Abstract
A diversity of GABAergic cell types exist within each brain area, and each cell type is thought to play a unique role in the modulation of principal cell output. Basket cells, whose axon terminals surround principal cell somata and proximal dendrites, have a privileged and influential position for regulating the firing of principal cells. This review explores the dichotomy of the two basket cell classes, cholecystokinin- (CCK) and parvalbumin (PV)-containing basket cells, beginning with differences at the level of the individual cell and subsequently focusing on two ways in which this intrinsic dichotomy is enhanced by extrinsic factors. Neuromodulatory influences, exemplified by the effects of the peptide CCK, dynamically enhance the differential functions of the two cell types. Specifications at the level of the postsynaptic principal cell, including input-specific differences in chloride handling and differences in long-range projection patterns of the principal cell targets, also enhance the distinct network function of basket cells. In this review, new findings will be highlighted concerning the roles of neuromodulatory control and postsynaptic long-range projection pattern in the definition of basket cell function.
The diversity of GABAergic interneurones and their roles in normal and abnormal network function are complex, and the interpretation of these roles is constantly evolving. The classification of different GABAergic cell types takes into account a number of factors which include axonal and dendritic connectivity, morphology, intrinsic electrophysiological properties, combinations of molecular markers, temporal firing characteristics during network oscillations and developmental origins. Basket cells (BCs) are GABAergic cells that synapse onto the somata and proximal dendrites of their principal cell targets. This perisomatic synaptic arrangement is thought to be of particular advantage in influencing the output of principal cells. BCs can be functionally and anatomically divided into two non-overlapping populations based on their immunopositivity to parvalbumin (PV) and cholecystokinin (CCK). PV BCs are also called fast-spiking basket cells because of their fast, non-accommodating firing patterns and fast membrane time constants, while CCK BCs are also called regular-spiking basket cells due to their accommodating firing patterns and slower time constants. A number of additional dichotomies have been shown to exist between these two BC classes (Fig. 1).
Figure 1. Summarizing intrinsic differences between PV- and CCK-containing basket cells and their synapses.
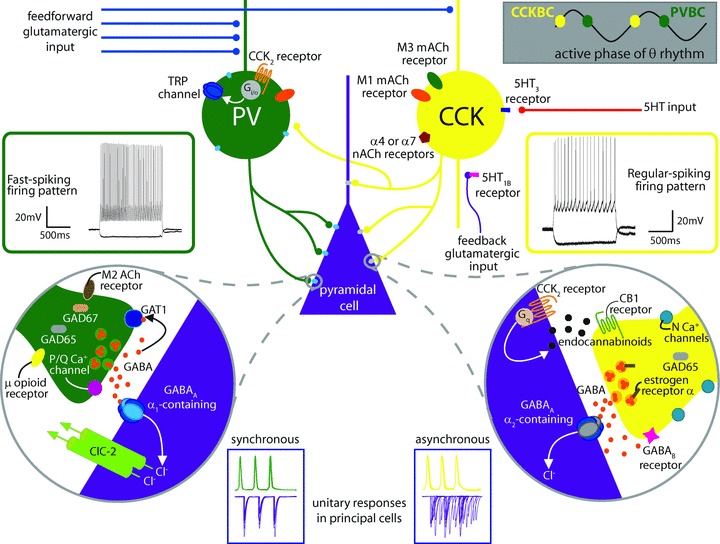
As described in the text, CCK BCs and PV BCs both target the perisomatic region of postsynaptic principal cells, but the distribution of CCK BC synapses is somewhat shifted toward the proximal dendrite, indicating a slight domain specificity. PV BCs (shown in green) have a fast spiking firing pattern (left, green inset) and are primarily activated by feedforward glutamatergic input (blue inputs) with high temporal fidelity while CCK BCs (shown in yellow) have regular spiking firing patterns (right, yellow inset) and because of their intrinsic proclivity for temporal summation are best activated by convergent feedforward and feedback (purple) glutamatergic input. PV BCs and CCK BCs are active at different phases of the theta cycle (upper right grey inset). CCK BCs express M1 (orange oval) and M3 (green oval) muscarinic acetylcholine (mACh) receptors as well as α7 or α4 nicotinic acetylcholine receptors (nACh, maroon pentagon) and 5HT3 receptors (dark blue rectangle). 5HT input (red input) can also affect 5HT1B receptors (pink rectangle) on CA1 pyramidal cell boutons to decrease feedforward excitation of CCK BCs. PV BCs express M1 receptors, extrasynaptic α1-containing GABAA receptors (light blue ovals) and CCK2 receptors (orange lines) coupled to a Gi/o pathway resulting in the opening of TRP channels (dark blue ovals) – non-selective cation channels that depolarize the cell (also see Fig. 2). At the same time, CCK2 receptors on pyramidal cells (orange lines, right circular inset) are coupled to Gq proteins which activate a molecular cascade leading to the synthesis of endocannabinoids (small black octagons). Endocannabinoids synthesized in response to this or other signals travel in a retrograde fashion to the CB1 receptors (green lines) on presynaptic CCK BC terminals to suppress release of GABA (small orange circles). As noted in the text, CCK BCs utilise N-type Ca2+ channels (light blue circles) located at a distance from the GABA release sites, express more GAD65 (grey rounded rectangle) than GAD 67, couple to mainly α2 subunit-containing GABAA receptors (grey oval), and express α oestrogen receptors (brown rectangle) and GABAB receptors (pink star) presynaptically. PV BC synapses (left circular inset) contain abundant GAD65 and GAD67 (tan rounded rectangle) and the GABA transporter GAT1 (blue rounded square), utilise P/Q-type Ca2+ channels (pink circle) located close to GABA release sites, and express μ opioid receptors (yellow oval) and M2 mACh receptors (brown oval), both of which inhibit release. PV BCs couple with mainly α1 subunit-containing GABAA receptors, and their inputs are modulated by the hyperpolarization-activated chloride channel, ClC-2 (green cylinders). Unitary responses in pyramidal cells (purple traces in blue insets, bottom) upon PV BC stimulation (green action potentials) are highly synchronous while responses to CCK BC stimulation (yellow action potentials) exhibit asynchronous release. Electrophysiological traces adapted by permission from Journal of Neuroscience; Lee SY, Földy C, Szabadics J & Soltesz I (2011). Cell-type-specific CCK2 receptor signalling underlies the cholecystokinin-mediated selective excitation of hippocampal parvalbumin-positive fast-spiking basket cells. J Neurosci31,10993–11002, copyright 2011.
In Part I of this review, differences related to the connectivity and intrinsic properties of the two types of BCs will be summarized. Subsequently, two additional ways in which other cell types can enhance the intrinsic dichotomous function of BCs will be discussed. Part II will discuss how specific neuromodulators, exemplified by the peptide CCK, can dynamically enhance the discrete functions of PV and CCK BCs. In Parts III and IV, two specific examples demonstrate that principal cells themselves can also participate in creating the distinct roles of BCs. Part III will describe how postsynaptic principal cell specialization allows differential handling of incoming BC inputs. Part IV delineates an evolving new dimension of specificity based on GABAergic cell targeting of specific excitatory cell subnetworks, groups of principal cells that have preferential connectivity depending on their long-range projection patterns.
Part I: The intrinsic dichotomy of basket cells
The two types of BCs (PV- and CCK-containing) have very different properties, making each particularly well-suited to perform different tasks in the regulation of principal cell output (Glickfeld & Scanziani, 2006; Freund & Katona, 2007). Both types are perisomatically targeting basket cells, but despite their similar morphologies, PV BCs and CCK BCs have different developmental origins, with PV BCs arising from the medial ganglionic eminence (MGE) and CCK BCs arising from the caudal ganglionic eminence (CGE) (Fishell, 2007; Tricoire et al. 2011). In general, PV BCs are considered to have qualities that are well-suited to control the precise timing and oscillatory activity of the network. CCK BCs, on the other hand, receive information from distinct sources and multiple modulatory systems, integrating these inputs over longer time windows to shape and respond to subtleties of principal cell output (Freund & Katona, 2007). However, while useful for framing the major roles of BCs, the simplified view of PV BCs as the timekeepers and CCK BCs as the modulators does not capture all of the distinct properties of BCs since, for example, both PV BCs and CCK BCs can be modulated by endogenously and exogenously applied substances. Some modulators affect only one BC population and others can affect both populations, but as Part II will describe in more detail, the impact of that modulation differs dramatically between the BC types. Here, the main differences known to exist between PV BCs and CCK BCs will briefly be summarized to provide a framework for Parts II–IV.
Inputs to basket cells
PV BCs receive numerous inputs, and in comparison CCK BCs receive fewer total inputs, though CCK BCs receive a higher proportion of inhibitory inputs (Matyas et al. 2004). Despite the relatively abundant inhibitory input to CCK BCs, the precise origins of GABAergic input to CCK BCs are not fully known–note that while unitary functional connections have been described from CCK BCs to PV BCs, direct functional connections from PV BCs to CCK BCs have yet to be observed (see Karson et al. 2009). Interestingly, at mossy fibre synapses in CA3, PV BCs receive frequent but small glutamatergic inputs, while CCK BCs receive infrequent but much larger inputs from mossy fibres (Szabadics & Soltesz, 2009). On the other hand in CA1, inputs to PV BCs are larger than inputs to CCK BCs, a discrepancy that may have to do with the respective coding functions of these brain regions (Gulyás et al. 1999; Glickfeld & Scanziani, 2006; Szabadics & Soltesz, 2009).
Glutamatergic inputs to PV BCs undergo NMDA-independent long-term potentiation (LTP) through calcium-permeable AMPA channels, while glutamatergic inputs to CCK BCs do not undergo LTP (Nissen et al. 2010). However, unlike their glutamatergic inputs, the numerous GABAergic inputs to CCK BCs do undergo LTP during theta burst stimulation, presumably leading to disinhibition of pyramidal cells during theta rhythm-associated behavioural states (Evstratova et al. 2011). Long-term plasticity of input to PV BCs and CCK BCs is also synapse specific, allowing the same presynaptic cell to be differentially modulated at individual synapses depending on the BC type it is contacting (Pelkey & McBain, 2008).
PV BCs have fast action potential-driven calcium events in their dendrites, and non-passive dendritic cable properties that enhance the temporal fidelity of dendritic signals and enable PV BCs to respond very quickly at the soma even to distal dendritic inputs (Aponte et al. 2008; Hu et al. 2010; Nörenberg et al. 2010). These properties enhance the ability of PV BCs to fire action potentials rapidly in response to incoming feed-forward glutamatergic inputs. CCK BCs, on the other hand, have larger, longer action potential-evoked dendritic calcium transients that may enhance their temporal integrative capacity (Evstratova et al. 2011). As we will see below, this property makes CCK BCs responsive to combined input from temporally and physically more separated inputs (Glickfeld & Scanziani, 2006).
Receptor expression
PV BCs express high levels of GABAAα1 receptors on their cell surface that can be located extrasynaptically (Nusser et al. 1995; Baude et al. 2007; Kasugai et al. 2010). In addition, as mentioned above, PV BCs and CCK BCs are sensitive to different neuromodulators. PV BCs express μ opioid receptors that can cause hyperpolarization (Neu et al. 2007; Glickfeld et al. 2008; Gulyás et al. 2010; Krook-Magnuson et al. 2011), as well as M1 muscarinic acetylcholine (mACh) receptors, and M2 mACh receptors on their axon terminals (Freedman et al. 1993; Hájos et al. 1998; Freund & Katona, 2007; Morales et al. 2008; Cea-del Rio et al. 2010). PV BCs also express CCK2 receptors, and as will be discussed in Part II, the effects of CCK on PV BCs and CCK BCs are quite different. Interestingly, CCK BCs have strong associations with modulatory systems involved in regulation of mood (Freund, 2003). Serotonergic (5HT) input influences CCK BCs directly through their 5HT3 receptors and can also selectively decrease feedback excitation of CCK BCs through 5HT1B receptors located on presynaptic terminals of CA1 pyramidal cells (McMahon & Kauer, 1997; Winterer et al. 2011). Acetylcholine (ACh) binds to α4 and α7 nicotinic ACh (nACh) receptors as well as M1 and M3 mACh receptors on CCK BCs (Freedman et al. 1993; Hájos et al. 1998; Freund & Katona, 2007; Morales et al. 2008; Cea-del Rio et al. 2010). CCK BCs also contain α oestrogen receptors, which may have implications in the fluctuation of CCK levels that is seen in tandem with hormonal cycles (Freund & Katona, 2007; Hart et al. 2007; Lee & Soltesz, 2011b). Cannabinoid type 1 (CB1) receptors, the main targets of the psychoactive drug marijuana, are present on presynaptic terminals of CCK BCs but not PV BCs (Katona et al. 1999; Freund & Katona, 2007), where they mediate suppression of GABA release in response to both tonic and on-demand production of endocannabinoids by postsynaptic pyramidal cells (Földy et al. 2006; Neu et al. 2007; Kano et al. 2009).
At the GABAergic basket cell synapse
While PV BCs express abundant GAD65 and GAD67, isoforms of the GABA synthesizing enzyme and PV BC synapses contain the GABA transporter GAT1, enhancing the temporal fidelity of their signals, CCK BC synapses express more GAD65 than GAD67 and have little GAT1 expression (Karson et al. 2009; Fish et al. 2011; Tricoire et al. 2011). CCK BCs express GABAB receptors on their presynaptic terminals, which can suppress release (Freund & Katona, 2007; Neu et al. 2007). In their axon terminals, PV BCs utilise only a few P/Q type calcium channels per release site, closely apposed to their release machinery allowing quick neurotransmitter release with a low probability of action potential-independent release. CCK BCs instead utilise N type calcium channels that can be modulated by G protein coupled receptors (GPCRs) and are located at a greater distance from release sites (Wilson et al. 2001; Hefft & Jonas, 2005; Glickfeld & Scanziani, 2006; Kerr et al. 2008; Bucurenciu et al. 2010).
Unitary events triggered by CCK-containing interneurones have a temporal jitter compared with PV BC-mediated events, which are much more precisely time-locked to incoming input (Hefft & Jonas, 2005; Daw et al. 2009). This asynchronous release property may give CCK-containing cells the ability to provide longer-lasting barrages of inhibition, suppressing local activity under certain circumstances such as periods of prolonged local network activity. The mechanism for asynchronous release is thought to be the expression of different Ca2+ sensor machinery isoforms at the synapses of CCK BCs, and asynchronous release can be modulated separately from synchronous release by muscarinic agonists which reduce asynchronous release, kainate receptor activation which selectively suppresses phasic release from CCK BCs and tonic cannabinoid type 1 (CB1) receptor activation at presynaptic CCK BC synapses (Kerr et al. 2008; Pelkey & McBain, 2008; Ali & Todorova, 2010; Daw et al. 2010; Lourenco et al. 2010).
PV BC synapses onto pyramidal cells primarily utilise α1 subunit-containing GABAA receptors which mediate the sedative and amnesic effects of benzodiazepines. Postsynaptic GABAA receptors at CCK BC synapses are primarily α2 subunit-containing receptors that are involved in mediating the anxiolytic effects of benzodiazepines (Freund & Katona, 2007). Additionally, Part III will detail the novel finding that PV BC inputs experience selective postsynaptic modulation by the Cl− transporter ClC-2.
Oscillatory and network properties
PV BCs and CCK BCs are activated at different phases of theta rhythm in anaesthetized animals (Klausberger & Somogyi, 2008). In addition, as mentioned above, PV BCs are predominantly activated in a feed-forward fashion in CA1 (Glickfeld & Scanziani, 2006), and are essential in the generation of gamma oscillations in the hippocampus (Soltesz & Deschênes, 1993; Ylinen et al. 1995; Hájos et al. 2000; Bartos et al. 2007; Sohal et al. 2009; Gulyás et al. 2010). In contrast, CCK BCs integrate incoming inputs over longer time windows, forming synapses with a higher failure rate and less temporal precision, making CCK BCs better suited to respond to coincident feed-forward and feed-back excitation from different sources (Klausberger et al. 2005; Glickfeld & Scanziani, 2006). Part IV will discuss how the axonal connectivity of CCK BCs and PV BCs can also be quite different depending on the subnetwork to which the principal cell target belongs.
Perisomatically versus dendritically targeting PV and CCK cells
It should be noted that both CCK- and PV-containing dendritically targeting cells also exist, with similarly dichotomous features. These cells have distinct properties both from their perisomatic-targeting counterparts as well as from each other. Compared with PV BCs, PV-containing, dendritically targeting bistratified cells fire at different phases of network oscillations, have different types of plasticity at incoming inputs, and do not directly respond to CCK (Klausberger & Somogyi, 2008; Nissen et al. 2010; Lee et al. 2011). While both perisomatically and dendritically targeting CCK-containing cells are modulated by endocannabinoids, express M1 and M3 mACh receptors, and exhibit asynchronous release, CCK-containing, dendritically targeting Schaffer collateral-associated cells experience significantly less modulation by endocannabinoids (either by tonic, depolarization-induced or mGluR-mediated production), display a unique biphasic membrane potential response to mACh receptor activation, and have differing specific asynchronous release properties (Hefft & Jonas, 2005; Daw et al. 2009; Lee et al. 2010; Cea-del Rio et al. 2011).
In summary, the complementary but distinct roles of PV BCs and CCK BCs are thought to make basket cells capable, together, of balancing the needs of principal cells in terms of both reliability and plasticity (Fig. 1) (Freund, 2003; Klausberger et al. 2005; Glickfeld & Scanziani, 2006; Soltesz, 2006; Freund & Katona, 2007; Lee & Soltesz, 2011b).
Part II: Neuromodulators exaggerate the dichotomy between BCs
Not only are PV BCs and CCK BCs affected differently by different neuromodulators, but a single neuromodulator can have opposing effects on the two basket cell populations. One example of this phenomenon comes from the study of the effects of the CCK peptide. In addition to being a marker of certain types of neurones in the brain, CCK is a biologically active peptide, classically known best for its role in the release of pancreatic enzymes of the gut. However, CCK is also an extremely abundant peptide in the brain where it modulates activity of both CCK and PV basket cells (Földy et al. 2007). Intriguingly, the effects of CCK on PV BCs and CCK BCs are strikingly different, although the receptor by which the effects are mediated, the CCK2 receptor, is the same (Földy et al. 2007; Lee et al. 2011).
How is the dichotomous modulation of PV BC and CCK BC activity by CCK achieved? CCK2 receptors are classically thought to involve a Gq-coupled molecular cascade. Indeed, with respect to the modulation of CCK BC activity, CCK2 receptors coupled to Gq are the major players (Földy et al. 2007; Lee & Soltesz, 2011a; Lee et al. 2011). In this case, CCK2 receptors are located postsynaptically on pyramidal cells. CCK binds to the Gq-coupled CCK2 receptor, activating a molecular cascade that culminates in the production of endocannabinoids by the pyramidal cell. Endocannabinoids then act as retrograde messengers, binding to CB1 receptors on presynaptic CCK BC terminals and suppressing transmitter release. Thus, the effect of CCK application on transmission from CCK BCs is a suppression of GABA release onto pyramidal cells (Földy et al. 2007). However, PV BCs respond differently to CCK application. In PV BCs, the application of CCK causes a robust depolarization and an increase in firing. Because of this, until recently, it was unclear whether the dramatic increase in PV BC firing in response to CCK application could directly suppress GABA release from CCK BCs through the spillover of GABA from PV BC terminals, activating presynaptic GABAB receptors on CCK BC terminals. However, paired recordings between CCK BCs and pyramidal cells demonstrated that the CCK-induced suppression of CCK BC release could be blocked by CB1 antagonists but not by GABAB antagonists, supporting the idea that CCK works to suppress release from CCK BCs indirectly through CCK receptors on pyramidal cells (Lee & Soltesz, 2011a).
Still, how does the CCK2 receptor concurrently mediate endocannabinoid release from pyramidal cells as well as depolarization of PV BCs? Recent evidence has shown that the differential effects of the CCK2 receptor on pyramidal cells and PV BCs are due to the coupling of this GPCR with different G proteins in the different cell types (Lee et al. 2011). Unlike the canonical Gq pathway in pyramidal cells, CCK2 receptors on PV BCs instead mediate depolarization of PV BCs by a Gi/o-coupled mechanism involving internal calcium stores and culminating in the activation of non-selective cation channels known as TRP channels, which depolarize the PV BCs (Lee et al. 2011).
In this way, the CCK peptide can mediate both suppression of inhibition by the CCK BC population and simultaneously increase inhibition by the PV BC population, both through CCK2 receptor activation (Fig. 2). A similarly dichotomous neuromodulation of BCs by a single substance can be observed with ACh. Cholinergic modulation of BCs has major effects on the oscillatory behaviour of the network, a property that has been reviewed elsewhere (Bartos et al. 2007; Lawrence, 2008; Cea-del Rio et al. 2010; Gulyás et al. 2010). The expression of different cholinergic receptors on PV BCs and CCK BCs was also mentioned in Part I. Briefly, CCK BCs appear to be more sensitive to synaptic release of ACh than PV BCs. The activation of M3 mACh and α4 and α7 nACh receptors expressed on CCK BCs can affect their firing pattern and increase firing rate (Freund & Katona, 2007; Cea-del Rio et al. 2010). Both PV BCs and CCK BCs express M1 mACh receptors which can depolarize the cells, while M2 mACh receptors on PV BC terminals suppress GABA release from PV BCs (Hájos et al. 1998; Cea-del Rio et al. 2010). An additional measure of modulation by ACh is provided by mACh receptors on pyramidal cells, which can also increase the production of endocannabinoids and selectively suppress release from CB1-expressing CCK BC terminals (Freund & Katona, 2007; Neu et al. 2007; Lawrence, 2008). The net effect of ACh, therefore, is multifold and the precise meaning of the specific type and localization of ACh inputs and receptors on BCs has yet to be fully understood. However, it is clear from the examples of both CCK and ACh that the concurrent but opposing modulation of the two basket cell populations by a single neuromodulator further exaggerates the dichotomy between them, underscoring the complex manner in which neuromodulation may dynamically shift inhibitory control between different GABAergic cell types.
Figure 2. Schematic representation of the divergent effects of CCK on PV and CCK basket cells through cell-type-dependent selectivity of CCK2 receptor signalling.
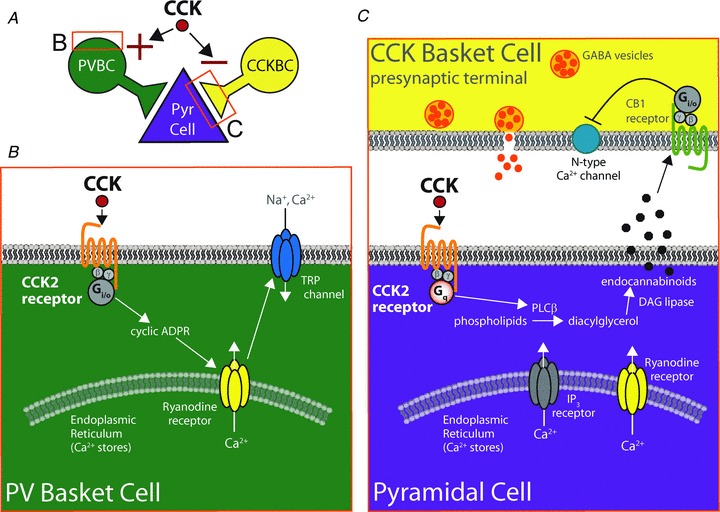
A, CCK signalling through the CCK2 receptor exerts opposing effects on PV and CCK basket cells, as described in B and C, with the net effect on PV BCs being depolarization and firing, and the net effect on CCK BCs being a suppression of GABA release. B, in PV BCs, CCK2 receptors couple to an unusual, pertussis toxin-sensitive pathway utilising a Gi/o-coupled mechanism involving cyclic ADP ribose, ryanodine receptors on intracellular calcium stores, and ultimately activating a non-selective cationic conductance through TRP channels. C, in pyramidal cells, CCK2 receptors signal through the more canonical Gq–PLC pathway in which PLCβ produces diacylglycerol (DAG) which is converted by DAG lipase to endocannabinoids that can travel in a retrograde fashion to cannabinoid type 1 (CB1) receptors on presynaptic CCK basket cell terminals, decreasing GABA release. Adapted by permission from Journal of Neuroscience; Lee SY, Földy C, Szabadics J & Soltesz I (2011). Cell-type-specific CCK2 receptor signalling underlies the cholecystokinin-mediated selective excitation of hippocampal parvalbumin-positive fast-spiking basket cells. J Neurosci31,10993–11002, copyright 2011.
Part III: Principal cells participate in generating functional BC dichotomies
As mentioned in Part I, the subunit composition of GABAA receptors postsynaptic to PV BC and CCK BC synapses differs, with PV BCs utilising primarily GABAAα1 and CCK BCs utilising primarily α2 subunit-containing receptors (Freund, 2003; Freund & Katona, 2007). Mounting evidence suggests that additional postsynaptic cell specializations enhance the dichotomous function of BCs. For example, postsynaptic chloride handling has recently been shown to be different for CCK BC and PV BC inputs.
The internal chloride concentration, {Cl−}i, of a cell can be controlled by a number of factors including the well-known transporters KCC2 and NKCC1. Expression differences in these transporters provide the basis for the depolarizing effects of GABA during development and also influence the reversal potential for GABAergic events in adult neurones (reviewed in Blaesse et al. 2009). The reversal potential for GABAergic events depends primarily, albeit not exclusively due to bicarbonate permeability, on {Cl−}i. Thus, the reversal potential for GABAergic events is thought of as largely dependent on the expression of Cl− transporters in the particular cell and the cellular compartment being studied. Alterations in EGABA outside of development and within particular cellular compartments have been explored before. The {Cl−}i at the axon initial segment, for example, may be different from other cellular compartments such that GABAergic input from axoaxonic cells could depolarize the cell (Szabadics et al. 2006; reviewed in Woodruff et al. 2010).
Földy et al. (2010) noted that both the current–voltage relationship, in which PV BC inputs showed an outward rectification limiting IPSC amplitude below ECl, and the reversal potential of unitary responses in paired recordings between CCK BCs or PV BCs and postsynaptic CA1 pyramidal cells, differed. This was surprising because both BC types target the somata and proximal dendrites of pyramidal cells, and the synapses intermingle at individual cell membranes. This means that CCK BC and PV BC-mediated responses would be expected to reflect a compartment with a relatively uniform {Cl−}i overall. The concentration of solutes within the pipette is considered to diffuse rapidly throughout the perisomatic cellular compartment, which is expected to abolish differences in EGABA by flooding the region with the desired {Cl−}. The mechanism of the observed differences in current–voltage response between BC types was found to be the hyperpolarization-activated chloride channel ClC-2 whose gating depends on {Cl−}i (Staley, 1994), and which is selectively more active at PV BC than CCK BC inputs. In fact, the differences in PV BC and CCK BC current–voltage curves were abolished in knockout mice lacking the ClC-2 gene, Clcn2 (Földy et al. 2010). It was also noted that there is a difference in the distribution of PV BC and CCK BC boutons, such that CCK BC synapses are shifted toward the proximal dendritic domain compared with PV BC synapses. The magnitude of the ClC-2 current itself was also larger at the soma than in the proximal dendrites, indicating that the difference between PV BC and CCK BC inputs could be due either to domain-specific targeting by basket cells or to synapse-specific expression of ClC-2. This study provided the first evidence that the postsynaptic principal cell can differentially modulate the local {Cl−}i on the basis of presynaptic cell type.
As described above, PV BCs are capable of firing very fast, well-timed action potentials during intense oscillatory activity while CCK BCs fire less frequently and integrate temporally more separated inputs. This would suggest that locations postsynaptic to PV BC synapses are subjected to a higher chloride load than CCK BC synapses. As ClC-2 is inwardly rectifying, meaning that it is significantly more active when the membrane potential is more hyperpolarized than ECl (as may occur due to GABAB receptor activation or other K+ conductances) or when {Cl−}i is high, the specific action of ClC-2 on PV BC inputs may serve as a protective mechanism to ensure that barrages of PV BC input do not cause local shifts in EGABA. Of course, an also interesting but not mutually exclusive idea is that ClC-2 mediates a residual outward conductance due to incomplete inward rectification of the channel at membrane potentials more depolarized than ECl (Ratté & Prescott, 2011), selectively adding a Cl− leak at PV BC inputs compared to CCK BC inputs.
The mechanism by which postsynaptic principal cells specifically designate the receptor subtypes and chloride transporters to match presynaptic BC inputs remains to be determined. The degree to which other non-BC GABAergic inputs to principal cells experience input-specific modulation will also be important to investigate. Since some interneurones also express ClC-2 (Rinke et al. 2010), the types of ClC-2-expressing cells and the impact of ClC-2 expression in interneurones will also be important topics for future studies. However, it is clear that the input-specific expression of ClC-2 provides an additional mechanism by which postsynaptic cells can play an active role in creating and maintaining dichotomy in BC function.
Part IV: BC dichotomy on the basis of postsynaptic principal cell projection pattern
In most studies of BCs, postsynaptic principal cells are considered a relatively homogeneous population for the sake of understanding specific differences in BC subtypes. However, within a single layer, principal cells can be decidedly non-homogeneous. For example, principal cells within a single layer may have many different long-range targets, express a number of different cellular markers or exhibit different functional activity during network oscillations (Baimbridge et al. 1991; Slomianka, 1992; Varga et al. 2010; Mizuseki et al. 2011). These differences, previously considered important primarily for understanding the macrocircuitry of the brain between regions, are increasingly being recognized as significant factors determining specific local microcircuitry as well.
The selective innervation of groups of topologically distant principal cells arising during similar ontogenetic stages has recently been described (Yu et al. 2009; Deguchi et al. 2011). Similarly, a number of studies have revealed groups of selectively connected principal cells in and between various brain regions, forming subnetworks with different connection preferences depending on long-range projection pattern (for review, see Krook-Magnuson et al. 2012). However, the selectivity of GABAergic cell types for these subnetworks of interconnected pyramidal cells is largely unexplored. This type of information is critical for building hypotheses regarding the network function of particular cell types, since it is still unclear whether specific GABAergic cells serve to unite or segregate outputs to different brain regions.
One particularly striking example of subnetwork specificity is the case of CCK BCs in the medial entorhinal cortex layer II (MEClayerII) (Varga et al. 2010). In this region, two non-overlapping immunohistochemical markers (reelin and calbindin) label two non-overlapping principal cell populations that project from MEClayerII to either ipsilateral dentate gyrus (reelin+ cells) or contralateral entorhinal cortex (calbindin+ cells). This allowed distinct principal cell subnetworks to be labelled more easily, on the basis of immunohistochemistry. Surprisingly, Varga et al. (2010) found that CCK-positive punctae selectively surround the somata of certain principal cells, while PV-positive punctae appear less selective (Fig. 3A). Immunohistochemistry to label the two principal cell subnetworks, using calbindin and reelin in addition to VGLUT3 at CCK BC terminals, revealed that CCK BCs pick out those cells (calbindin+ cells, red) that project to the contralateral entorhinal cortex, avoiding the cells (reelin+ cells, blue) that project to the ipsilateral dentate gyrus (Fig. 3B; Varga et al. 2010). The organization of PV-positive punctae suggests that PV BCs may contact and regulate the activity of both MEClayerII principal cell subnetworks.
Figure 3. Subnetwork-specific targeting by CCK BCs in the medial entorhinal cortex layer II (MEClayerII).
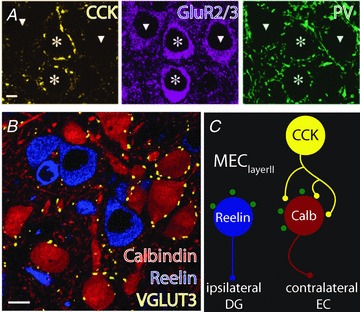
A, in medial entorhinal cortex layer II (MEClayerII), CCK-positive punctae (yellow) surround only certain principal cells (labelled using GluR2/3, purple, targeted cells noted by stars) and avoid others (noted by triangles) while PV punctae (green) do not appear selective. B, the cells targeted by CCK BC terminals (labelled using a marker of CCK BC boutons, VGLUT3, yellow) selectively innervate the calbindin-containing principal cells (red) that project to the contralateral entorhinal cortex (EC), avoiding reelin-containing principal cells (blue) that form the perforant path to the ipsilateral dentate gyrus (DG). C, summary diagram representing the arrangement of perisomatic boutons in MEClayerII. Perisomatic PV-containing punctae (green) are seen surrounding both principal cell populations while CCK BCs select only calbindin-containing principal cells. Parts A and B adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience Varga C, Lee SY & Soltesz I (2010). Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci13, 822–824, copyright 2010.
The degree to which there may be quantitative or individual cell preferences for postsynaptic targets amongst PV BCs remains to be determined, and the generalizability of this finding to other brain regions must be further explored. Nevertheless, additional divergence of BC function is already suggested by these studies: not only do CCK BCs and PV BCs have different intrinsic properties and respond differently to neuromodulators, but they may also have differential connectivity with regard to specific subnetworks of principal cells. This observation raises two direct and very important questions that will need to be addressed in future studies. First, what is the purpose of CCK BC selectivity for contralaterally projecting cells in MEClayerII? MEClayerII is an important region in spatial navigation, containing grid cells and border cells (for review, see Derdikman & Moser, 2010). It will be very interesting to see which subnetworks these cell types belong to, and is interesting to speculate that CCK BC function may play a role in coordinating spatial information between hemispheres. Second, are certain GABAergic cell types more specific for excitatory subnetworks than others, and are CCK BCs generally specific for certain subnetworks or is CCK BC selectivity brain region specific? Other evidence suggests that certain GABAergic cells within other brain regions may also be more selective for principal cell subnetworks than previously realized (Farinas & DeFelipe, 1991; Yoshimura & Callaway, 2005; Otsuka & Kawaguchi, 2009; Varga et al. 2010). While PV BC punctae seem to be less specific in MEClayerII as well as in other brain regions (Packer & Yuste, 2011), axoaxonic cells do appear to select callosal or cortical rather than thalamic-projecting pyramidal cells in the cat visual cortex (Farinas & DeFelipe, 1991). These findings emphasize that a complete understanding of GABAergic microcircuitry may also require a thorough consideration of the long-range projection patterns of the target cells in that brain area, and that the excitatory cell subnetwork may reveal additional divergence in the functions of the two BC types as well as other GABAergic cells.
Part V: Summary and outlook
The analysis of dichotomous BC function provides an opportunity to evaluate previously unknown types of network specificity. Throughout this review, the unique and important differential roles of PV BCs and CCK BCs in controlling output from principal cells have been discussed. BC dichotomy involves not only many differences in intrinsic properties and direct connectivity, but can also be enhanced by dynamic neuromodulation or by differences in the postsynaptic cell targets.
In addition to their roles in normal brain activity, BCs and their modulation have been suggested to play roles in a number of diseases, and thus understanding the roles of specific types of GABAergic cells will be important for developing future therapeutics either through pharmacology or targeted optogenetic technologies (Kravitz et al. 2010). Disruptions in gamma oscillations, mediated by PV BCs, and their relationship to theta oscillations have been implicated in schizophrenia (Lisman & Buzsaki, 2008; Lewis et al. 2011), and α1 subunit-containing GABAA receptors, the main GABAA receptors targeted by PV BCs, are reduced in schizophrenic subjects (Glausier & Lewis, 2011). The CCK peptide itself has been implicated in normal sleep rhythms and can cause anxiety when applied in normal subjects, while decreased levels of CCK are also observed in schizophrenia (reviewed in Lee & Soltesz, 2011b). The delayed actions of antidepressants, which typically block the reuptake of 5HT, have recently been shown to involve the action of 5HT1B receptors, which are located on the glutamatergic feedback inputs to CCK BCs (Winterer et al. 2011). In epilepsy, a selective decrease of CCK BC terminals are seen in CA1 (Wyeth et al. 2010), while CB1 receptor expression on GABAergic terminals is increased relative to CB1 receptors on glutamatergic terminals (reviewed in Armstrong et al. 2009). Dysfunction of PV BCs in the dentate of epileptic animals may also contribute to hyperexcitability (Zhang & Buckmaster, 2009). Therefore, BCs are involved in the underlying pathology of a number of neurological disorders and the divergent network effects of PV BC and CCK BC types could represent a future therapeutic target.
In summary, this review has highlighted new ways in which the dichotomy between basket cells is achieved and enhanced by specific mechanisms. A number of questions may arise from the observations discussed above:
1. While CCK BCs and PV BCs are active during different phases of theta oscillations (and are also differentially modulated during sharp wave ripples and gamma oscillations) in anaesthetized animals (Klausberger & Somogyi, 2008), one major future direction will be to determine whether CCK BCs and PV BCs are differentially modulated during specific behavioural states and particular network oscillations in behaving animals (Isaacson & Scanziani, 2011). This type of study is now possible using in vivo single cell recordings in awake, behaving animals (Harvey et al. 2009; Lee et al. 2009), and the results will add an additional essential dimension to the classification of GABAergic cell types.
2. Is the differential effect of ClC-2 on PV BCs and CCK BCs due to synapse-specific {Cl−}i differences or the different but overlapping domains of CCK BC and PV BC axons? Do particular principal or even GABAergic cell types, as suggested by ClC-2 expression in some interneurones (Rinke et al. 2010), utilise domain or synapse-specific expression of ClC-2 to enhance aspects of incoming information depending on cell type, subnetwork or brain region?
3. Does selectivity for specific principal cell subnetworks represent an additional difference in the function of BCs – with CCK BCs enhancing the independent processing of separate streams of information through a brain region and PV BCs serving to coordinate the activity of distant brain regions? To what extent do other types of GABAergic cells exhibit subnetwork specificity in different brain regions?
4. Similarly, given the dichotomous effects of neuromodulators such as CCK on CCK BCs and PV BCs and the possible differential targeting of CCK BCs to specific subnetworks, is it possible that neuromodulators may serve as switches to increase or decrease coordinated activity between distant brain regions?
The exploration of these ideas will yield insights into the ways in which differential functions of BC types as well as the functions of other specific types of interneurones not only coordinate the microcircuitry within brain regions but also influence the coordination of distant brain regions during specific cognitive or behavioural states.
Acknowledgments
This work was supported by the US National Institutes of Health grants NS35915 and NS74432. The authors thank Dr Soo Yeun Lee for helpful comments on the manuscript.
Glossary
Abbreviations
- ACh
acetylcholine
- BC
basket cell
- CB1
cannabinoid type 1
- CCK
cholecystokinin
- CGE
caudal ganglionic eminence
- MGE
medial ganglionic eminence
- nACh
nicotinic ACh
- PV
parvalbumin
References
- Ali AB, Todorova M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur J Neurosci. 2010;31:1196–1207. doi: 10.1111/j.1460-9568.2010.07165.x. [DOI] [PubMed] [Google Scholar]
- Aponte Y, Bischofberger J, Jonas P. Efficient Ca2+ buffering in fast-spiking basket cells of rat hippocampus. J Physiol. 2008;586:2061–2075. doi: 10.1113/jphysiol.2007.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C, Morgan RJ, Soltesz I. Pursuing paradoxical proconvulsant prophylaxis for epileptogenesis. Epilepsia. 2009;50:1657–1669. doi: 10.1111/j.1528-1167.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimbridge KG, Peet MJ, McLennan H, Church J. Bursting response to current-evoked depolarization in rat CA1 pyramidal neurons is correlated with lucifer yellow dye coupling but not with the presence of calbindin-D28k. Synapse. 1991;7:269–277. doi: 10.1002/syn.890070404. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Baude A, Bleasdale C, Dalezios Y, Somogyi P, Klausberger T. Immunoreactivity for the GABAA receptor α1 subunit, somatostatin and Connexin36 distinguishes axoaxonic, basket, and bistratified interneurons of the rat hippocampus. Cereb Cortex. 2007;17:2094–2107. doi: 10.1093/cercor/bhl117. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Bucurenciu I, Bischofberger J, Jonas P. A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nat Neurosci. 2010;13:19–21. doi: 10.1038/nn.2461. [DOI] [PubMed] [Google Scholar]
- Cea-del Rio CA, Lawrence JJ, Erdelyi F, Szabo G, McBain CJ. Cholinergic modulation amplifies the intrinsic oscillatory properties of CA1 hippocampal cholecystokinin-positive interneurons. J Physiol. 2011;589:609–627. doi: 10.1113/jphysiol.2010.199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cea-del Rio CA, Lawrence JJ, Tricoire L, Erdelyi F, Szabo G, McBain CJ. M3 muscarinic acetylcholine receptor expression confers differential cholinergic modulation to neurochemically distinct hippocampal basket cell subtypes. J Neurosci. 2010;30:6011–6024. doi: 10.1523/JNEUROSCI.5040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw MI, Pelkey KA, Chittajallu R, McBain CJ. Presynaptic kainate receptor activation preserves asynchronous GABA release despite the reduction in synchronous release from hippocampal cholecystokinin interneurons. J Neurosci. 2010;30:11202–11209. doi: 10.1523/JNEUROSCI.6334-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi Y, Donato F, Galimberti I, Cabuy E, Caroni P. Temporally matched subpopulations of selectively interconnected principal neurons in the hippocampus. Nat Neurosci. 2011;14:495–504. doi: 10.1038/nn.2768. [DOI] [PubMed] [Google Scholar]
- Derdikman D, Moser EI. A manifold of spatial maps in the brain. Trends Cogn Sci. 2010;14:561–569. doi: 10.1016/j.tics.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Evstratova A, Chamberland S, Topolnik L. Cell type-specific and activity-dependent dynamics of action potential-evoked Ca2+ signals in dendrites of hippocampal inhibitory interneurons. J Physiol. 2011;589:1957–1977. doi: 10.1113/jphysiol.2010.204255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, DeFelipe J. Patterns of synaptic input on corticocortical and corticothalamic cells in the cat visual cortex. II. The axon initial segment. J Comp Neurol. 1991;304:70–77. doi: 10.1002/cne.903040106. [DOI] [PubMed] [Google Scholar]
- Fish KN, Sweet RA, Lewis DA. Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex. 2011;21:2450–2460. doi: 10.1093/cercor/bhr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G. Perspectives on the developmental origins of cortical interneuron diversity. Novartis Found Symp. 2007;288:21–35. discussion 35–44, 96–28. [PubMed] [Google Scholar]
- Földy C, Lee SH, Morgan RJ, Soltesz I. Regulation of fast-spiking basket cell synapses by the chloride channel ClC-2. Nat Neurosci. 2010;13:1047–1049. doi: 10.1038/nn.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Földy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10:1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- Földy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Wetmore C, Stromberg I, Leonard S, Olson L. α-Bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J Neurosci. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABAA receptor α1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Atallah BV, Scanziani M. Complementary modulation of somatic inhibition by opioids and cannabinoids. J Neurosci. 2008;28:1824–1832. doi: 10.1523/JNEUROSCI.4700-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hájos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hájos N, Papp EC, Acsady L, Levey AI, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1998;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-α-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461:941–946. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Hu H, Martina M, Jonas P. Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science. 2010;327:52–58. doi: 10.1126/science.1177876. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Sieghart W, Shigemoto R, Somogyi P. Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling. Eur J Neurosci. 2010;32:1868–1888. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr AM, Reisinger E, Jonas P. Differential dependence of phasic transmitter release on synaptotagmin 1 at GABAergic and glutamatergic hippocampal synapses. Proc Natl Acad Sci U S A. 2008;105:15581–15586. doi: 10.1073/pnas.0800621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O’Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Luu L, Lee SH, Varga C, Soltesz I. Ivy and neurogliaform interneurons are a major target of μ-opioid receptor modulation. J Neurosci. 2011;31:14861–14870. doi: 10.1523/JNEUROSCI.2269-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Varga C, Lee SH, Soltesz I. New dimensions of interneuronal specialization unmasked by principal cell heterogeneity. Trends Neurosci. 2012 doi: 10.1016/j.tins.2011.10.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ. Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci. 2008;31:317–327. doi: 10.1016/j.tins.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Lee AK, Epsztein J, Brecht M. Head-anchored whole-cell recordings in freely moving rats. Nat Protoc. 2009;4:385–392. doi: 10.1038/nprot.2009.5. [DOI] [PubMed] [Google Scholar]
- Lee SH, Foldy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci. 2010;30:7993–8000. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Soltesz I. Requirement for CB1 but not GABAB receptors in the cholecystokinin mediated inhibition of GABA release from cholecystokinin expressing basket cells. J Physiol. 2011a;589:891–902. doi: 10.1113/jphysiol.2010.198499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Földy C, Szabadics J, Soltesz I. Cell-type-specific CCK2 receptor signaling underlies the cholecystokinin-mediated selective excitation of hippocampal parvalbumin-positive fast-spiking basket cells. J Neurosci. 2011;31:10993–11002. doi: 10.1523/JNEUROSCI.1970-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Soltesz I. Cholecystokinin: a multi-functional molecular switch of neuronal circuits. Dev Neurobiol. 2011b;71:83–91. doi: 10.1002/dneu.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Fish KN, Arion D, Gonzalez-Burgos G. Perisomatic inhibition and cortical circuit dysfunction in schizophrenia. Curr Opin Neurobiol. 2011;21(6):866–872. doi: 10.1016/j.conb.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco J, Cannich A, Carta M, Coussen F, Mulle C, Marsicano G. Synaptic activation of kainate receptors gates presynaptic CB1 signaling at GABAergic synapses. Nat Neurosci. 2010;13:197–204. doi: 10.1038/nn.2481. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons are excited via serotonin-gated ion channels. J Neurophysiol. 1997;78:2493–2502. doi: 10.1152/jn.1997.78.5.2493. [DOI] [PubMed] [Google Scholar]
- Matyas F, Freund TF, Gulyas AI. Convergence of excitatory and inhibitory inputs onto CCK-containing basket cells in the CA1 area of the rat hippocampus. Eur J Neurosci. 2004;19:1243–1256. doi: 10.1111/j.1460-9568.2004.03225.x. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Diba K, Pastalkova E, Buzsaki G. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci. 2011;14:1174–1181. doi: 10.1038/nn.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Hein K, Vogel Z. Hippocampal interneurons co-express transcripts encoding the α7 nicotinic receptor subunit and the cannabinoid receptor 1. Neuroscience. 2008;152:70–81. doi: 10.1016/j.neuroscience.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu A, Földy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen W, Szabo A, Somogyi J, Somogyi P, Lamsa KP. Cell type-specific long-term plasticity at glutamatergic synapses onto hippocampal interneurons expressing either parvalbumin or CB1 cannabinoid receptor. J Neurosci. 2010;30:1337–1347. doi: 10.1523/JNEUROSCI.3481-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörenberg A, Hu H, Vida I, Bartos M, Jonas P. Distinct nonuniform cable properties optimize rapid and efficient activation of fast-spiking GABAergic interneurons. Proc Natl Acad Sci U S A. 2010;107:894–899. doi: 10.1073/pnas.0910716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Roberts JD, Baude A, Richards JG, Sieghart W, Somogyi P. Immunocytochemical localization of the α1 and β2/3 subunits of the GABAA receptor in relation to specific GABAergic synapses in the dentate gyrus. Eur J Neurosci. 1995;7:630–646. doi: 10.1111/j.1460-9568.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons. J Neurosci. 2009;29:10533–10540. doi: 10.1523/JNEUROSCI.2219-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ. Target-cell-dependent plasticity within the mossy fibre–CA3 circuit reveals compartmentalized regulation of presynaptic function at divergent release sites. J Physiol. 2008;586:1495–1502. doi: 10.1113/jphysiol.2007.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratté S, Prescott S. ClC-2 channels regulate neuronal excitability, not intracellular chloride levels. J Neurosci. 2011;31:15838–15843. doi: 10.1523/JNEUROSCI.2748-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke I, Artmann J, Stein V. ClC-2 voltage-gated channels constitute part of the background conductance and assist chloride extrusion. J Neurosci. 2010;30:4776–4786. doi: 10.1523/JNEUROSCI.6299-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomianka L. Neurons of origin of zinc-containing pathways and the distribution of zinc-containing boutons in the hippocampal region of the rat. Neuroscience. 1992;48:325–352. doi: 10.1016/0306-4522(92)90494-m. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I. Diversity in the Neuronal Machine – Order and Variability in Interneuronal Microcircuits. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Soltesz I, Deschênes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Staley K. The role of an inwardly rectifying chloride conductance in postsynaptic inhibition. J Neurophysiol. 1994;72:273–284. doi: 10.1152/jn.1994.72.1.273. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Soltesz I. Functional specificity of mossy fiber innervation of GABAergic cells in the hippocampus. J Neurosci. 2009;29:4239–4251. doi: 10.1523/JNEUROSCI.5390-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci. 2011;31:10948–10970. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C, Lee SY, Soltesz I. Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci. 2010;13:822–824. doi: 10.1038/nn.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Winterer J, Stempel AV, Dugladze T, Földy C, Maziashvili N, Zivkovic AR, Priller J, Soltesz I, Gloveli T, Schmitz D. Cell-type-specific modulation of feedback inhibition by serotonin in the hippocampus. J Neurosci. 2011;31:8464–8475. doi: 10.1523/JNEUROSCI.6382-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Anderson SA, Yuste R. The enigmatic function of chandelier cells. Front Neurosci. 2010;4:201. doi: 10.3389/fnins.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyeth MS, Zhang N, Mody I, Houser CR. Selective reduction of cholecystokinin-positive basket cell innervation in a model of temporal lobe epilepsy. J Neurosci. 2010;30:8993–9006. doi: 10.1523/JNEUROSCI.1183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Soltesz I, Bragin A, Penttonen M, Sik A, Buzsaki G. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus. 1995;5:78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458:501–504. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Buckmaster PS. Dysfunction of the dentate basket cell circuit in a rat model of temporal lobe epilepsy. J Neurosci. 2009;29:7846–7856. doi: 10.1523/JNEUROSCI.6199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


