Abstract
Cortical neuronal network operations depend critically on the recruitment of GABAergic interneurons and the properties of their inhibitory output signals. Recent evidence indicates a marked difference in the signalling properties of two major types of perisomatic inhibitory interneurons, the parvalbumin- and the cholecystokinin-containing basket cells. Parvalbumin-expressing basket cells are rapidly recruited by excitatory synaptic inputs, generate high-frequency trains of action potentials, discharge single action potentials phase-locked to fast network oscillations and provide fast, stable and timed inhibitory output onto their target cells. In contrast, cholecystokinin-containing basket cells are recruited in a less reliable manner, discharge at moderate frequencies with single action potentials weakly coupled to the phases of fast network oscillations and generate an asynchronous, fluctuating and less timed inhibitory output. These signalling modes are based on cell type-dependent differences in the functional and plastic properties of excitatory input synapses, integrative qualities and in the kinetics and dynamics of inhibitory output synapses. Thus, the two perisomatic inhibitory interneuron types operate with different speed and precision and may therefore contribute differently to the operations of neuronal networks.
Marlene Bartos studied Biology at the University of Braunschweig (Germany), and received her PhD at the Technical University of Munich on network activity patterns in invertebrates. She moved to the lab of Dr Mike Nusbaum at the University of Pennsylvania as postdoc (Philadelphia, USA) where she examined mechanisms underlying network synchronization in a ‘simple’ neuronal network of crabs. She continued her work by focusing on synaptic mechanisms underlying information processing during brain rhythms in a more ‘complex’ network in the hippocampus of rodents as Assistant Professor at the University of Freiburg, Germany (head of the Department Dr Peter Jonas). In 2007 she took up a Professorship at the School of Medical Sciences, University of Aberdeen (UK) and continued her work at the University of Freiburg in 2010.
 |
Introduction
Neuronal network functions depend markedly on the signalling characteristics of GABAergic inhibitory cells. Interneurons form a diverse population and can be subdivided into several types on the basis of their physiological properties, neurochemical marker content, somatodendritic location and most notably, the location of their axon terminals (Freund & Buzsáki, 1996). Among the various interneuron types, perisomatic inhibitory interneurons (PIIs) have attracted greatest attention because they comprise one of the largest interneuron subpopulation in cortical circuits (Freund & Buzsáki, 1996). Furthermore, they form soma-near inhibitory synapses (Klausberger & Somogyi, 2008), an optimal location to control the timing and frequency of action potential generation in target cells (Cobb et al. 1995; Miles et al. 1996; Pouille & Scanziani, 2001). The control of spike timing is an important requirement for the synchronization of principal cell assemblies and the emergence of fast network activity patterns (Bartos et al. 2007). In the hippocampus, theta oscillations (3–12 Hz) co-emerge with gamma rhyhms (30–120 Hz) during exploratory behaviour (Bragin et al. 1995; Buzsáki & Draguhn, 2004). They have been postulated to act as temporal reference signals for the processing of information and thus may underlie cognitive functions such as learning, memory formation and retrieval of stored information (Buzsáki & Draguhn, 2004). Finally, changes in the number and distribution of interneurons, specifically PIIs, seem to relate to neurological disorders, such as schizophrenia (Lewis et al. 2005) and epilepsy (Sayin et al. 2003; Wyeth et al. 2010).
Although PIIs show defined morphological properties, namely dense axonal collaterals in the principal cell layers (Freund & Buzsáki, 1996), they can also be classified on the basis of their neurochemical content into two major types, the parvalbumin (PV)- and the cholecystokinin (CCK)-expressing PIIs (Fig. 1). PV-PIIs in turn subdivide into two subgroups, the axo-axonic cells (AAs) and the basket cells (BCs). AAs contact the axon initial segment of postsynaptic principal cells whereas BCs form terminals at the soma and proximal dendrites of their target neurons (Freund & Buzsáki, 1996; Klausberger & Somogyi, 2008). Intrinsic and synaptic characteristics of PV-BCs and CCK-BCs are very different. First, PV-BCs discharge trains of short duration action potentials at high frequencies (>150 Hz at physiological temperatures; Fig. 1A; Doischer et al. 2008), whereas CCK-BCs fire accommodating spike trains at moderate frequencies (Fig. 1B; Lee et al. 2011). Second, PV-BCs have a low input resistance and fast membrane time constant (τm) of ∼10 ms (Doischer et al. 2008). In contrast, CCK-cells have higher input resistances and a twofold slower τm (Glickfeld & Scanziani, 2006; Cea-del Rio et al. 2010). Third, type 1 cannabinoid receptors (CB1R) are densely expressed at presynaptic terminals of CCK-positive cells but are absent at PV-cell output synapses (Katona et al. 1999). CB1Rs reduce GABAergic transmission after binding endogenous cannabinoids released from postsynaptic principal cells in an activity-dependent manner, a phenomenon well known as depolarization-induced suppression of inhibition (DSI; Fig. 1C; Katona et al. 1999; Glickfeld & Scanziani, 2006; Neu et al. 2007; Daw et al. 2009). Thus, the combination of dissimilar passive and active membrane properties together with the cell type-specific expression of DSI supports the notion that the two BC types may contribute differentially to information processing and the emergence of rhythmic activity patterns in the hippocampus.
Figure 1. Morphological and physiological characteristics of parvalbumin- and cholecystokinin-expressing basket cells.

A, top, parvalbumin expressing (PV)-BCs show a high frequency and non-accommodating discharge pattern and selectively express the calcium binding protein paravalbumin (PV) (right). Bottom, reconstruction of an intracellularly labeled PV-BC in CA1 with axon collaterals mostly restricted to the pyramidal cell layer (Str. Pyr.) B, top, cholecystokinin expressing (CCK)-BCs show a slow and accommodating train of action potentials when depolarized by suprathreshold current injection and express high levels of the neuropeptide cholecystokinin (CCK) as revealed by antibody labelling (right). Bottom, reconstruction of an intracellularly labeled CCK-BC shows that there are no apparent morphological differences to PV-BCs. C, left, representative paired recording of a presynaptic CCK-BC and the postsynaptic principal cell. Single action potentials (top) evoke unitary IPSCs (bottom). CCK-BCs can be identified by their sensitivity to depolarization induced suppression of inhibition (DSI; green; control IPSC, black; recovered IPSC, grey). Right, same experimental configuration as left with a presynaptic PV-BC. Note, the lack of DSI at PV-BC output synapses. Bottom, schematic illustration of the molecular mechanisms underlying DSI. Strong activation of pyramidal cells evokes the release of endogenous cannabinoids that retrogradely activate G-protein coupled cannabinoid receptors (CB1Rs) at CCK-BC terminals, thereby inhibiting GABA release. A and B reproduced from Lee et al. (2011) with permission from the Society for Neuroscience; C reproduced from Glickfeld & Scanziani, (2006) with permission from Macmillan Publishers Ltd, Nature Neuroscience©2006.
The functional roles of PIIs are determined by the sequence of events ranging from synaptic inputs signal integration and interneuron recruitment to GABA release at their output synapses. Following this temporal order, we will review some of the major differences in the molecular and cellular mechanisms which underlie these events in the two BC types. Finally, we will address the question of how the two cell types are involved in the emergence of fast (gamma) brain rhythms and the formation of principal cell assemblies. We will focus on the rodent hippocampus, although some of our conclusions may also be extrapolated to other cortical areas.
Differential strength and stability of PV-BC and CCK-BC excitatory inputs
The hippocampus shows a distinct laminar structure with a layer-specific distribution of afferent pathways and local axon collaterals. PV- and CCK-BCs have very similar bitufted dendritic projections which span many layers of the hippocampus (Fig. 1; Freund & Buzsáki, 1996). This structural arrangement allows both BC types in the three hippocampal regions (dentate gyrus {DG}, CA1, CA3) to receive inputs from all major excitatory pathways (Fig. 2A; see Fig. 1 in Bartos et al. 2011), including feedforward excitatory inputs from long-range extra- and intra-hippocampal projections and feedback recurrent excitation from local principal cells (Glickfeld & Scanziani, 2006; Sambandan et al. 2010). Recruitment of BCs by afferent pathways elicits short-latency feedforward inhibition which determines action potential timing (Pouille & Scanziani, 2001), whereas, activation by local recurrent pathways will produce feedback inhibition and limit the duration of the discharge period in the target cells. Thus, BCs can be recruited by different combinations of excitatory inputs and may thereby serve dynamic functions in the circuitry. However, PV-BCs seem to act as rapid signalling devices which produce precisely timed inhibition in their postsynaptic cells (Jonas et al. 2004; Doischer et al. 2008). On the contrary, CCK-BCs in CA1 can only discharge at lower frequencies (Klausberger et al. 2005; Tukker et al. 2007) and respond less readily to excitatory inputs. Efficient recruitment of cells depends on several factors including strength and dynamics of excitatory inputs and the mechanisms underlying integration of the input signals (Fig. 2). All these and may underlie their observed signalling differences factors seem to vary between PV-BCs and CCK-BCs.
Figure 2. Synaptic excitation, integration and recruitment of basket cells.

A, top left, schematic illustration of the major excitatory pathways converging onto PV- (blue) and CCK-BCs (red) in CA1. Middle left, extracellular stimulation of Schaffer collaterals induce EPSCs with amplitudes several times larger in CB1R-negative expressing (CB1R−) PV-BCs than in CB1R-positive expressing (CB1R+) CCK-BCs. EPSCs recorded in BCs were normalized to simultaneously recorded EPSCs in pyramidal cells (Pyr). Bottom left, plot summarizing the relationship between EPSCs recorded in both BC types and in pyramidal cells upon perforant path (PP) or Schaffer collateral (SC) stimulation. Top right, stimulation of the alveus at 20 Hz induces EPSCs with markedly stronger multiple-pulse depression in postsynaptic CCK-BCs (red traces) than in PV-BCs (blue traces). Bottom right, suprathreshold alveus stimulation at 20 Hz only transiently recruits CCK-BCs but allows continuous action potential generation in PV-BCs. B, top left, schematic illustration of the experimental design. Top right, integration of synaptic inputs was tested by SC stimulation at t = 0 ms (black arrow) paired with alveus stimulation at step-wise increasing time delays in relation to SC activation. Bottom, summary plot showing that CCK-BCs summate both inputs over longer inter-stimulus intervals than PV-BCs. Reproduced from Glickfeld & Scanziani (2006) with permission from Macmillan Publishers Ltd, Nature Neuroscience©2006.
Electron microscopical investigations showed that asymmetrical, putative glutamatergic synapses are distributed along the somatodendritic domain of interneurons with ∼90% of the terminals contacting dendritic domains (Gulyás et al. 1999). More importantly, PV-cells receive ∼3 times more asymmetrical inputs than CCK-cells (Gulyás et al. 1999; Mátyás et al. 2004). Consistent with these observations, extracellular stimulation of the Schaffer collateral pathway, the perforant path (PP), or recurrent axons of local principal cells induces excitatory postsynaptic currents (EPSCs) in CB1R-negative expressing BCs with peak amplitudes ∼7-fold larger than in CB1R-positive expressing BCs (Fig. 2A; Glickfeld & Scanziani, 2006). Thus, CA1 PV-BCs seem to receive a stronger excitatory input than their corresponding CCK-BC partners, which is consistent with the observed precise timing and reliable recruitment of PV-BCs (Glickfeld & Scanziani, 2006; Tukker et al. 2007; Oren et al. 2010). However, contrasting data have been recently determined in the CA3 area. Paired recordings between presynaptic mossy fibre (MF) boutons, the output synapses from DG granule cells, and postsynaptic BCs, show that unitary IPSCs at PV-BC synapses have ∼7-fold smaller peak amplitudes than their CCK-BC partners (Szabadics & Soltesz, 2009). Moreover, the probability of obtaining paired recordings is ∼3 times higher for MF–PV-BC pairs indicating a higher degree of convergence of excitatory inputs on PV- than on CCK-BCs (Szabadics & Soltesz, 2009). It would be interesting to examine whether the apparent dissimilarity in strength of excitatory inputs between CA1 and CA3 (MF vs. Schaffer collaterals) leads to circuit-dependent recruitment and different functional roles of the two BC types.
During physiological states, such as the emergence of rhythmic activity patterns in cortical networks, interneurons receive excitatory inputs at high frequencies. Under this condition EPSCs are subjected to short-term modulation. Indeed, repetitive activation of afferent fibres in CA1 or the DG at high frequencies (20 Hz) results in a decline in the peak amplitude of subsequently evoked EPSCs in both PV- and CCK-BCs (Fig. 2A; Glickfeld & Scanziani, 2006; Sambandan et al. 2010). The extent of multiple-pulse depression is, however, markedly higher in CCK- than in PV-BCs and contributes to weak and less frequent discharge of CA1 CCK-cells but reliable activation of PV-BCs during repetitive activation of afferent pathways (Fig. 2A; Glickfeld & Scanziani, 2006). It may also underlie the observed episodic discharge of CCK-BCs but reliable activity of PV-BCs during fast rhythmic activity patterns in vivo and in vitro (Fig. 5A; Klausberger et al. 2005; Tukker et al. 2007; Gulyás et al. 2010).
Figure 5. Differential contribution of PV-BCs and CCK-BCs in the emergence of network oscillations.

A, left, cell attached recordings from identified CA3 PV- and CCK-BCs and local field potential recordings during in vitro carbachol-evoked gamma oscillations in hippocampal slices. Middle, bar graphs summarizing the number of action potentials per gamma cycle and their phase-coupling strength of both BC types. Right, bath-application of DAMGO, a μ-opioid agonist that selectively inhibits GABA release at PV-BC output synapses, abolishes carbachol-induced gamma oscillations. B, left, top, diagram of the experimental design: in vivo extracellular recording of gamma oscillations in the prefrontal cortex of mice during activation of channelrhodopsin (ChR2)-expressing pyramidal cell (PY) with blue light and simultaneous activation of halorhodopsin (eNpHR)-containing PV-BCs with yellow light. Left, bottom, activation of eNpHR reduces action potential generation in PV-BCs. Right, top, filtered local field potential recordings in absence (red) or presence (black) of PV-cell activity. Right, bottom, summary plot of the PV-BC effect on gamma power. C, schematic diagram illustrating the hypothetical contribution of CCK-BCs on sparse coding in CA1 principal cells (PC). Timing of action potentials generated in PCs, CCK-BCs and PV-BCs is shown in relation to the phase of fast hippocampal network oscillations. A reproduced from Gulyás et al. (2010) with permission from the Society for Neuroscience; B reproduced from Sohal et al. (2009) with permission from Macmillan Publishers Ltd, Nature©2009; and C reproduced from Klausberger et al. (2005) with permission from the Society for Neuroscience.
Differential synaptic integration and recruitment of PV-BCs and CCK-BCs
Efficiency and precision in the recruitment of cells depends on the processing and integration of synaptic inputs. PV-BCs reliably translate rapid excitatory inputs into a fast short-latency inhibitory output (Jonas et al. 2004; Doischer et al. 2008). In contrast, CCK-BCs respond less readily to afferent signals. Differences in spatiotemporal processing of EPSPs at interneuron dendrites may play a key role in these cell type-dependent signalling characteristics. As EPSPs propagate from the dendrites to the soma they will be attenuated and strongly decelerated. The degree of filtering depends, however, on several factors including the location of the synapse, the geometrical parameters of the dendrites and the passive and active membrane properties (Johnston & Wu, 1995). PV-BCs have a short morphotonic length (measure of morphological dendritic parameters; Larkman et al. 1992) due to their large dendritic diameters (Emri et al. 2001; Nörenberg et al. 2010; Bartos et al. 2011). Thus, attenuation and deceleration are less pronounced than in other interneuron types (Emri et al. 2001). The morphotonic compactness also indicates that in PV-BCs attenuation is not as much dependent on synapse location along a given dendrite (Nörenberg et al. 2010). PV-BCs have a low input resistance (Rin∼80 MΩ; measured in response to somatic subthreshold current pulses) and a fast membrane time constant (τm) of ∼10 ms, which points to a low specific membrane resistance (Rm, resistance for a given membrane area; ∼10–15 kΩ; Bartos et al. 2002; Glickfeld & Scanziani, 2006; Nörenberg et al. 2010). The combination of a large dendritic diameter with low Rm will support fast propagation of EPSPs (Geiger et al. 1997; Doischer et al. 2008; Nörenberg et al. 2010). A consequence of a fast τm is that propagating EPSPS will arrive at the soma with short half-durations and thereby define narrow time windows for temporal summation (Fig. 2B; Glickfeld & Scanziani, 2006; Nörenberg et al. 2010). In contrast, CCK-BCs have a much higher Rin (∼150 MΩ) and longer τm (∼20 ms; Glickfeld & Scanziani, 2006; Cea-del Rio et al. 2010) than PV-BCs implying a higher Rm. Consequently, EPSPs will be less attenuated but will arrive at the soma with longer half-durations. CCK-BCs can therefore integrate inputs over longer time scales and be recruited during less frequent but repetitive depolarizing signals or by convergent inputs from various pathways (Fig. 2B; Glickfeld & Scanziani, 2006). This property is important for CCK-BCs as they receive slowly conducting signals from subcortical areas (Freund & Katona, 2007; Cea-del Rio et al. 2010). Similar mechanisms of input integration and BC recruitment may also be of relevance in the CA3 area, where PV-BCs receive weaker but more numerous MF inputs than CCK-BCs (Szabadics & Soltesz, 2009).
Several lines of evidence indicate that voltage-dependent conductances are key factors determining spatiotemporal signal processing within neuron dendrites (Johnston et al. 2003; Gulledge et al. 2005). However, limited information is available on the active membrane properties of distinct interneuron types including CCK-BCs (see Bartos et al. 2011) with the exception of PV-BCs in the DG. They express low densities of voltage-dependent Na+ conductances at their dendrites, but are equipped with high densities of voltage-dependent K+, predominantly Kv3-type, channels (Hu et al. 2010). Interestingly, K+ conductances facilitate the decay time course of EPSPs at apical dendrites of these cells and thereby enhance temporal precision in recruitment in PV-BCs (Hu et al. 2010). Furthermore, the density of K+ channels declines with distance from the soma, indicating that acceleration of EPSPs will be more profound at proximal than at distal dendrites.
In summary, interneurons are equipped with distinct passive and active membrane properties which allow cell type-specific processing of excitatory inputs. Fast, high frequency EPSPs will support precisely timed and reliable activation of PV-BCs but require coincident arrival of the inputs. In contrast, slow EPSPs will induce a less precise and unreliable recruitment of CCK-BCs. Thus CCK-BCs may be better suited to respond to slow and loosely correlated convergent inputs.
Differential glutamate receptor expression in PV-BCs and CCK-BCs
The majority of excitatory synaptic terminals at interneurons contain postsynaptic AMPA receptors (AMPARs). AMPARs are heterotetramers, composed of combinations of four subunits: GluR1, GluR2, GluR3 and GluR4 (Dingledine et al. 1999). Unlike pyramidal cells, which express AMPARs made up of GluR1 and GluR2, fast-spiking presumably PV-BCs in the DG express GluR1, GluR4 and low levels of GluR2 (Geiger et al. 1995). AMPARs lacking GluR2 are characterized by fast deactivation kinetics. Indeed, AMPAR-mediated excitatory postsynaptic currents (EPSCs) at MF to fast-spiking BC synapses have large peak conductances and rapid time courses with decay time constants in the submillisecond range (∼0.8 ms; Geiger et al. 1997). AMPARs lacking the GluR2 subunit show a Ca2+ permeability (CP) several times larger than GluR2 containing Ca2+ impermeable AMPARs (CI-AMPARs; Geiger et al. 1995). CP-AMPARs have inwardly rectifying current–voltage (I–V) relationships due to intracellular polyamine block at depolarized membrane potentials (Bowie & Mayer, 1995; Donevan & Rogawski, 1995), and can be blocked by philanthotoxin (PhTx). In contrast CI-AMPARs are characterized by a linear I–V and are insensitive to PhTx (Fig. 3; Tóth & McBain, 1998; Nissen et al. 2010; Sambandan et al. 2010). CP-AMPARs are abundantly expressed at MF synapses contacting PV-BCs in the DG and at recurrent inputs from CA1 pyramidal cells onto PV-BCs (Fig. 3A; Nissen et al. 2010; Sambandan et al. 2010). Conversely, synapses formed by CA1 pyramidal cells onto CCK-BCs express postsynaptic CI-AMPARs (Fig. 3B; Nissen et al. 2010). Although glutamate evoked currents in principal cells containing the GluR2 subunit show a slower deactivation than the ones evoked in fast-spiking BCs (Geiger et al. 1995), differences in kinetic properties of synaptically evoked EPSCs among the two interneuron types in CA1 (extracellular stimulation; Glickfeld & Scanziani, 2006) and CA3 (MF-BC paired recordings; Szabadics & Soltesz, 2009) are not evident. One possible explanation could be the differential expression of auxiliary transmembrane AMPAR-regulatory proteins (TARPs) in the two BC types. TARPs are powerful modulators of AMPAR channel proteins (Jackson & Nicoll, 2011). It has been shown that TARP association enhances the mean channel conductance of homomeric GluR2-lacking AMPARs (Soto et al. 2009) and slows down the kinetics of AMPAR deactivation and desensitization (Jackson & Nicoll, 2011). However, the extent of these effects is highly TARP type dependent (Jackson et al. 2011) and may vary among neuron types including PV-BCs and CCK-BCs.
Figure 3. Distinct AMPA receptor subunit compositions determine different plastic properties in PV-BCs and CCK-BCs.
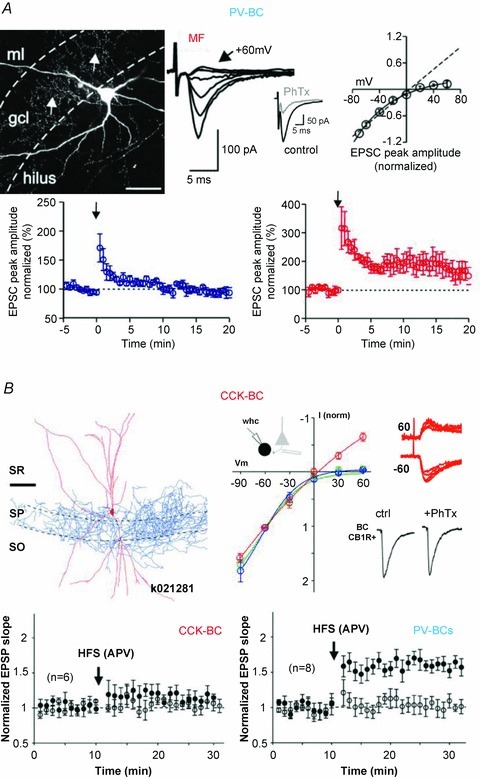
A, top left, intracellular labelling of a PV-BC in rat dentate gyrus (DG). Top right, extracellular stimulation of mossy fibres (MFs) activates AMPAR-mediated EPSCs with inwardly rectifying current–voltage (I–V) relationships. EPSCs mediated by Ca2+ permeable (CP)-AMPARs as indicated by their sensitivity to philantotoxin (PhTx). Bottom, associative high frequency stimulation of MFs induces long term potentiation at PV-BC (red) input synapses, but the same protocol applied to the perforant path is unable to do so (blue). B, top left, reconstruction of a CA1 CCK-BC. Top right, extracellular stimulation at the alveus induces EPSCs in CCK-BCs with linear I–V relation (red line and traces). EPSCs are mediated by Ca2+ impermeable (CI)-AMPARs as further indicated by the lack of PhTx sensitivity. Bottom, CA1 PV-BCs express long term potentiation after applying a non-associative high frequency stimulation to recurrent pyramidal cell (PC) inputs. In contrast, the same stimulation does not induce plastic changes at PC to CCK-BC synapses. Scale bars 100 μm. A reproduced from Sambandan et al. (2010) and B from Nissen et al. (2010) with permission from the Society for Neuroscience.
Distinct kinetic properties of AMPARs in PV- and CCK-BCs will influence synaptic integration and the time window for action potential generation, but differences in Ca2+ signalling via AMPARs will determine input specificity and may contribute to the induction of plastic changes in synaptic strength (Kullmann & Lamsa, 2007; Sambandan et al. 2010).
Differential expression of synaptic plasticity in PV-BCs and CCK-BCs
NMDA receptors (NMDARs) play a key role in the induction of synaptic plasticity in principal cells (Collingridge et al. 1983). In contrast, NMDAR-dependent long-lasting synaptic potentiation (LTP) was only rarely observed at input synapses onto interneurons (Lamsa et al. 2005). This fits with the low density of NMDARs in hippocampal interneurons in comparison to principal cells, which is lowest in PV-expressing cells (Nyíri et al. 2003). Consistent with the high heterogeneity in interneuron types, several forms of synaptic plasticity have been identified in GABAergic cells (Isaac et al. 2007; Kullmann & Lamsa, 2007; Nissen et al. 2010; Sambandan et al. 2010). However, PV-expressing cells seem to share one common mechanism for the induction of long-lasting synaptic plasticity: the activation of postsynaptic CP-AMPARs (Pelkey et al. 2005; Lamsa et al. 2007; Oren et al. 2009; Nissen et al. 2010; Sambandan et al. 2010). Associative LTP, which requires the pairing of strong presynaptic activation with postsynaptic action potential generation, was observed at recurrent MF inputs at PV-BCs in the DG (Fig. 3A). However, the same protocol was unable to induce any plastic changes when PP inputs, which are apparently devoid of CP-AMPAR, were activated (Sambandan et al. 2010). Furthermore, high frequency stimulation of recurrent collaterals induces LTP when paired with postsynaptic hyperpolarization in PV-BCs in CA1 (Fig. 3B; Lamsa et al. 2007; Oren et al. 2009; Nissen et al. 2010). In contrast, CCK-BCs in CA1 seem to lack synaptic plasticity at their recurrent principal cell inputs (Fig. 3B; Nissen et al. 2010), even when using the same protocol that was effective at inducing LTP in their PV-BC partners. The lack of synaptic plasticity correlates with the expression of CI-AMPARs at these specific inputs.
CP-AMPAR activation can also lead to long lasting depression (LTD). Induction of LTD is evident at recurrent synapses onto PV-expressing bistratified cells in CA1, which contact dendritic domains of their target cells (Nissen et al. 2010). Similarly, high frequency stimulation of recurrent CA3 collaterals onto stratum radiatum interneurons and MF inputs on CA3 stratum lucidum interneurons produces LTD (Laezza et al. 1999; Pelkey et al. 2005). Reduction in synaptic strength at these synapses depends on postsynaptic Ca2+ influx through CP-AMPARs and the coincident activation of presynaptic metabotropic GluR7. Taken together, synaptic plasticity adjusts the strength of synaptic communication between pyramidal cells and interneurons in a cell type-specific manner. It boosts recruitment of PV-BCs and thus fast perisomatic inhibitory output onto large neuronal cell populations, and at the same time reduces dendritic inhibition onto their target cells. Shifting the inhibitory weight from dendritic towards perisomatic inhibition may be an important mechanism in supporting high coherence of network oscillations.
Different timing and stability of PV-BC and CCK-BC output signalling
The functional characteristics of PV-BC and CCK-BC output synapses are markedly different (Fig. 4). Action potentials of PV-BCs have short half-durations (<0.4 ms) and propagate rapidly (∼0.25 m s−1) with minimal failure rate along the axon (Doischer et al. 2008). Thus, they support a rapid and reliable transduction of signals from the input to the release site. PV-BCs form 10–12 synapses at CA1 principal cells (∼50% at the soma and 50% at proximal dendrites; Buhl et al. 1994) and three to seven terminals at postsynaptic BCs (Bartos et al. 2001). Unitary IPSCs at PV-BC output synapses occur with minimal fluctuation in synaptic latency and peak amplitude and are generated with low failure rates (<0.5%), hence providing a stable inhibitory output onto target cells (Fig. 4A; Bartos et al. 2002; Hefft & Jonas, 2005). This temporal precision is generated by a highly synchronized GABA release (half-duration of quantal release ∼300 μs; Kraushaar & Jonas, 2000), mediated by the tight coupling of the Ca2+ source, the P/Q-type Ca2+ channels, and the Ca2+ sensor (Hefft & Jonas, 2005; Bucurenciu et al. 2008). Both, precision in timing of GABA release and high release probability (∼0.6; Kraushaar & Jonas, 2000), are largely determined by the time course of the presynaptic Ca2+ transient, which in turn is shaped by the half-duration of the presynaptic action potential. Although the size and kinetics of Ca2+ transients were not directly measured at PV-BC output synapses, imaging studies indicate that GABA release is evoked by low numbers of presynaptic Ca2+ channels (Bucurenciu et al. 2010). This will minimize asynchronous release and support phasic and precise transmission at PV-BC output synapses.
Figure 4. PV- and CCK-BCs differ in basic properties of GABA release.

A, top, paired recordings from a presynaptic PV-BC and a postsynaptic granule cell (GC; left) and a CCK-interneuron to GC pair (right) in the DG. Note differences in the timing of IPSCs and fluctuations in the amplitude. Bottom, 50 Hz trains of action potentials in presynaptic BCs induce fast and synchronized IPSCs at PV-BC to GC synapses but asynchronous IPSCs at CCK-interneuron output synapses. B, top, bar graph summarizing the ratio of synchronous to asynchronous GABA release ratio (synch./asynch. release) evoked by trains of 25 action potentials at 50 Hz for different presynaptic interneuron types (BSC, bistratified cell; TLC, trilaminar cell). Note, asynchronous release depends on the nature of the presynaptic interneuron but is independent of the identity of the target cell. Bottom, naturally occurring spike trains (5 bursts of 5 spikes at 50 Hz) in CCK expressing interneurons generate a continuous barrage of IPSCs in postsynaptic pyramidal cells (PCs). A reproduced from Hefft & Jonas (2005) with permission from Macmillan Publishers Ltd, Nature Neuroscience©2005; B reproduced from Daw et al. (2009) with permission from the Society for Neuroscience.
In contrast, CCK-BCs fire single action potentials with a half-duration of >0.5 ms that progressively broaden during repetitive activation (Cea-del Rio et al. 2010), resulting in a less timed transduction of the signal from the input to the output site. Although action potential propagation velocity has not been measured in CCK-BCs, unitary IPSCs evoked at their output synapses occur after longer delays than at PV-BC synapses (Hefft & Jonas 2005; Glickfeld & Scanziani 2006). CCK-BCs form ∼2 times fewer synapses at CA1 principal cells than PV-BCs (Nyíri et al. 2001) with 50% of them located at the soma and the remaining 50% at the dendrites (Klausberger et al. 2005). IPSCs mediated by these synapses show strong fluctuations in latency and amplitude as well as a high failure rate (∼16%), thereby generating an unstable inhibitory output signal (Fig. 4A; Hefft & Jonas, 2005; Daw et al. 2009). The low temporal precision can be largely explained by weak coupling between N-type Ca2+ channels and the release sites (Hefft & Jonas, 2005). GABAergic transmission at CCK-BC output synapses is further characterized by a marked asynchronous component, which results in a form of tonic inhibition (Fig. 4; Hefft & Jonas, 2005; Daw et al. 2009; Ali & Todorova, 2010). The time course of the asynchronous release rate depends on the number and frequency of presynaptic action potentials (Daw et al. 2009) and can vary between 58 ms for one and 295 ms for 10 action potentials in DG granule cells (Hefft & Jonas, 2005).
Paired recordings between interconnected CCK-cells in CA3 and CA1 (Daw et al. 2009), CCK-cell to PV-BC paired recordings in CA1 (Karson et al. 2009), and studies on synapses among CCK-interneurons in stratum lacunosmum-moleculare–radiatum of CA1 (Ali & Todorova, 2010) revealed that asynchronous release is a general property of synapses formed by CCK-cells and independent of the nature of the target neuron. However, Daw et al. (2009) and Karson et al. (2009) report that the ratio between synchronous and asynchronous release is independent of the target cell, whereas Ali & Todorova (2010) found that this ratio is larger for postsynaptic interneurons than for principal cells. The modulation of synchronous and asynchronous release by CB1Rs is a key characteristic of the function of output synapses formed by CCK-cells. Interestingly, the synchronicity ratio of GABA release at lacunosum-moleculare–radiatum interneuron output synapses is increased by the CB1R inverse agonist AM-251 but decreased by the agonist anandamide (Ali & Todorova, 2010). It would be interesting to examine whether synchronous and asynchronous release are also differentially altered during DSI.
In summary, PV- and CCK-BCs differ markedly in the timing and stability of inhibitory output signalling. These properties vary depending on the molecular and structural equipment of their output synapses. Inhibitory signalling correlates with the properties of excitatory inputs and the integration characteristics of the two cell types. The rapid input–output relationship indicates that PV-BCs act as fast signalling devices whereas CCK-BCs act as slow processing units.
Computational roles of PV-BCs and CCK-BCs in neuronal networks
The output synapses of BCs are proposed to play a key role in the emergence of gamma oscillations (Cobb et al. 1995; Buzsáki & Draguhn, 2004; Bartos et al. 2007). Fast-spiking PV-BCs discharge ∼1 action potential per cycle time-locked to the descending phases of gamma oscillations in acute hippocampal slice preparations and in anaesthetized rodents (Fig. 5A; Bragin et al. 1995; Csicsvari et al. 2003; Klausberger et al. 2005; Mann et al. 2005; Oren et al. 2010; Gulyás et al. 2010). PV-BCs are highly interconnected by gap junctions (∼20%; Bartos et al. 2002) and chemical synapses (∼10%; Bartos et al. 2002, 2007; Doischer et al. 2008). Fast transmission of filtered action potentials via gap junctions will provide a rapid postsynaptic depolarization which will be followed by a delayed synaptic inhibition. Experimental and theoretical studies have shown that this mechanism boosts the generation of gamma oscillations in interneuron networks (Whittington et al. 1995; Vida et al. 2006). Thus, reliable recruitment of a few PV-BCs will synchronize their activity via electrical and chemical synapses and spread a coherent inhibitory output onto the circuitry. A regular and phasic inhibition will set time windows for action potential generation in principal cells (Pouille & Scanziani, 2001), a key mechanism for the synchronization of principal cell assemblies (Csicsvari et al. 2003).
To directly determine the role of PV-BCs in the generation of gamma oscillations, recent new technologies have been developed to modulate their activity in a circuit-specific manner. Cre-recombinase-dependent expression of channelrhodopsin 2 (ChR2), a cation channel activated by blue light (∼470 nm), or the enhanced Natronomonas pharaonis halorhodopsin (eNpHR), a Cl− pump driven by yellow light (∼590 nm), through injection of adeno-associated viruses (AAVs) in PV-Cre mice, allows the selective activation or silencing of PV-BCs, respectively (Fig. 5B; Cardin et al. 2009; Sohal et al. 2009). Light-driven activation of fast-spiking PV-ChR2-cells specifically boosts gamma band oscillations (Cardin et al. 2009), whereas light-driven hyperpolarization of PV-eNpHR-cells results in a marked reduction in gamma power (Sohal et al. 2009). Thus, PV-BCs are significant elements in the emergence of behaviourally related gamma rhythms. To examine the relevance of PV-BCs in cognitive functions a new strategy was developed to specifically silence their output synapses (Murray et al. 2011). Block of GABA release was accomplished by AAV-driven expression of tetanus toxin light chain in the CA1 area of PV-Cre mice, which cleaves VAMP2 and stops vesicle fusion. The lack of phasic inhibition produced impaired spatial working memory in these mice but left spatial reference memory unperturbed (Murray et al. 2011).
The unreliable and less precise output of CCK-BCs suggests a weaker contribution to the generation of fast brain rhythms. Indeed, CCK-BCs fire action potentials sporadically during gamma oscillations with lower phase coupling strength than their PV-BC partners, both in vitro and in vivo (Fig. 5A; Tukker et al. 2007; Klausberger & Somogyi, 2008; Gulyás et al. 2010). What could be the functional role of CCK-BCs in neuronal networks? Previous investigations have shown that a fluctuating inhibitory signal may regulate the balance between excitation and inhibition, implementing a gain control mechanism (Mitchell & Silver, 2003). Furthermore, the tonic form of inhibition could act as an offset which increases the excitatory drive needed to reach action potential threshold in principal cells. CCK-BCs could provide both mechanisms to the network. During explorative behavior-induced gamma oscillations, principal neurons are sparsely active (Leutgeb et al. 2007). However, once the animal passes the centre of the place field, place cells discharge bursts of action potentials at ∼20 Hz (Leutgeb et al. 2007). Under these conditions, principal cells can release endogenous cannabinoids and silence GABA release at inputs from CCK-cells (Neu et al. 2007). CCK-BCs discharge action potentials during the ascending phase of single gamma cycles and overlap markedly with the activity of CA1 principal cells in vivo (Fig. 5C; Tukker et al. 2007; Klausberger & Somogyi, 2008). This spatially restricted disinhibition (via CB1Rs) of strongly recruited principal cells but maintained inhibition of weakly active or silent principal cells (inhibited by CCK-cells) will support the ‘winner takes all’ situation and enhance the signal-to-noise ratio at the network level. Such a mechanism could play an important role in processing and encoding of information in principle cell assemblies (Fig. 5C; Klausberger & Somogyi, 2008).
Which tools are available to examine the functional role of CCK-BCs in neuronal networks? Pharmacological modulation of CB1R as a mean to dissect the participation of CCK-cells in cortical function is unspecific due to their expression in both CCK-interneurons and principal cells (Ali & Todorova 2010, Holderith et al. 2011). Therefore, circuit- and cell type-specific loss-of-function studies using newly developed CCK-Cre mouse lines, in which Cre is expressed solely in GABAergic cells (Taniguchi et al. 2011), would be highly favourable to answer this important question. For example, stereotaxic injection of recombinant AAVs encoding proteins that interfere with GABA release (e.g. tetanus toxin light chain, Murray et al. 2011) in defined cortical areas of CCK-Cre-expressing mice would allow a circuit-specific loss of CCK-cell output signalling.
Concluding remarks
The signalling characteristics of PV-BCs and CCK-BCs are highly dissimilar. This is largely based on differences in the strength, dynamics and incidence of excitatory synaptic inputs, their integrative properties, and the functional characteristics of their inhibitory synaptic outputs. Although the importance of PV-BCs in gamma oscillogenesis and cognition is well supported, detailed investigations are needed to address the contribution of CCK-BCs in network dynamics and formation of cell assemblies. Here we have highlighted BCs, but interneurons appear in many flavours which may reflect their different functions in cortical networks. The input–output relations of the various interneuron types are not well understood. Thus, future investigations both in vitro and in vivo are required to uncover the full breadth of functional interneuron diversity. New techniques, such as whole-cell recordings in the behaving animal, genetic tools for functional ablation of defined interneuron types together with optogenetic manipulations provide exciting possibilities to address these challenging questions.
Acknowledgments
We thank Jonas-Frederic Sauer and Sha Savanthrapadian for critical comments on the manuscript. The work relevant to this Review was supported by the SFB 505 C6 (M.B.), the Lichtenberg Award (M.B.), the University of Aberdeen (M.B.), the Schram Award (M.B.) and FRIAS at the University of Freiburg (M.B.).
Glossary
Abbreviations
- AA
axo-axonic cell
- BC
basket cell
- CB1R
cannabinoid receptor type 1
- CCK
cholecystokinin
- ChR2
channelrhodopsin 2
- CI-AMPAR
Ca2+-impermeable AMPA receptor
- CP-AMPAR
Ca2+-permeable AMPA receptor
- DG
dentate gyrus
- DSI
depolarization-induced suppression of inhibition
- eNpHR
enhanced Natronomonas pharaonis halorhodopsin
- GluR
glutamate receptor
- MF
mossy fibre
- PII
perisomatic inhibitory interneuron
- PP
perforant path
- PV
parvalbumin
- Pyr
pyramidal cells
- TARP
transmembrane AMPAR regulatory protein
- SC
Schaffer collateral
References
- Ali AB, Todorova M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur J Neurosci. 2010;21:1196–1207. doi: 10.1111/j.1460-9568.2010.07165.x. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Geiger JR, Jonas P. Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J Neurosci. 2001;21:2687–2698. doi: 10.1523/JNEUROSCI.21-08-02687.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JRP, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Nat Acad Sci USA. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Bartos M, Alle H, Vida I. Role of microcircuit structure and input integration in hippocampal interneuron recruitment and plasticity. Neuropharmacology. 2011;60:730–739. doi: 10.1016/j.neuropharm.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jandó G, Nádasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Bucurenciu I, Bischofberger J, Jonas P. A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nat Neurosci. 2010;13:19–21. doi: 10.1038/nn.2461. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cea-del Rio CA, Lawrence JJ, Tricoire L, Erdelyi F, Szabo, McBain CJ. M3 muscarinic acetylcholine receptor expression confers differential cholinergic modulation to neurochemically distinct hippocampal basket cell subtypes. J Neurosci. 2010;30:6011–6024. doi: 10.1523/JNEUROSCI.5040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, Bartos M. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;26:12956–12968. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci U S A. 1995;92:9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emri Z, Antal K, Gulyás AI, Megías M, Freund TF. Electrotonic profile and passive propagation of synaptic potentials in three subpopulations of hippocampal CA1 interneurons. Neuroscience. 2001;104:1013–1026. doi: 10.1016/s0306-4522(01)00136-1. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Geiger JRP, Lübke J, Roth A, Frotscher M, Jonas P. Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron. 1997;18:1009–1023. doi: 10.1016/s0896-6273(00)80339-6. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol. 2005;65:205–206. doi: 10.1002/neu.20144. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Megías M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neuroscience. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, Szabó GG, Ulbert I, Holderith N, Monyer H, Erdélyi F, Szabó G, Freund TF, Hájos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Holderith N, Németh B, Papp Ol, Veres JM, Nagy GA, Hájos N. Cannabinoids atenuate hippocampal gamma oscillations by supressing excitatory synaptic input onto CA3 pyramidal neurons and fast spiking basket cell. J Physiol. 2011;589:4921–4934. doi: 10.1113/jphysiol.2011.216259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Martina M, Jonas P. Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science. 2010;327:52–58. doi: 10.1126/science.1177876. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary sub-units. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Milstein AD, Soto D, Farrant M, Cull-Candy SG, Nicoll RA. Probing TARP modulation of AMPA receptor conductance with polyamine toxins. J Neurosci. 2011;31:7511–7520. doi: 10.1523/JNEUROSCI.6688-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Wu SM-S. Foundations of Cellular Neurophysiology. MIT Press, Cambridge, MA, USA; 1995. [Google Scholar]
- Johnston D, Christie BR, Frick A, Gray R, Hoffman DA, Schexnayder LK, Watanabe S, Yuan LL. Active dendrites, potassium channels and synaptic plasticity. Philos Trans R Soc Lond B Biol Sci. 2003;358:667–674. doi: 10.1098/rstb.2002.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron Diversity series: Fast in, fast out-temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O’Neill J, Huck JHJ, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraushaar U, Jonas P. Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse. J Neurosci. 2000;20:5594–5607. doi: 10.1523/JNEUROSCI.20-15-05594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science. 1999;285:1411–1414. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci. 2005;8:916–924. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman AU, Major G, Stratford KJ, Jack JJB. Dendritic morphology of pyramidal neurones of the visual cortex of the rat. IV: Electrical geometry. J Comp Neurol. 1992;323:137–152. doi: 10.1002/cne.903230202. [DOI] [PubMed] [Google Scholar]
- Lee SY, Földy C, Szabadics J, Soltesz I. Cell-type-specific CCK2 receptor signaling underlies the cholecystokinin-mediated selective excitation of hippocampal parvalbumin-positive fast-spiking basket cells. J Neurosci. 2011;31:10993–11002. doi: 10.1523/JNEUROSCI.1970-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Mátyás F, Freund TF, Gulyás AI. Convergence of excitatory and inhibitory inputs onto CCK-containing basket cells in the CA1 area of the rat hippocampus. Eur J Neurosci. 2004;19:1243–1256. doi: 10.1111/j.1460-9568.2004.03225.x. [DOI] [PubMed] [Google Scholar]
- Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Sauer J-F, Riedel G, McClure C, Ansel L, Cheyne L, Bartos M, Wisden W, Wulff P. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu A, Földy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen W, Szabo A, Somogyi J, Somogyi P, Lamsa KP. Cell type-specific long-term plasticity at glutamatergic synapses onto hippocampal interneurons expressing either parvalbumin or CB1 cannabinoid receptor. J Neurosci. 2010;30:1337–1347. doi: 10.1523/JNEUROSCI.3481-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörenberg A, Hu H, Vida I, Bartos M, Jonas P. Non-uniform cable properties optimize rapid signaling in fast-spiking GABAergic interneurons. Proc Natl Acad Sci U S A. 2010;107:894–899. doi: 10.1073/pnas.0910716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyíri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of α2-subunit containing GABAA receptors in synapses of hippocampal pyramidall cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Nyíri G, Stephenson FA, Freund TF, Somogyi P. Large variability in synaptic N-methyl-D-aspartate receptor density on interneurons and a comparison with pyramidal-cell spines in the rat hippocampus. Neuroscience. 2003;119:347–363. doi: 10.1016/s0306-4522(03)00157-x. [DOI] [PubMed] [Google Scholar]
- Oren I, Nissen W, Kullmann DM, Somogyi P, Lamsa KP. Role of ionotropic glutamate receptors in long-term potentiation in rat hippocampal CA1 oriens-lacunosum moleculare interneurons. J Neurosci. 2009;29:939–950. doi: 10.1523/JNEUROSCI.3251-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren I, Hájos N, Paulsen O. Identification of the current generator underlying cholinergically induced gamma frequency field potential oscillations in the hippocampal CA3 region. J Physiol. 2010;588:785–797. doi: 10.1113/jphysiol.2009.180851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron. 2005;46:89–102. doi: 10.1016/j.neuron.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Sambandan S, Sauer JF, Vida I, Bartos M. Associative plasticity at excitatory synapses facilitates recruitment of fast-spiking interneurons in the dentate gyrus. J Neurosci. 2010;30:11826–11837. doi: 10.1523/JNEUROSCI.2012-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin U, Osting S, Hagen J, Rutecki P, Sutula T. Spontaneous seizures and loss of axo-axonic and axo-somatic inhibition induced by repeated brief seizures in kindled rats. J Neurosci. 2003;23:2759–2768. doi: 10.1523/JNEUROSCI.23-07-02759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Coombs ID, Renzi M, Zonouzi M, Farrant M, Cull-Candy SG. Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, γ-5. Nat Neurosci. 2009;12:277–285. doi: 10.1038/nn.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Soltesz I. Functional specificity of mossy fiber innervation of GABAergic cells in the hippocampus. J Neurosci. 2009;29:4239–4251. doi: 10.1523/JNEUROSCI.5390-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of Cre driver for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci. 1998;1:572–578. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27:8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida I, Bartos M, Jonas P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron. 2006;49:107–117. doi: 10.1016/j.neuron.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JGR. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Wyeth MS, Zhang N, Mody I, Houser CR. Selective reduction of cholecystokinin-positive basket cell innervation in a model of temporal lobe epilepsy. J Neurosci. 2010;30:8993–9006. doi: 10.1523/JNEUROSCI.1183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


