally into extrastriate visual areas and also are detected during the performance of complex visual attention tasks, without obvious memory encoding demands (43). Such findings suggest these areas can be imaged and may be involved in high level perceptual demands and perhaps influenced by novelty and encoding manipulations, but it is unlikely that these activations are the common denominator connecting imaging studies with a “declarative memory system.” It seems more likely that they reflect more extensive visual processing occurring for novel stimuli that may not be directly related to the memory storage and consolidation processes per se, much in the same manner that increased activation of the left prefrontal cortex likely signifies more elaborate encoding of verbal information but not necessarily storage or consolidation.
The absence of consistent findings relating hippocampal formation activity to verbal encoding is a puzzle. The resolution may have to do with the technical considerations discussed earlier. Current neuroimaging techniques are likely most sensitive to acute changes in neuronal activity associated with temporally isolated processing events. It seems possible that critical memory-related processes subserved by medial temporal lobe structures may not include acute and differential activation during the initial encoding of information or may involve relatively sparse neural changes in relation to encoding (ref. 44; see ref. 28 for discussion). If such a possibility is correct, certain aspects of medial temporal lobe contributions to memory may simply be invisible to present human neuroimaging techniques (but see refs. 42 and 45 for alternative possibilities and refs. 42 and 46–50 for discussions of medial temporal lobe findings in relation to retrieval).
Long Term Memory Retrieval.
At a task level, two kinds of retrieval task can be studied: implicit and explicit retrieval (51, 52). The distinction between implicit and explicit retrieval tasks refers only to an operational definition of what subjects are required or encouraged to do during test and not to underlying memory systems or mechanisms (53). Implicit retrieval includes tasks in which a prior encoding episode affects performance on a task regardless of whether there is any intention to retrieve information from the past episode. Most effects on memory in everyday life are of this sort, in which we use the products of past learning without directly attempting to “revive” them from that past. Explicit retrieval refers to the intentional and conscious retrieval of information. Implicit retrieval, by its definition, is not necessarily a single entity; in fact, it seems more likely that it represents a diverse set of processes that allow the facilitation of information processing and control of overt behaviors through learning. Thus, any discussion of implicit retrieval has to be limited to a small domain of findings. For our purposes, focus is given to the phenomenon of priming.
Implicit Memory Retrieval (Priming).
When an item is repeated across a set of tasks, performance on the later task often improves. This facilitation in performance (priming) has been the focus of a number of functional neuroimaging studies. The first studies extended directly from the behavioral literature (54–57) by examining how prior exposure to words (e.g., “course”) facilitated the word stem completion task (e.g., complete word stems such as “cou”). By using PET, Squire and colleagues (refs. 50 and 58; see also ref. 47, 48, and 92) showed that posterior visual brain areas were highly active during visually guided word stem completion. Critically, certain bilateral visual areas were less active if subjects had experienced study words before the PET scans (see ref. 58 for discussion).
We and others (59) have observed such reductions during repeated processing of picture objects. In our study, conducted in collaboration with Rotte and Dale, single colored pictures of objects were presented, and subjects made a decision as to whether the objects tended to move on their own (e.g., bike) or to stay still (e.g., tree). New and repeated items were intermixed randomly and then analyzed by using event-related fMRI procedures (60–62). Subjects responded to repeated items significantly faster (811 ms) than new items (939 ms). Contrasting the activation patterns obtained for the new items with those for repeated items revealed reduced activation in extrastriate cortex for the repeated items, generalizing the phenomenon observed with word stem completion to the pictorial domain (Fig. 2). Together, these findings from word stem completion and picture repetition suggest a neural correlate of priming: Perceptual processing of a stimulus is more efficient after exposure to that stimulus, producing quicker response times and requiring less neural activity in brain areas activated for task performance (58). Such observations are reminiscent of primate single-unit studies that have detected suppression of activity in inferotemporal regions when object stimuli are repeated implicitly (63, 64). The functional neuroimaging and single-unit studies are quite possibly revealing different manifestations of the same underlying neuronal changes (65).
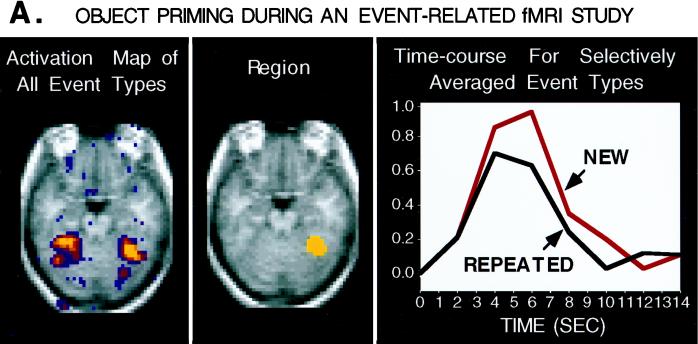
Figure 2.
A horizontal section shows averaged (n = 4) fMRI data for an object–classification task in which items were either new or repeated (primed). These activation maps are based on the t statistic and are derived from continuous runs of intermixed trials (mean intertrial interval = 8 s). Event-related fMRI techniques were used to make activation maps (61) and to remove the overlapping contributions of adjacent trials (60). The time course for a region of right extrastriate cortex (peak coordinate = 32, -48, -16) is shown for the new and repeated trials separately. Repeated items show a reduced level of activation relative to new items, suggesting a neural correlate of priming.
One question that arises from the word stem completion findings is why only perceptual regions showed significant priming effects, as indicated by reductions in activation, after prior exposure to the stimulus items. On the one hand, word stem completion entails many task demands, such as lexical search, that activate areas outside visual cortex (58, 66). Yet these areas showed minimal, if any, reductions in activation. On the other hand, the behavioral literature on priming suggests that priming-related facilitation on word stem completion and similar tasks is sensitive to alterations in the perceptual format of the stimuli, indicating that the effect likely depends, in part, on perceptual processing stages (52, 67). The resolution might lie in the fact that these early PET studies repeated items across repetitions but did not repeat the exact task demands. For example, in the studies of Buckner et al. (58), during the initial exposure to the target words, subjects performed a semantic decision task (rate how much they liked the meaning of the words) that was quite different from the word stem completion task subsequently used to assess the priming effect. Thus, conceptual, higher level task demands such as lexical search did not overlap across repetitions (much as in typical behavioral studies of word stem completion priming). Several more recent studies, however, have suggested that conceptual priming effects can be observed in high order brain regions under the appropriate conditions.
Raichle et al. (68) explored item repetition across a PET word (verb) generation task requiring semantic access. They found robust decreases in left prefrontal areas associated with increasing task facilitation across many item repetitions. Gabrieli and colleagues (23, 27) examined direct item repetition after a single exposure in a semantic decision task. Subjects viewed words and decided whether the words were abstract or concrete. In some instances, the same words were repeated. fMRI revealed that repetition was correlated with reduced activation in left prefrontal regions. Thus, priming effects can be observed in higher order brain regions when appropriate task processes overlap across item repetitions. Moreover, such an effect can be observed during word stem completion, a task typically thought to benefit mostly from perceptual priming. We demonstrated this across a series of three fMRI experiments in which we repeated visual (e.g., “cou”) (69) or auditory (e.g., the sound “pur”)‖ (35) word stem cues across repetitions. Critically, subjects always engaged the same task across repetitions: word stem completion. These conditions, which repeat the perceptual word stem cues as well as the exact lexical search demands, showed robust left prefrontal activation reductions. These reductions showed nearly identical locations across the visual and auditory variants, consistent with the notion that word stem completion can show an amodal, abstract priming effect when conceptual demands are held constant across repetitions.
Direct manipulations of task demands across repetitions have supported further the notion that the reductions are indeed process-specific (23, 39). Wagner et al. (39), for example, tested whether left prefrontal reductions would be observed under conditions in which both the stimulus items and the conceptual task demands re-occur across repetitions (i.e., the exact task repeats) but not during situations in which the stimulus items, but not the task, repeat. They found significant left prefrontal reductions only when both the item and task repeated but not when only items repeated.
Taken collectively, the existing data are consistent, in general terms, with the theoretical idea put forth by the transfer appropriate processing framework (67, 70, 71). Within this framework, facilitation across tasks under priming conditions is believed to occur when the same kinds of processing are drawn on across the item repetitions. Schacter and Tulving (72) have proposed a related idea that specific subsystems can be biased via priming, depending on where the processing benefit occurs. The functional neuroimaging data take these frameworks one step further by specifically revealing anatomic substrates of these facilitation effects. In instances in which perceptual processes overlap across repetitions, priming-related reductions manifest themselves in visual processing areas. When conceptual processes such as lexical or semantic retrieval are the source of facilitation, higher order brain regions in the left prefrontal cortex, among others, show reduced activation.
Current work is attempting to delineate, in more anatomic detail, where these priming benefits occur. Halgren, Dale, and colleagues (73), for example, further showed that the posterior visual cortex priming reductions tend to be in extrastriate areas involved in comparatively higher level visual processing, beyond early visual areas defined by retinotopy (74, 75) and perhaps as late in the visual processing stream as anterior TE. Such precise anatomic delineation undoubtedly will help to further link primate and human studies to better elucidate memory phenomena (65).
There is additional work to determine the origins of such priming effects. In normal human subjects, a complicating factor is the possibility that subjects will adopt explicit retrieval strategies regardless of task instructions, a phenomenon called “explicit intrusion” or “explicit contamination” (76). Researchers have explored two ways to address such an issue. The initial task in which items are first exposed can be constructed to minimize explicit awareness of information (e.g., by using shallow encoding). By doing so, subjects are unlikely to adopt explicit retrieval strategies because they do not have conscious access to the initial exposure of the items. By using such a procedure, Schacter et al. (47) observed activation reductions in perceptual processing regions during priming, suggesting that such reductions are not a consequence of explicit contamination. Unfortunately, this approach does not work for item repetition across conceptual processing tasks. Conceptual tasks, by design, are exactly those kinds of tasks that normally allow “deep” long term memory encoding (see earlier sections). To circumvent this further difficulty, researchers have turned to a patient group, organic amnesiacs, with impaired long term memory. Amnesic patients, in these situations, can be used to gain insight into whether observed neuroimaging correlates of conceptual priming are related to explicit contamination. Gabrieli et al. (38), and later ourselves in collaboration with his group, Verfaellie, and Schacter, explored whether conceptual priming effects would be observed in amnesic patients. Left prefrontal activation reductions were observed during conceptual priming in amnesia (Fig. 1), suggesting that, even when explicit retrieval strategies are ineffective, the proposed correlates of conceptual priming still can be observed. The implication of this finding is that, in normal subjects, priming related activation reductions arise from task components involving implicit retrieval and are not a by-product of explicit contamination.
Explicit Memory Retrieval.
Unlike the previous section on priming that discusses memory effects that occur regardless of whether there is an intent to retrieve from memory, explicit retrieval refers to situations in which a subject is actively and intentionally trying to gain access to past information (52). Squire and colleagues (50, 58) conducted an early set of PET studies to directly address this intentional retrieval demand. Subjects were asked to use word stems (e.g., “cou”) as cues to retrieve earlier presented study words (e.g., “courage”). Such a manipulation differs from the priming situation discussed above. In the priming (or implicit retrieval) paradigm, subjects were asked to generate any word that was a possible completion of the stems, but they nonetheless often unintentionally produced the study words. In the explicit retrieval conditions, subjects actively tried to remember the earlier study words. When these active intentional retrieval processes were engaged, a pathway of brain areas highly similar to the areas observed during implicit verbal retrieval was activated. However, in addition, several new brain areas including the anterior prefrontal cortex (right > left) and posterior medial parietal cortex also were activated, with these areas perhaps subserving, in some way, demands related to explicit retrieval.
A number of more recent studies of explicit retrieval also have found anterior prefrontal areas to be recruited across a wide range of tasks spanning both recognition (34, 77) and recall (28, 78, 79) and using various kinds of experimental materials, including words (58, 77) and pictures of faces and objects (33, 34, 79), and even during illusory recognition (80). The consistent observation of prefrontal activation and the tendency toward right greater than left lateralization was highlighted by the proposal of a model of hemispheric asymmetry in relation to memory function by Tulving and colleagues (9, 81). Their model states that there is preferential involvement of the left prefrontal cortex during encoding and of the right prefrontal cortex during episodic (explicit) retrieval. This important observation provides a useful heuristic, but it should be noted that the areas tending to show differential activation during encoding and retrieval are not homologous regions and are often activated together, as in the case of the explicit retrieval variant of word stem completion discussed above (58, 66). The more accurate characterization is probably that explicit retrieval tends to additionally recruit anterior prefrontal cortex at or near Brodmann area 10 (sometimes bilaterally but often right > left).
In view of this convergent finding, it is not surprising that researchers moved quickly to try to determine the exact role that this area might play in explicit retrieval. Explicit retrieval involves many demands that can be separated broadly into processes related to memory search (attempt) and processes related to retrieval success (recognition or recall). The concept of search captures, heuristically, the set of processes by which we attempt effortfully to gain access to past information whereas retrieval success refers to processes engaged when we succeed at gaining access to past information. The two represent general concepts and are useful only as heuristics. Nonetheless, they have provided the basis for a number of experiments that have sought to examine whether brain areas, particularly prefrontal areas, are activated differentially more by memory search or by success (47, 82–85). Unfortunately, consensus has not been reached. Both possibilities have been proposed: (i) that they are differentially involved in effortful processes related to retrieval search or attempt (47, 82) and (ii) that they are activated more by retrieval success (83). We recently have collected fMRI data that both agrees with the findings of Rugg et al. (83) and possibly explains some of the divergence across studies.
We first asked whether these anterior prefrontal areas could be detected with fMRI and whether they would be activated differentially during a retrieval condition that required much effort and was rarely successful (a situation created by the use of a shallow encoding task; 1005-ms response time, 47% correct) vs. a condition in which retrieval was almost always successful and required less effort (deep encoding task; 875-ms response time, 85% correct). Both conditions, which were examined in blocks of many successive trials of the same types, showed robust activation of the right anterior prefrontal cortex in nearly the same areas as have been found to be activated in numerous PET studies. Furthermore, consistent with the results of Rugg et al. (83), there was a tendency toward greater activation in the blocked condition with more retrieval success. However, there is a complicating factor in these studies: Many trials of a similar type are presented together, and it is possible that subjects may perceive changes in the likelihood of different trial types (and their requisite responses) across the blocks and so adopt different strategies across blocks. Such a block “context” effect is confounded in many neuroimaging studies but is particularly problematic for our blocked trial fMRI study because two kinds of trial are intermixed with quite different probabilities across the blocks. Thus, the context of the blocks might have been contributing to modulation of the anterior prefrontal cortex. This prompted us to explore, in a second study, whether the activations were related truly to retrieval success.
For this second study, we adopted recently developed procedures for selectively averaging fMRI responses to individual trials (61, 62, 86–88). By intermixing the trials and post hoc separating the trials in which subjects report failing to recognize items (unsuccessful retrieval) vs. those instances in which there was successful retrieval, we could ask whether differential activation of the anterior prefrontal cortex was associated exclusively with those items that were retrieved successfully. It is important to note that, because the trials were intermixed randomly, subjects could not predict or alter strategies differentially across the successful and unsuccessful retrieval events. The findings showed that significant right anterior prefrontal activation was elicited by trials in which subjects correctly designated that items were new (a form of “unsuccessful” retrieval) as well as during successful retrieval and, more importantly, that there were no differences across these two trial types (89). We believe this represents an appropriate test of the retrieval success hypothesis and shows that, in isolation, either kind of event type can activate anterior prefrontal cortex. Moreover, the combination of the blocked fMRI study we initially conducted and the second selective trial averaging study also suggests a possible interpretation of the past results: The anterior prefrontal cortex (right > left) is activated by many explicit retrieval tasks and can do so independently of retrieval success per se. However, as we and others have shown in paradigms that contain blocks of trials of a particular type, modulations of these regions may be induced, perhaps because of shifts in subject-initiated retrieval strategies (85).
CONCLUSIONS
At the outset, we noted that neuroimaging techniques are extremely powerful because they can provide information regarding particular brain regions involved in actually performing diverse types of cognitive tasks, including memory tasks. The findings discussed above illustrate this point. Tasks that promote long term memory encoding have tended to activate areas in the left prefrontal cortex as well as the anterior cingulate and the right-lateral cerebellum. These areas also are activated by word generation tasks and certain verbal working memory tasks, indicating the interdependency of the processing demands across these tasks that we often, possibly erroneously, think of as separate. Explicit memory retrieval has been associated with additional activation of several brain areas, including the anterior prefrontal cortex (often right > left), although the specific contribution that these areas make to retrieval remains largely unknown. Implicit retrieval manifesting priming has revealed reductions in brain areas activated for task performance, perhaps reflecting the facilitation of local processing regions as a consequence of item repetition.
It is also worthwhile to note that, although comparisons of the brain regions involved in specific tasks may in many cases be undertaken with the aim of identifying regions that are uniquely associated with a particular task, the nature of these comparisons will, unavoidably, also entail consideration of commonalties of activation. Regions may contribute to several kinds of cognitive tasks. Examining the correlations of brain activation with functional tasks may thus have an important side benefit of focusing attention on neural systems and their interactions across task types (90), such as was the case noted for left prefrontal involvement in long term memory encoding, elaborate word generation, and working memory.
Future attempts to reliably identify and understand the bases of both the differences and commonalities in activation patterns across tasks are thus likely to advance our understanding of the complex, and pervasive, function of memory in our lives. Such advances may arise directly, through providing new information on neural systems and correlates of memory, and indirectly: Efforts to interpret and integrate the functional neuroimaging findings may enhance our understanding of the tasks themselves and what it is, precisely, that we are doing—or not doing—when we deliberately set about to remember something (explicit memory) or use the products of past learning without ever becoming aware of an intention to remember (implicit memory) or engage in any of a multitude of other cognitive endeavors such as perception or attention for which memory is important.
Acknowledgments
We thank Bruce Rosen and Daniel Schacter for collaboration, support, and discussion and Nicholas Szumski for help with the preparation of this manuscript. Mieke Verfaellie, Michael Rotte, Anders Dale, Anthony Wagner, and John Gabrieli were also collaborators on several of the studies presented. Endel Tulving, Cheryl Grady, Anthony Wagner, Michael Posner, Steven Petersen, and an anonymous reviewer all provided valuable comments on earlier drafts of this manuscript. Support was provided by the McDonnell Center for Higher Brain Function, the Dana Foundation, the Human Frontiers Science Program, and National Institutes of Health grants: National Institute on Deafness and Other Communication Disorders DC03245 to R.L.B., National Institute of Mental Health AG-08377 to Steven Petersen, and National Institute on Aging AG08441 to Daniel Schacter in support of W.K.
ABBREVIATIONS
- PET
positron-emission tomography
- fMRI
functional MRI
Footnotes
Koutstaal, W., Buckner, R. L., Schacter, D. & Rosen, B. R. Fourth Annual Meeting of the Cognitive Neuroscience Society, March 23–25, 1997, Boston, MA, p. 68.
References
- 1.Posner M I, Raichle M E. Images of Mind. New York: Scientific American Books; 1994. [Google Scholar]
- 2.Tulving E. Elements of Episodic Memory. New York: Oxford Univ. Press; 1983. [Google Scholar]
- 3.Gluck M A, Myers C E. Annu Rev Psychol. 1997;48:481–514. doi: 10.1146/annurev.psych.48.1.481. [DOI] [PubMed] [Google Scholar]
- 4.Polster M R, Nadel L, Schacter D L. J Cognit Neurosci. 1991;3:95–116. doi: 10.1162/jocn.1991.3.2.95. [DOI] [PubMed] [Google Scholar]
- 5.Raichle M E. In: The Handbook of Physiology: Section 1. The Nervous System: Vol. V. Higher Functions of the Brain: Part 1. Plum F, Mountcastle V, editors. Bethesda, MD: Am. Physiol. Assoc.; 1987. pp. 643–674. [Google Scholar]
- 6.Kwong K K, Belliveau J W, Chesler D A, Goldberg I E, Weisskoff R M, Poncelet B P, Kennedy D N, Hoppel B E, Cohen M S, Turner R. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa S, Tank D W, Menon R, Ellerman J M, Kim S G, Merkle H, Ugurbil K. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen B R, Buckner R L, Dale A M. Proc Natl Acad Sci USA. 1998;95:773–780. doi: 10.1073/pnas.95.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tulving E, Kapur S, Craik F I M, Moscovitch M, Houle S. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner R L, Tulving E. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 10. Amsterdam: Elsevier; 1995. pp. 439–466. [Google Scholar]
- 11.Bartlett F C. Remembering: A Study in Experimental and Social Psychology. Cambridge: Cambridge Univ. Press; 1932. [Google Scholar]
- 12.Craik F I M, Lockhart R S. J Verb Learn Verb Behav. 1972;11:671–684. [Google Scholar]
- 13.Fuster J M. Memory in the Cerebral Cortex. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- 14.Robinson D L, Rugg M D. Biol Psychol. 1988;26:111–116. doi: 10.1016/0301-0511(88)90016-6. [DOI] [PubMed] [Google Scholar]
- 15.Dale A, Ahlfors S P, Aronen H J, Belliveau J W, Houtilainen M, Ilmoniemi R J, Kennedy W A, Korvenoja A, Liu A K, Reppas J B, Rosen B R, Sereno M I, Simpson G V, Standertskjold-Nordenstam C-G, Virtanen J, Tootell R B H. Soc Neurosci Abstr. 1995;21:1275. [Google Scholar]
- 16.Dale, A. M., Halgren, E., Lewine, J. D., Buckner, R. L., Paulson, K., Marinkovic, K. & Rosen, B. R. (1997) NeuroImage S592.
- 17.Snyder A Z, Abdullaev Y G, Posner M I, Raichle M E. Proc Natl Acad Sci USA. 1995;92:1689–1693. doi: 10.1073/pnas.92.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George J S, Aine C J, Mosher J C, Schmidt D M, Ranken D M, Schlitt H A, Wood C C, Lewine J D, Sanders J A, Belliveau J W. J Clin Neurophysiol. 1995;12:406–431. doi: 10.1097/00004691-199509010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Badgaiyan R D, Posner M I. J Neurosci. 1997;17:4904–4913. doi: 10.1523/JNEUROSCI.17-12-04904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinze H J, Mangun G R, Burchert W, Hinrichs H, Scholtz M, Munte T F, Gos A, Scherg M, Johannes S, Hundeshagen H, et al. Nature (London) 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- 21.Petersen S E, Fox P T, Posner M I, Mintun M A, Raichle M E. Nature (London) 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 22.Wise R, Chollet F, Hadar U, Friston K J, Hoffner E, Frackowiak R S J. Brain. 1991;114:1803–1817. doi: 10.1093/brain/114.4.1803. [DOI] [PubMed] [Google Scholar]
- 23.Demb J B, Desmond J E, Wagner A D, Vaidya C J, Glover G H, Gabrieli J D E. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein D, Milner B, Zatorre R J, Meyer E, Evans A C. Proc Natl Acad Sci USA. 1995;92:2899–2903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin A, Haxby J V, Lalonde F M, Wiggs C L, Ungerleider L G. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- 26.Kapur S, Craik F I M, Tulving E, Wilson A A, Houle S, Brown G M. Proc Natl Acad Sci USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabrieli J D E, Desmond J E, Demb J B, Wagner A D, Stone M V, Vaidya C J, Glover G H. Psychol Sci. 1996;7:278–283. [Google Scholar]
- 28.Fletcher P C, Frith C D, Grasby P M, Shallice T, Frackowiak R S J, Dolan R J. Brain. 1995;118:401–416. doi: 10.1093/brain/118.2.401. [DOI] [PubMed] [Google Scholar]
- 29.Desmond J E, Gabrieli J D E, Sobel N, Rabin L A, Wagner A D, Seger C A, Glover G H. Soc Neurosci Abstr. 1996;22:1111. [Google Scholar]
- 30.Fiez J A, Raife E A, Balota D, Schwarz J P, Raichle M E, Petersen S E. J Neurosci. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith E E, Jonides J. Cognit Psychol. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- 32.Braver T S, Cohen J D, Nystrom L E, Jonides J, Smith E E, Noll D C. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 33.Grady C L, McIntosh A R, Horwitz B, Maisog J M, Ungerleider L G, Mentis M J, Pietrini P, Schapiro M B, Haxby J V. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- 34.Haxby J V, Ungerleider L G, Horwitz B, Maisog J M, Rapoport S L, Grady C L. Proc Natl Acad Sci USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koutstaal, W. J. Mem. Lang. 37, 555–583.
- 36.Moscovitch M, Winocur G. Ann NY Acad Sci. 1995;769:119–150. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- 37.Rabinowitz J C, Craik F I M, Ackerman B P. Can J Exp Psychol. 1982;36:325–344. [Google Scholar]
- 38.Gabrieli J D E, Sullivan E V, Desmond J E, Stebbins G T, Vaidya C J, Keane M M, Wagner A D, Zarella M M, Glover G H, Pfefferbaum A. Soc Neurosci Abstr. 1996;22:632. [Google Scholar]
- 39.Wagner A D, Buckner R L, Koutstaal W, Schacter D L, Gabrieli J D E, Rosen B R. Fourth Annual Meeting of the Cognitive Neuroscience Society. 1997. p. 68. [Google Scholar]
- 40.Tulving E, Markowitsch H J, Craik F I M, Habib R, Houle S. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 41.Stern C E, Corkin S, Gonzalez R G, Guimaraes A R, Baker J R, Jennings P J, Carr C A, Sugiura R M, Vedantham V, Rosen B R. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabrieli J D, Brewer J B, Desmond J E, Glover G H. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- 43.Corbetta M, Miezin F M, Dobmeyer S, Shulman G L, Petersen S E. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClelland J L, McNaughton B L, O’Reilly R C. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 45.Martin A, Wiggs C L, Haxby J V, Weisberg J A. NeuroImage. 1997;5:S628. [Google Scholar]
- 46.Fletcher P C, Frith C D, Rugg M D. Trends Neurosci. 1997;20:213–223. doi: 10.1016/s0166-2236(96)01013-2. [DOI] [PubMed] [Google Scholar]
- 47.Schacter D L, Alpert N M, Savage C R, Rauch S L, Albert M S. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Backman L, Almkvist O, Andersson J, Nordberg A, Winblad B, Reineck R, Langstrom B. J Cognit Neurosci. 1997;9:378–391. doi: 10.1162/jocn.1997.9.3.378. [DOI] [PubMed] [Google Scholar]
- 49.Schacter D L, Reiman E, Uecker A, Polster M R, Yun L S, Cooper L A. Nature (London) 1995;376:587–590. doi: 10.1038/376587a0. [DOI] [PubMed] [Google Scholar]
- 50.Squire L R, Ojemann J G, Miezin F M, Petersen S E, Videen T O, Raichle M E. Proc Natl Acad Sci USA. 1992;89:1837–1341. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roediger H L, III, McDermott K B. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 8. Amsterdam: Elsevier; 1993. pp. 63–131. [Google Scholar]
- 52.Schacter D L, Chiu C-Y P, Ochsner K N. Annu Rev Neurosci. 1993;16:159–182. doi: 10.1146/annurev.ne.16.030193.001111. [DOI] [PubMed] [Google Scholar]
- 53.Schacter D L. J Exp Psychol Learn Mem Cognit. 1987;13:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- 54.Graf P, Squire L R, Hadden P. Science. 1982;218:1243–1244. doi: 10.1126/science.7146909. [DOI] [PubMed] [Google Scholar]
- 55.Graf P, Mandler G. J Verb Learn Verb Behav. 1984;23:553–568. [Google Scholar]
- 56.Bowers J, Schacter D L. J Exp Psychol Learn Mem Cognit. 1990;15:404–416. doi: 10.1037//0278-7393.16.3.404. [DOI] [PubMed] [Google Scholar]
- 57.Marsolek C J, Kosslyn S M, Squire L R. J Exp Psychol Learn Mem Cognit. 1992;18:492–508. doi: 10.1037//0278-7393.18.3.492. [DOI] [PubMed] [Google Scholar]
- 58.Buckner R L, Petersen S E, Ojemann J G, Miezin F M, Squire L R, Raichle M E. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin A, Lalonde F M, Wiggs C L, Weisberg J, Ungerleider L G, Haxby J V. Soc Neurosci Abstr. 1995;21:1497. [Google Scholar]
- 60.Ganis G, Kutas M, Schendan H E, Dale A M. Fourth Annual Meeting of the Cognitive Neuroscience Society. 1997. p. 42. [Google Scholar]
- 61.Dale A M, Buckner R L. Hum Brain Map. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 62.Buckner R L, Bandettini P A, O’Craven K M, Savoy R L, Petersen S E, Raichle M E, Rosen B R. Proc Natl Acad Sci USA. 1996;93:14878–14883. doi: 10.1073/pnas.93.25.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Miller E K, Desimone R. J Neurophysiol. 1994;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- 64.Riches I P, Wilson F A, Brown M W. J Neurosci. 1991;11:1763–1779. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ungerleider L G. Science. 1995;270:769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- 66.Buckner R L. Psychonom Bull Rev. 1996;3:149–158. doi: 10.3758/BF03212413. [DOI] [PubMed] [Google Scholar]
- 67.Roediger H L, III, Weldon M S, Challis B H. In: Varieties of Memory and Consciousness: Essays in Honor of Endel Tulving. Roediger H L, Craik F I M, editors. Hillsdale, NJ: Erlbaum; 1989. pp. 2–41. [Google Scholar]
- 68.Raichle M E, Fiez J A, Videen T O, MacLoed A-M K, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 69.Buckner R L, Koutstaal W, Schacter D L, Petersen S E, Raichle M E, Rosen B R. Fourth Annual Meeting of the Cognitive Neuroscience Society. 1997. p. 67. [Google Scholar]
- 70.Blaxton T A. J Exp Psychol Learn Mem Cognit. 1989;15:657–668. [Google Scholar]
- 71.Morris C D, Bransford J P, Franks J J. J Verb Learn Verb Behav. 1977;16:519–533. [Google Scholar]
- 72.Schacter D L, Tulving E. Memory Systems 1994. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- 73.Halgren E, Buckner R L, Marinkovic K, Rosen B R, Dale A M. Fourth Annual Meeting of the Cognitive Neuroscience Society. 1997. p. 34. [Google Scholar]
- 74.Sereno M I, Dale A M, Reppas J B, Kwong K K, Belliveau J W, Brady T J, Rosen B R, Tootell R B H. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 75.Tootell R B H, Dale A M, Sereno M I, Malach R. Trends Neurosci. 1996;19:481–489. doi: 10.1016/S0166-2236(96)10053-9. [DOI] [PubMed] [Google Scholar]
- 76.Schacter D L. In: Memory Systems 1994. Schacter D L, Tulving E, editors. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- 77.Tulving E, Kapur S, Markowitsch H J, Craik F I M, Habib R, Houle S. Proc Natl Acad Sci USA. 1994;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andreasen N C, O’Leary D S, Ardnt S, Cizadlo T, Hurtig R, Rezai K, Watkins G L, Boles Ponto L L, Hichwa R D. Proc Natl Acad Sci USA. 1995;92:5111–5115. doi: 10.1073/pnas.92.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buckner R L, Raichle M E, Miezin F M, Petersen S E. J Neurosci. 1996;16:6219–6235. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schacter D L, Buckner R L, Koutstaal W, Dale A M, Rosen B R. Fourth Annual Meeting of the Cognitive Neuroscience Society. 1997. p. 68. [Google Scholar]
- 81.Nyberg L, Cabeza R, Tulving E. Psychonom Bull Rev. 1996;3:135–148. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- 82.Kapur S, Craik F I M, Jones C, Brown G M, Houle S, Tulving E. NeuroReport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 83.Rugg M D, Fletcher P C, Frith C D, Frackowiak R S J, Dolan R J. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- 84.Nyberg L, Tulving E, Habib R, Nilsson L-R, Kapur S, Houle S, Cabeza R, McIntosh A R. NeuroReport. 1995;7:249–252. [PubMed] [Google Scholar]
- 85.Wagner A D, Gabrieli J D E, Desmond J E, Joaquim S, Glover G H. Soc Neurosci Abstr. 1996;22:719. [Google Scholar]
- 86.Konishi S, Yoneyama R, Itagaki H, Uchida I, Nakajima K, Kato H, Okajima K, Koizumi H, Miyashita Y. NeuroReport. 1996;8:19–23. doi: 10.1097/00001756-199612200-00005. [DOI] [PubMed] [Google Scholar]
- 87.Zarahn E, Aguirre G, D’Esposito M. NeuroImage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- 88.Josephs O, Turner R, Friston K. Hum Brain Map. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 89.Buckner R L, Koutstaal W, Dale A M, Schacter D L, Wagner A D, Rosen B R. Soc Neurosci Abstr. 1997;23:531. [Google Scholar]
- 90.Roediger H L, III, Buckner R L, McDermott K B. In: Unitary vs. Multiple Systems Accounts of Memory. Foster J K, Jelicic M, editors. New York: Oxford Univ. Press; 1998. [Google Scholar]
- 91.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 92.Blaxton T A, Bookheimer S, Zeffiro T A, Figlozzi C M, Gaillard W D, Theodore W H. Can J Exp Psychol. 1996;50:42–56. doi: 10.1037/1196-1961.50.1.42. [DOI] [PubMed] [Google Scholar]